- Record: found
- Abstract: found
- Article: found
A Proof of Concept, Phase II Randomized European Trial, on the Efficacy of ALF-5755, a Novel Extracellular Matrix-Targeted Antioxidant in Patients with Acute Liver Diseases
Read this article at
Abstract
Objective
No efficient medical treatment is available for severe acute hepatitis (SAH) except N-acetylcysteine for acetaminophen-induced acute liver failure. The human C-type lectin Reg3α, referred to as ALF-5755, improved survival in an animal model of acute liver failure and was well tolerated in a phase 1 trial in humans. We performed a phase 2a trial of ALF5755 in non-acetaminophen induced SAH.
Design
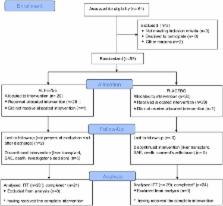
double-blind, randomized, placebo-controlled study. The primary end-point was the improvement in the coagulation protein synthesis assessed by the change of Prothrombin (PR) during the 72 hours following treatment initiation calculated as PRH0 minus PRH72 divided by 72 (PR slope H0H72). Intention to treat (ITT) and per-protocol (PP) analysis of the entire group and the Hepatitis B virus (HBV)/AIH (auto-immune hepatitis) sub-group were done separately.
Results
57 patients were included. Twenty-eight received ALF-5755, 29 the placebo. Etiologies were: Hepatitis A (n = 10), HBV (n = 13), AIH (n = 9), drug-induced (n = 8), other (n = 17). On the whole group, nor the PR slope H0H72 (0.18±0.31 vs 0.25±0.32), nor the transplant-free survival rate at day 21 (75 vs 86%) differed between groups. Conversely, in the HBV-AIH subgroup, in which ALF was more severe, PR slope H0-H72 was higher in the ALF-5755 arm, the difference being significant in PP analysis (0.048±0.066 vs -0.040±0.099, p = 0.04); the median length of hospitalization was lower in the ALF-5755 group (8 vs 14 days, p = 0.02).
Conclusion
ALF-5755 was not efficient in a ITT analysis performed on the whole sample; however it led to a significant, although moderate, clinical benefit in a PP analysis of the sub-group of patients with HBV or AIH related SAH. As HBV is the major cause of SAH in Asia and Africa and AIH a growing cause, this study emphasizes the need to pursuit the evaluation of this novel medical treatment of SAH.
Related collections
Most cited references17
- Record: found
- Abstract: found
- Article: not found
Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure.
- Record: found
- Abstract: found
- Article: not found
Acute liver failure: Summary of a workshop.
- Record: found
- Abstract: found
- Article: found