- Record: found
- Abstract: found
- Article: not found
Signaling memory from cell shape
in-brief
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
The shape of cells controls the speed and duration of signaling and can maintain short-term
signaling memory in cellular subdomains, report Craske et al. on page 1147.
Figure
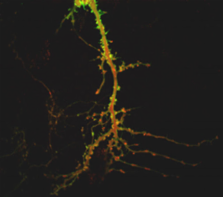
Glutamate triggers translocation of PKCγ (green) into spines. The postsynaptic marker
PSD95 is in red.
In response to stimulation, signaling molecules move through cells to newly available
binding sites at a rate consistent with random diffusion. In round cells, such diffusible
signals reach different regions of the membrane at nearly the same time. To find out
what happens in cells with complex morphologies, such as neurons that have thick and
thin branches and spines, Craske et al. employed confocal video microscopy of YFP-labeled
protein kinase C (PKC) movement following glutamate stimulation.PKC moved rapidly
to the plasma membrane of the soma and thick branches, but arrived at the membrane
of thin branches and spines several seconds later. Moreover, PKC remained in the membrane
of thin branches and spines well after it had retreated from the membrane around the
soma.
Mathematical modeling indicated that diffusion could account for the pattern of PKC
localization when cell shape was taken into account. The bulk of the cytosol, and
thus the majority of PKC, is in the cell body before cell stimulation. Following stimulation,
PKC movement was unimpeded in the soma and thick branches, but the larger surface
area to volume ratio of thin branches meant that there was not enough PKC close by
to fill the binding sites in the membrane. Thus, PKC continued to drift in from other
parts of the cell after binding in the membrane of the soma and thick branches was
complete. In the case of the spines, modeling showed that the narrow necks constrained
PKC movement and slowed accumulation and dispersal.
The differential rate of accumulation of PKC means that some regions of the cell could
remain semi-active for five to ten seconds after signaling was complete in the soma.
These cell regions would retain a memory of recent signaling events and would be primed
for subsequent ones. The team expects such signaling memory will be found in other
membrane-targeted proteins.
Related collections
Author and article information
Comments
Comment on this article
scite_
