- Record: found
- Abstract: found
- Article: found
Surrogate endpoints in global health research: still searching for killer apps and silver bullets?
editorial
8 March 2018
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
In clinical research, there is widespread acceptance that surrogate endpoints may
not translate to long-term benefits.1–3 Clinical epidemiologists highlight the hazards
of surrogate measures (eg, biomarkers, laboratory test results and short-term improvements
in health) that substitute for outcomes which are important for patients (eg, avoiding
premature death or severe disability). For example, in cardiovascular research, improvements
in parameters such as blood pressure or cholesterol may not improve outcomes such
as deaths. Improvements in surrogate endpoints may not correlate with real outcomes
of interest (and may even increase the risk of death, in some cases). And there are
many examples and case studies in the literature that illustrate the hazards of using
surrogates in clinical epidemiology.1–3
In comparison, in global health, we are often stunned when interventions that showed
improvements in surrogate endpoints do not lead to lives being saved. Take, for example,
the new tuberculosis (TB) detection technology, Xpert MTB/RIF(R) (Cepheid Inc, Sunnyvale,
California, USA), an automated, molecular test for TB and drug resistance. Xpert MTB/RIF
was first endorsed by WHO in 20104 and has since been rolled out in many countries
with over 23 million tests conducted in the past 6 years.5 While the test is rapid,
accurate and much superior to tests that have been in use for decades,6 some pragmatic
randomised controlled trials (RCTs) did not show improvements in long-term outcomes
such as reduction in mortality.7 8 These results have prompted media headlines such
as ‘improved diagnostics fail to halt the rise of tuberculosis.”9
The recent RCT in India of the WHO Safe Childbirth Checklist presents another example.
The WHO Safe Childbirth Checklist is a quality-improvement tool to promote systematic
adherence to practices that have been associated with improved childbirth outcomes.10
In a large-scale study in 24 districts in India, adherence of birth attendants to
essential birth practices was higher in facilities that participated in the coaching-based
WHO Safe Childbirth Checklist programme than in those that did not. But maternal and
perinatal mortality and maternal morbidity did not differ significantly between the
two groups.10 Again, this prompted media headlines such as ‘a birth checklist fails
to reduce deaths in rural India’11 and ‘a lifesaving childbirth tool was successfully
introduced in India—but saved no lives’.12
There are many more such examples in global health, from complex water and sanitation
interventions, to TB vaccine trials, where surrogate endpoints do not align well with
long-term outcomes.13 14 But given the weak health systems in many low-income and
middle-income countries, it is surprising that global health researchers and journalists
have great expectations that new tools, widgets, drones and checklists will save lives
and are then stunned and disappointed when they do not. These ‘technological’ innovations
often improve surrogate endpoints but may fail to meaningfully improve clinical outcomes
in part because such outcomes improve only when a series of causal events are improved
or completed. Often, the entire cascade of events in healthcare needs to improve;
merely improving one or two steps (eg, diagnosis or process of care) may not lead
to improvements in overall outcomes or result in sustained benefit.
In addition, there are innovations for which the expectation of improved health outcomes
may not be necessary; especially innovations that aim to facilitate the patient–provider
interface through improved coordination and integration of care (eg, using text message
reminders, video consultations, remote monitoring and medication adherence technologies).15–17
For example, while a patient’s health may not improve simply because they are able
to consult their general practitioner via Skype, such innovations may make the process
and experience of care more convenient, save the costs of travel and forfeited work
and reduce care-seeking delays. But again, important as they are, these benefits are
only points in the causal cascade that link innovations to improved health outcomes,
and indicators of these benefits (rather than health outcomes) may be sufficient to
determine whether an innovation is effective.
It is important that global health researchers are realistic when choosing indicators
of effectiveness—an innovation designed to reduce costs or improve convenience should
be evaluated primarily based on those indicators. For example, the purpose of a TB
diagnostic test is to rapidly and accurately identify patients with TB. Once this
is done, other factors become more prominent (see figure 1),18 for example, what treatment
is initiated and why (empirical vs test and treat), how quickly, treatment completion
rates and treatment of comorbidities. These steps in the care cascade are often weak
in many settings.7 19–21 In that case, is it fair to expect a TB test to save lives?
