- Record: found
- Abstract: found
- Article: found
Electrical Guidance of Human Stem Cells in the Rat Brain
Read this article at
Summary
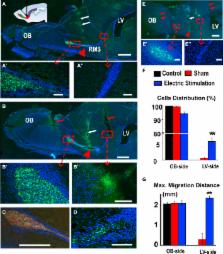
Limited migration of neural stem cells in adult brain is a roadblock for the use of stem cell therapies to treat brain diseases and injuries. Here, we report a strategy that mobilizes and guides migration of stem cells in the brain in vivo. We developed a safe stimulation paradigm to deliver directional currents in the brain. Tracking cells expressing GFP demonstrated electrical mobilization and guidance of migration of human neural stem cells, even against co-existing intrinsic cues in the rostral migration stream. Transplanted cells were observed at 3 weeks and 4 months after stimulation in areas guided by the stimulation currents, and with indications of differentiation. Electrical stimulation thus may provide a potential approach to facilitate brain stem cell therapies.
Graphical Abstract
Highlights
-
•
Developed a technology and device delivering electric current to the brain in vivo
-
•
Achieved stable delivery of currents to brain with monitoring and safety concerns
-
•
Exhibited effective guidance of migration of transplanted human NSCs in live brain
-
•
Demonstrated enhanced motility, survival, and differentiation of the guided hNSCs
Abstract
In this article, Zhao and colleagues report a novel technology delivering directional electric currents which mobilizes and guides human neural stem cells through the brain in vivo, demonstrating an effective and safe approach to facilitate stem cell therapy, with significant implications for a wide range of brain diseases.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Neuronal replacement from endogenous precursors in the adult brain after stroke.
- Record: found
- Abstract: found
- Article: not found
Corridors of Migrating Neurons in Human Brain and Their Decline during Infancy
- Record: found
- Abstract: found
- Article: not found
