- Record: found
- Abstract: found
- Article: not found
New single-molecule speckle microscopy reveals modification of the retrograde actin flow by focal adhesions at nanometer scales
Read this article at
Abstract
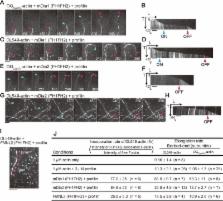
This paper introduces a new, easy-to-use method of fluorescence single-molecule speckle microscopy for actin with nanometer-scale accuracy. This new method reveals that actin flows in front of mature focal adhesions (FAs) are fast and biased toward FAs, suggesting that mature FAs are actively engaged in pulling and remodeling the local actin network.
Abstract
Speckle microscopy directly visualizes the retrograde actin flow, which is believed to promote cell-edge protrusion when linked to focal adhesions (FAs). However, it has been argued that, due to rapid actin turnover, the use of green fluorescent protein–actin, the lack of appropriate analysis algorithms, and technical difficulties, speckle microscopy does not necessarily report the flow velocities of entire actin populations. In this study, we developed a new, user-friendly single- molecule speckle (SiMS) microscopy using DyLight dye-labeled actin. Our new SiMS method enables in vivo nanometer-scale displacement analysis with a low localization error of ±8–8.5 nm, allowing accurate flow-velocity measurement for actin speckles with lifetime <5 s. In lamellipodia, both short- and long-lived F-actin molecules flow with the same speed, indicating they are part of a single actin network. These results do not support coexistence of F-actin populations with different flow speeds, which is referred to as the lamella hypothesis. Mature FAs, but not nascent adhesions, locally obstruct the retrograde flow. Interestingly, the actin flow in front of mature FAs is fast and biased toward FAs, suggesting that mature FAs attract the flow in front and actively remodel the local actin network.
Related collections
Most cited references56
- Record: found
- Abstract: found
- Article: not found
Actin, a central player in cell shape and movement.
- Record: found
- Abstract: found
- Article: not found
Feature point tracking and trajectory analysis for video imaging in cell biology.
- Record: found
- Abstract: found
- Article: not found
