- Record: found
- Abstract: found
- Article: found
The Kallikrein-Kinin System: A Novel Mediator of IL-17-Driven Anti- Candida Immunity in the Kidney

Read this article at
Abstract
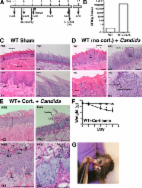
The incidence of life-threatening disseminated Candida albicans infections is increasing in hospitalized patients, with fatalities as high as 60%. Death from disseminated candidiasis in a significant percentage of cases is due to fungal invasion of the kidney, leading to renal failure. Treatment of candidiasis is hampered by drug toxicity, the emergence of antifungal drug resistance and lack of vaccines against fungal pathogens. IL-17 is a key mediator of defense against candidiasis. The underlying mechanisms of IL-17-mediated renal immunity have so far been assumed to occur solely through the regulation of antimicrobial mechanisms, particularly activation of neutrophils. Here, we identify an unexpected role for IL-17 in inducing the Kallikrein (Klk)-Kinin System (KKS) in C. albicans-infected kidney, and we show that the KKS provides significant renal protection in candidiasis. Microarray data indicated that Klk1 was upregulated in infected kidney in an IL-17-dependent manner. Overexpression of Klk1 or treatment with bradykinin rescued IL-17RA -/- mice from candidiasis. Therapeutic manipulation of IL-17-KKS pathways restored renal function and prolonged survival by preventing apoptosis of renal cells following C. albicans infection. Furthermore, combining a minimally effective dose of fluconazole with bradykinin markedly improved survival compared to either drug alone. These results indicate that IL-17 not only limits fungal growth in the kidney, but also prevents renal tissue damage and preserves kidney function during disseminated candidiasis through the KKS. Since drugs targeting the KKS are approved clinically, these findings offer potential avenues for the treatment of this fatal nosocomial infection.
Author Summary
Candida albicans is the causative agent of oropharyngeal candidiasis (OPC, thrush), dermal and vaginal candidiasis. However, the most severe C. albicans-induced disease is disseminated candidiasis, a frequent nosocomial infection associated with a high mortality rate. During disseminated candidiasis, C. albicans form invasive hyphae that damage target organs, particularly kidney and liver. Previous studies have identified an essential role of interleukin-17 (IL-17) in controlling systemic infection through regulation of neutrophils. We show here for the first time that IL-17 also regulates the renal protective Kallikrein-kinin system (KKS). Our discovery of a connection between IL-17 and the KKS suggests a new, previously unanticipated avenue for the treatment of renal damage in disseminated candidiasis. These findings have potential translational significance , as agonists of the KKS are in routine clinical use. Therefore, these results not only identify downstream mediators that could serve as novel drug targets, but could possibly be used to guide decisions on whether targeting these mediators could be a useful therapeutic option in conjunction with current antifungal therapies.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: found
Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis
- Record: found
- Abstract: found
- Article: not found
Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice.

- Record: found
- Abstract: found
- Article: found