- Record: found
- Abstract: found
- Article: found
Layer 2/3 pyramidal cells in the medial prefrontal cortex moderate stress induced depressive behaviors
Read this article at
Abstract
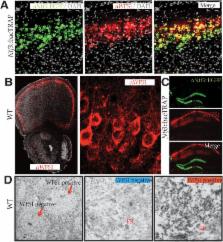
Major depressive disorder (MDD) is a prevalent illness that can be precipitated by acute or chronic stress. Studies of patients with Wolfram syndrome and carriers have identified Wfs1 mutations as causative for MDD. The medial prefrontal cortex (mPFC) is known to be involved in depression and behavioral resilience, although the cell types and circuits in the mPFC that moderate depressive behaviors in response to stress have not been determined. Here, we report that deletion of Wfs1 from layer 2/3 pyramidal cells impairs the ability of the mPFC to suppress stress-induced depressive behaviors, and results in hyperactivation of the hypothalamic–pituitary–adrenal axis and altered accumulation of important growth and neurotrophic factors. Our data identify superficial layer 2/3 pyramidal cells as critical for moderation of stress in the context of depressive behaviors and suggest that dysfunction in these cells may contribute to the clinical relationship between stress and depression.
eLife digest
Around 16% of people will experience an episode of major depression at some point in their lives, with symptoms including a loss of motivation, a reduced enjoyment of previously pleasurable activities, and disturbances in sleep and appetite. Multiple genes and environmental factors have been implicated in depression, and one of the strongest risk factors for developing the disorder is exposure to stress.
Stress and depression affect many of the same brain regions, most notably the prefrontal cortex—an area that is involved in decision making, problem solving and regulating emotions. Shrestha et al. therefore reasoned that a good way of obtaining insights into the relationship between stress and depression would be to study prefrontal cortex cells that express genes that have been linked to depression.
One such gene is Wfs1. Mutations in this gene cause a rare disorder called Wolfram syndrome, in which affected individuals experience a wide range of symptoms that often include severe depression. Shrestha et al. identified a specific population of cells in the prefrontal cortex that express Wfs1. When subjected to a stressful event, such as being restrained, mice that had been genetically modified to lack this gene in their prefrontal cortex were more likely to exhibit depression-like behaviors than non-modified mice. The genetically modified mice also released more stress hormones when restrained and produced different amounts of a number of proteins that regulate the growth and signaling of neurons.
Shrestha et al. propose that these proteins act on neural circuits that control how the mice respond to stress. Furthermore, changes in the levels or the distribution of these proteins may increase the likelihood that a stressful event will trigger behaviors associated with depression. Further experiments are required to investigate the possibility that using drugs to manipulate cells that express Wfs1 could protect against the harmful effects of stress, or even treat existing episodes of depression.
Related collections
Most cited references54
- Record: found
- Abstract: not found
- Article: not found
Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing
- Record: found
- Abstract: found
- Article: not found
A gene expression atlas of the central nervous system based on bacterial artificial chromosomes.
- Record: found
- Abstract: found
- Article: not found