- Record: found
- Abstract: found
- Article: found
Receptor-G Protein Interaction Studied by Bioluminescence Resonance Energy Transfer: Lessons from Protease-Activated Receptor 1
Read this article at
Abstract
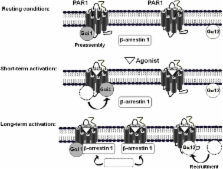
Since its development, the bioluminescence resonance energy transfer (BRET) approach has been extensively applied to study G protein-coupled receptors (GPCRs) in real-time and in live cells. One of the major aspects of GPCRs investigated in considerable details is their physical coupling to the heterotrimeric G proteins. As a result, new concepts have emerged, but few questions are still a matter of debate illustrating the complexity of GPCR-G protein interactions and coupling. Here, we summarized the recent advances on our understanding of GPCR-G protein coupling based on BRET approaches and supported by other FRET-based studies. We essentially focused on our recent studies in which we addressed the concept of preassembly vs. the agonist-dependent interaction between the protease-activated receptor 1 (PAR1) and its cognate G proteins. We discussed the concept of agonist-induced conformational changes within the preassembled PAR1-G protein complexes as well as the critical question how the multiple coupling of PAR1 with two different G proteins, Gαi1 and Gα12, but also β-arrestin 1, can be regulated.
Related collections
Most cited references69
- Record: found
- Abstract: found
- Article: not found
Crystal structure of rhodopsin: A G protein-coupled receptor.
- Record: found
- Abstract: found
- Article: not found
Thrombin signalling and protease-activated receptors.
- Record: found
- Abstract: found
- Article: not found