Likewise, it is not fair to expect that adherence to a childbirth checklist would
save lives. The purpose of a checklist is to ensure that essential tasks are done
during childbirth. But what if pregnant women do not come to health facilities on
time, or when referred for urgent hospital care they are unable to reach hospitals,
which may even lack facilities for Caesarean section or blood transfusion?12
Figure 1
A framework for outlining the pathways through which new tuberculosis (TB) tests can
result in improved patient outcomes. Source: Schumacher et al
18 PLoS ONE 2016 (open access under Creative Commons license).
We need to be more strategic about using surrogate endpoints in global health. First,
because some innovations are developed essentially to influence such surrogate endpoints;
second, because health system factors may predictably intervene in the care cascade
and third because waiting for long-term outcomes could delay the introduction of useful
innovations. On the other hand, we must not use surrogate endpoints naively, given
the dangers inherent in such endpoints. We must learn from clinical epidemiologists
who argue that, ‘researchers should avoid surrogate endpoints unless they have been
validated’2 and caution us that ‘the use of surrogate outcomes should be limited to
situations where a surrogate has demonstrated robust ability to predict meaningful
benefits’.3
Global health researchers should design innovative studies to show if and how surrogate
endpoints alter subsequent causal events or influence patient outcomes. If we care
about reducing mortality after use of a TB test or a childbirth checklist, then we
should also ensure that health systems are able to deliver subsequent life-saving
activities in the care cascade; a shift from a fixation on tools to patient-centred
solutions; from trials of standalone innovations to evaluations of complex, multisectoral
health interventions. Such studies do not have to be large RCTs with mortality as
the main outcome, given the methodological challenges of conducting RCTs, when (unlike
for drug or vaccines) the effectiveness of the innovation being trialled (eg, a diagnostic,
checklist, or text message reminder) depends on events further downstream in the care
cascade, which in turn depend on health system context.22–24
RCTs may have little value in evaluating innovations such as the WHO Childbirth Checklist
for which there is already strong and widely accepted evidence for the effectiveness
of each of their component interventions.10 Even if an RCT were to show improved maternal
and neonatal outcomes in one setting, it is unclear that the intervention would have
had a similar effect elsewhere, given that implementation and health system context
vary significantly.25 Indeed, causal pathways in public health interventions are often
long and complex, and RCT results are subject to effect modification.26 Unfortunately,
when such positive effects are found in RCTs, the result is often promoted as though
the findings of the study would be applicable everywhere. And despite the limitations
of RCTs or outcomes used in RCTs in global health, donors and guideline development
groups (eg, Grading of Recommendations, Assessment, Development and Evaluations27
often prioritise evidence from RCTs, even when RCTs may not be necessary or appropriate
for the innovation being considered for policy.
We therefore propose two ways forward. First, map out the exact point in the cascade
of care pathway in which an innovation is inserted and theorise how it may make a
difference and what barriers may impede its effects on health outcomes. Using the
TB example, while figure 1 shows a conceptual causal pathway through which a diagnostic
can have an impact,18 figure 2 shows an actual, messy pathway that patients navigate
within a real world, fragmented health system,28 thus identifying assumptions that
must hold, and barriers that must be overcome, for a diagnostic test to fulfil its
potential. Second, use theory-driven heath systems and implementation research29 30
on the adoption of innovations to confirm or refute assumptions of how an innovation
might work along the mapped-out care pathway and examine the impact of innovations
on the surrogate endpoints along the care cascade. Such implementation research can
provide rich insights into how we can optimise the impact and transferability of innovations,
depending on context.31–33
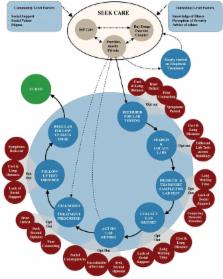
Figure 2
How patients navigate the diagnostic ecosystem in a fragmented health system in India. Source:
Yellapa et al
28 Global Health Action 2017 (open access under Creative Commons license).
We need to explicitly lower unreasonable expectations of the impact of innovations,
when surrogate endpoints are used, and when findings (including of RCTs) may not be
transferable beyond specific and similar context. We need to explain the difference
between surrogate endpoints and patient outcomes to policymakers and also to journalists
to make sure their reporting is factual and honest. Neither the Xpert MTB/RIF test
nor the WHO Safe Childbirth Checklist should be given up just because results of RCTs
on their effect on mortality are not favourable. New tools have their place and are
urgently needed in global health. Searching for silver bullets and killer apps are
worthwhile endeavours, but we must not expect them to be ‘silver’ or ‘killer’ when
introduced into systems that are suboptimal. If we care about making a real difference
in global health, we also need to work on strengthening health systems to ensure holistic,
effective and long-lasting solutions for patients and communities.
Related collections
Most cited references13
- Record: found
- Abstract: not found
- Article: not found
Surrogate end points in clinical trials: are we being misled?
Thomas Fleming (1996)

- Record: found
- Abstract: found
- Article: found
The South African Tuberculosis Care Cascade: Estimated Losses and Methodological Challenges
Pren Naidoo, Grant Theron, Molebogeng Rangaka … (2017)
- Record: found
- Abstract: found
- Article: not found