- Record: found
- Abstract: found
- Article: found
Hedonic Eating and the “Delicious Circle”: From Lipid-Derived Mediators to Brain Dopamine and Back
Read this article at
Abstract
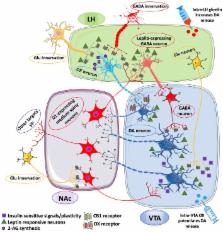
Palatable food can be seductive and hedonic eating can become irresistible beyond hunger and negative consequences. This is witnessed by the subtle equilibrium between eating to provide energy intake for homeostatic functions, and reward-induced overeating. In recent years, considerable efforts have been devoted to study neural circuits, and to identify potential factors responsible for the derangement of homeostatic eating toward hedonic eating and addiction-like feeding behavior. Here, we examined recent literature on “old” and “new” players accountable for reward-induced overeating and possible liability to eating addiction. Thus, the role of midbrain dopamine is positioned at the intersection between selected hormonal signals involved in food reward information processing (namely, leptin, ghrelin, and insulin), and lipid-derived neural mediators such as endocannabinoids. The impact of high fat palatable food and dietary lipids on endocannabinoid formation is reviewed in its pathogenetic potential for the derangement of feeding homeostasis. Next, endocannabinoid signaling that regulates synaptic plasticity is discussed as a key mechanism acting both at hypothalamic and mesolimbic circuits, and affecting both dopamine function and interplay between leptin and ghrelin signaling. Outside the canonical hypothalamic feeding circuits involved in energy homeostasis and the notion of “feeding center,” we focused on lateral hypothalamus as neural substrate able to confront food-associated homeostatic information with food salience, motivation to eat, reward-seeking, and development of compulsive eating. Thus, the lateral hypothalamus-ventral tegmental area-nucleus accumbens neural circuitry is reexamined in order to interrogate the functional interplay between ghrelin, dopamine, orexin, and endocannabinoid signaling. We suggested a pivotal role for endocannabinoids in food reward processing within the lateral hypothalamus, and for orexin neurons to integrate endocrine signals with food reinforcement and hedonic eating. In addition, the role played by different stressors in the reinstatement of preference for palatable food and food-seeking behavior is also considered in the light of endocannabinoid production, activation of orexin receptors and disinhibition of dopamine neurons. Finally, type-1 cannabinoid receptor-dependent inhibition of GABA-ergic release and relapse to reward-associated stimuli is linked to ghrelin and orexin signaling in the lateral hypothalamus-ventral tegmental area-nucleus accumbens network to highlight its pathological potential for food addiction-like behavior.
Related collections
Most cited references165
- Record: found
- Abstract: found
- Article: not found
Addiction-like reward dysfunction and compulsive eating in obese rats: Role for dopamine D2 receptors
- Record: found
- Abstract: found
- Article: not found
Reward, dopamine and the control of food intake: implications for obesity.
- Record: found
- Abstract: not found
- Article: not found