- Record: found
- Abstract: found
- Article: found
Radiation‐induced myocardial fibrosis: Mechanisms underlying its pathogenesis and therapeutic strategies
Read this article at
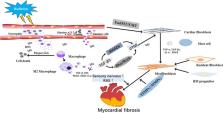
Abstract
Radiation‐induced myocardial fibrosis (RIMF) is a potentially lethal clinical complication of chest radiotherapy (RT) and a final stage of radiation‐induced heart disease (RIHD). RIMF is characterized by decreased ventricular elasticity and distensibility, which can result in decreased ejection fraction, heart failure and even sudden cardiac death. Together, these conditions impair the long‐term health of post‐RT survivors and limit the dose and intensity of RT required to effectively kill tumour cells. Although the exact mechanisms involving in RIMF are unclear, increasing evidence indicates that the occurrence of RIMF is related to various cells, regulatory molecules and cytokines. However, accurately diagnosing and identifying patients who may progress to RIMF has been challenging. Despite the urgent need for an effective treatment, there is currently no medical therapy for RIMF approved for routine clinical application. In this review, we investigated the underlying pathophysiology involved in the initiation and progression of RIMF before outlining potential preventative and therapeutic strategies to counter this toxicity.
Related collections
Most cited references113
- Record: found
- Abstract: found
- Article: not found
Cardiac Fibrosis: The Fibroblast Awakens.
- Record: found
- Abstract: found
- Article: not found
Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac fibrosis.
- Record: found
- Abstract: found
- Article: not found