- Record: found
- Abstract: found
- Article: found
Hyperalgesia and Reduced Offset Analgesia During Spinal Anesthesia
Abstract
Introduction
Spinal anesthesia induces short-term deafferentation and causes connectivity changes in brain areas involved in endogenous pain modulation. We determined whether spinal anesthesia alters pain sensitivity and offset analgesia. Offset analgesia is a manifestation of endogenous pain modulation and characterized by profound analgesia upon a small decrease in noxious stimulation.
Methods
In this randomized controlled crossover trial, static thermal pain responses and offset analgesia were obtained in 22 healthy male volunteers during spinal anesthesia and control conditions (absence of spinal anesthesia). Pain responses and offset analgesia were measured on a remote skin area above the upper level of anesthesia (C8/Th1).
Results
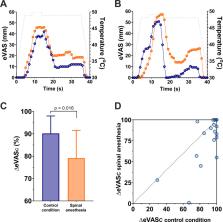
Following spinal injection of the local anesthetic, the average maximum anesthesia level was Th6. Static pain scores at C8/Th1 were higher during spinal anesthesia compared to control: 59.1 ± 15.0 mm (spinal anesthesia) versus 51.7 ± 19.7 mm (control; p = 0.03). Offset analgesia responses were decreased during spinal analgesia: pain score decrease 79 ± 27% (spinal anesthesia) versus 90 ± 17% (control; p = 0.016).
Discussion
We confirmed that spinal anesthesia-induced deafferentation causes hyperalgesic responses to noxious thermal stimulation and reduced offset analgesia at dermatomes remote and above the level of deafferentation. While these data suggest that the reduction of offset analgesia has a central origin, related to alterations in brain areas involved in inhibitory pain control, we cannot exclude alternative (peripheral) mechanisms.
Most cited references25
- Record: found
- Abstract: found
- Article: not found
Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy.
- Record: found
- Abstract: found
- Article: not found
Large-scale reorganization of the somatosensory cortex following spinal cord injuries is due to brainstem plasticity.
- Record: found
- Abstract: found
- Article: not found