- Record: found
- Abstract: found
- Article: found
Evaluation of Hedgehog Pathway Inhibitors as a Therapeutic Option for Uterine Leiomyosarcoma Using the Xenograft Model
Read this article at
Abstract
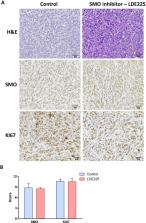
Uterine leiomyosarcoma (LMS) contributes to a significant proportion of uterine cancer deaths. It is a rare and high-risk gynecological cancer. LMS is challenging to the treatment due to the resistance of several therapies. The activation of the Hedgehog (HH) pathway has been reported in several types of female cancers. Uterine LMS presents an upregulation of the crucial HH signaling pathway members such as SMO and GLI1. Although targeting the HH pathway exhibited a potent inhibitory effect on the phenotype of uterine LMS in vitro, the effect of the HH inhibitors on LMS growth in vivo has not been identified. The present study aimed to assess the effect of Hedgehog pathway inhibitors (SMO-LDE225 and GLI-Gant61) as a therapeutic option in the xenograft model of uterine LMS. The results demonstrated that LDE225 treatment did not show any inhibitory effect on LMS tumor growth; however, treatment with GLI inhibitor (Gant61) induced a remarkable tumor regression with a significant decrease in Ki67 expression, compared to control ( p < 0.01). Moreover, administration of Gant61 decreased the expression of GLI1, GLI target genes BMP4 and c-MYC ( p < 0.05), indicating that the HH pathway is implicated in the LMS experimental model. In conclusion, our studies demonstrate for the first time that GLI inhibitor (Gant61), but not SMO inhibitor (LDE225), shows a potent inhibitory effect on LMS tumor growth and concomitantly suppresses the expression of GLI1- and GLI-targeted genes using the xenograft model of uterine LMS.
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial.
- Record: found
- Abstract: found
- Article: not found
The mechanisms of Hedgehog signalling and its roles in development and disease.
- Record: found
- Abstract: found
- Article: not found