- Record: found
- Abstract: found
- Article: found
Aquaporins Are Differentially Regulated in Canine Cryptorchid Efferent Ductules and Epididymis
Read this article at
Abstract
Simple Summary
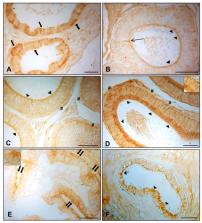
The distribution and expression of aquaporins (AQPs) in the testes and spermatozoa of several animal species play important roles in spermatogenesis and spermatozoon transit in this region. The aim of this study was to evaluate AQP7, AQP8, and AQP9 localization and expression in the efferent ductules and epididymal regions (the caput, corpus, and cauda) of normal and cryptorchid dogs. The results from immunohistochemistry, Western blotting, and real-time reverse transcription polymerase chain reaction (RT-PCR) show regional tissue distributions, particularly at the level of the epithelium of efferent ductules and both the regions caput and cauda of the canine cryptorchid epididymis. These findings support the hypothesis that these channel proteins respond differently to multiple stimuli that cause cryptorchidism (hormones, heat, osmolarity, etc.) and participate in the mechanisms of cell “resilience” or apoptosis taking place in the epididymis.
Abstract
The efferent ductules and the epididymis are parts of the male reproductive system where spermatozoa mature. Specialized epithelial cells in these ducts contribute to the transport of fluids produced by spermatozoa’s metabolic activity. Aquaporins (AQPs) have been demonstrated to be expressed in the spermatozoan membrane and testis epithelial cells, where they contribute to regulating spermatozoan volume and transit through environments of differing osmolality. Due to the lack of detailed literature regarding AQP expression in the canine male genital tract, the aim of this study was to investigate both the distribution and expression of AQP7, AQP8, and AQP9 in the efferent ductules and epididymal regions (caput, corpus, and cauda) from normal and cryptorchid dogs by using immunohistochemistry, Western blotting, and real-time reverse transcription polymerase chain reaction (RT-PCR). Our results show different patterns for the distribution and expression of the examined AQPs, with particular evidence of their upregulation in the caput and downregulation in the cauda region of the canine cryptorchid epididymis. These findings are associated with a modulation of Hsp70 and caspase-3 expression, suggesting the participation of AQPs in the luminal microenvironment modifications that are peculiar characteristics of this pathophysiological condition.
Related collections
Most cited references83
- Record: found
- Abstract: found
- Article: not found
Structure and function of aquaporin water channels

- Record: found
- Abstract: found
- Article: found
Regulation of epithelial function, differentiation, and remodeling in the epididymis
- Record: found
- Abstract: found
- Article: not found