- Record: found
- Abstract: found
- Article: found
Anti-Inflammatory and Neuroprotective Effects of Co-UltraPEALut in a Mouse Model of Vascular Dementia
Read this article at
Abstract
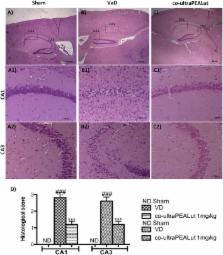
Vascular dementia (VaD), the second most common cause of cognitive impairment in the population, is a disease that results from reduction in regional cerebral blood flow and involves oxidative stress and inflammation. Co-ultramicronized PEALut (co-ultra PEALut) is a new compound with beneficial effects, which include anti-inflammatory and antioxidant properties. Recently, co-ultraPEALut has been shown to exhibit neuroprotective effects in models of Parkinson’s disease, cerebral ischemia and Alzheimer’s disease. However, its effects on VaD remain unknown. Therefore, the purpose of the present study was to highlight the potential neuroprotective actions of co-ultraPEALut containing N-palmitoylethanolamine (PEA) and the antioxidant flavonoid luteolin (Lut) (10:1 by mass) in a mouse model of VaD induced by bilateral carotid arteries occlusion. At 24 h after VaD induction, mice were orally treated with 1 mg/kg co-ultraPEALut daily for 15 days. On the 15th day, brain tissues were processed for histological, immunohistochemical, Western blot, and immunofluorescent analysis. Our results clearly demonstrate that co-ultraPEALut improved learning, memory ability, locomotor activity, and the reciprocal social interaction. In addition, the mice subjected to VaD and treated with the co-ultraPEALut showed a reorganization of CA1 and CA3 regions of the hippocampus and restored the number of hippocampal neurons as evidenced by NeuN expression, a specific marker of neurons. Furthermore following carotid arteries ligation, mice treated with co-ultraPEALut showed a modification of proinflammatory, proapoptotic proteins and of oxidative stress as evidenced by the expression of IκB-α, NF-κB p65, Bax, Bcl-2, inducible nitric oxide synthase, and cyclooxygenase-2. In order, co-ultraPEALut treatment restored VaD-induced loss of brain-derived neurotrophic factor and neurotrophins 3 (NT-3) expression in mice. These results confirmed that the neuroprotective effects of co-ultraPEALut were associated with its anti-inflammatory and antioxidant properties.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
Behavioural phenotyping assays for mouse models of autism.
- Record: found
- Abstract: found
- Article: not found
The orphan receptor GPR55 is a novel cannabinoid receptor.
- Record: found
- Abstract: found
- Article: not found