- Record: found
- Abstract: found
- Article: found
Antibiotic Exposure Has Sex-Dependent Effects on the Gut Microbiota and Metabolism of Short-Chain Fatty Acids and Amino Acids in Mice
Read this article at
Abstract
Accumulating evidence shows that the gut microbiota regulates host metabolism by producing a series of metabolites, such as amino acids, bile acids, fatty acids, and others. These metabolites have a positive or negative effect on host health. Antibiotic exposure can disrupt the gut microbiota and thereby affect host metabolism and physiology. However, there are a limited number of studies addressing whether antibiotic effects on the gut microbiota and host metabolism are sex dependent. In this study, we uncovered a sex-dependent difference in antibiotic effects on the gut microbiota and metabolome in colonic contents and tissues in mice. These findings reveal that sex-dependent effects need to be considered for antibiotic use in scientific research or clinical practice. Moreover, this study will also give an important direction for future use of antibiotics to modify the gut microbiome and host metabolism in a sex-specific manner.
ABSTRACT
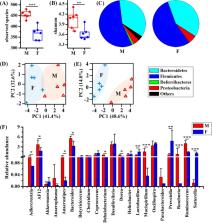
The gut microbiota has the capability to regulate homeostasis of the host metabolism. Since antibiotic exposure can adversely affect the microbiome, we hypothesized that antibiotic effects on the gut microbiota and host metabolism are sex dependent. In this study, we examined the effects of antibiotic treatments, including vancomycin (Vanc) and ciprofloxacin-metronidazole (CiMe), on the gut microbiome and metabolome in colonic contents and tissues in both male and female mice. We found that the relative abundances and structural composition of Firmicutes were significantly reduced in female mice after both Vanc and CiMe treatments but in male mice only after treatment with Vanc. However, Vanc exposure considerably altered the relative abundances and structural composition of representatives of the Proteobacteria especially in male mice. The levels of short-chain fatty acids (SCFAs; acetate, butyrate, and propionate) in colonic contents and tissues were significantly decreased in female mice after both antibiotic treatments, while these reductions were detected in male mice only after Vanc treatment. However, another SCFA, formate, exhibited the opposite tendency in colonic tissues. Both antibiotic exposures significantly decreased the levels of alanine, branched-chain amino acids (BCAAs; leucine, isoleucine, and valine) and aromatic amino acids (AAAs; phenylalanine and tyrosine) in colonic contents of female mice but not in male mice. Additionally, female mice had much greater correlations between microbe and metabolite than male mice. These findings suggest that sex-dependent effects should be considered for antibiotic-induced modifications of the gut microbiota and host metabolism.
IMPORTANCE Accumulating evidence shows that the gut microbiota regulates host metabolism by producing a series of metabolites, such as amino acids, bile acids, fatty acids, and others. These metabolites have a positive or negative effect on host health. Antibiotic exposure can disrupt the gut microbiota and thereby affect host metabolism and physiology. However, there are a limited number of studies addressing whether antibiotic effects on the gut microbiota and host metabolism are sex dependent. In this study, we uncovered a sex-dependent difference in antibiotic effects on the gut microbiota and metabolome in colonic contents and tissues in mice. These findings reveal that sex-dependent effects need to be considered for antibiotic use in scientific research or clinical practice. Moreover, this study will also give an important direction for future use of antibiotics to modify the gut microbiome and host metabolism in a sex-specific manner.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
FLASH: fast length adjustment of short reads to improve genome assemblies.
- Record: found
- Abstract: found
- Article: not found
Elevated circulating branched chain amino acids are an early event in pancreatic adenocarcinoma development
- Record: found
- Abstract: found
- Article: not found