- Record: found
- Abstract: found
- Article: found
Design, synthesis, and computational studies on dihydropyrimidine scaffolds as potential lipoxygenase inhibitors and cancer chemopreventive agents
Abstract
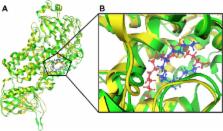
Dihydropyrimidine scaffold has a wide range of potential pharmacological activities such as antiviral, antitubercular, antimalarial, anti-inflammatory, and anticancer properties. 5-Lipoxygenase enzyme is an enzyme responsible for the metabolism of arachidonic acid to leukotrienes. The elevated levels of this enzyme and its metabolites in cancer cells have a direct relation on the development of cancer when compared to normal cells. The development of novel lipoxygenase inhibitors can have a major role in cancer therapy. A series of substituted 1,4-dihydropyrimidine analogues were synthesized and characterized by 1H-NMR, 13C-NMR, and HRMS. Molecular docking against lipoxygenase enzyme (protein data bank code =3V99) was done using Molecular Operating Environment 2013.08 and Leadit 2.1.2 softwares and showed high affinities. The synthesized compounds were tested for their lipoxygenase inhibitory activity and showed inhibition ranging from 59.37%±0.66% to 81.19%±0.94%. The activity was explained by a molecular docking study. The title compounds were also tested for cytotoxic activity against two human cancer cell lines Michigan Cancer Foundation-7 and human melanoma cells and a normal peripheral blood mononuclear cell line.
Most cited references29
- Record: found
- Abstract: found
- Article: not found
A fast flexible docking method using an incremental construction algorithm.
- Record: found
- Abstract: found
- Article: not found
Recent advances in the Biginelli dihydropyrimidine synthesis. New tricks from an old dog.

- Record: found
- Abstract: found
- Article: found