- Record: found
- Abstract: found
- Article: found
DNA methylation map in circulating leukocytes mirrors subcutaneous adipose tissue methylation pattern: a genome-wide analysis from non-obese and obese patients
Read this article at
Abstract
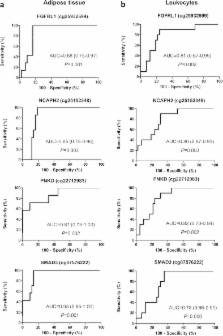
The characterization of the epigenetic changes within the obesity-related adipose tissue will provide new insights to understand this metabolic disorder, but adipose tissue is not easy to sample in population-based studies. We aimed to evaluate the capacity of circulating leukocytes to reflect the adipose tissue-specific DNA methylation status of obesity susceptibility. DNA samples isolated from subcutaneous adipose tissue and circulating leukocytes were hybridized in the Infinium HumanMethylation 450 BeadChip. Data were compared between samples from obese (n = 45) and non-obese (n = 8–10) patients by Wilcoxon-rank test, unadjusted for cell type distributions. A global hypomethylation of the differentially methylated CpG sites (DMCpGs) was observed in the obese subcutaneous adipose tissue and leukocytes. The overlap analysis yielded a number of genes mapped by the common DMCpGs that were identified to reflect the obesity state in the leukocytes. Specifically, the methylation levels of FGFRL1, NCAPH2, PNKD and SMAD3 exhibited excellent and statistically significant efficiencies in the discrimination of obesity from non-obesity status (AUC > 0.80; p < 0.05) and a great correlation between both tissues. Therefore, the current study provided new and valuable DNA methylation biomarkers of obesity-related adipose tissue pathogenesis through peripheral blood analysis, an easily accessible and minimally invasive biological material instead of adipose tissue.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Pharmacological management of obesity: an endocrine Society clinical practice guideline.
- Record: found
- Abstract: found
- Article: not found
Validation of a DNA methylation microarray for 450,000 CpG sites in the human genome.
- Record: found
- Abstract: found
- Article: not found