- Record: found
- Abstract: found
- Article: found
PI 3-kinase-dependent phosphorylation of Plk1–Ser99 promotes association with 14-3-3γ and is required for metaphase–anaphase transition

Read this article at
Abstract
Polo-like kinase 1 (Plk1) controls multiple aspects of mitosis and is activated through its phosphorylation at Thr210. Here we identify Ser99 on Plk1 as a novel mitosis-specific phosphorylation site, which operates independently of Plk1–Thr210 phosphorylation. Plk1–Ser99 phosphorylation creates a docking site for 14-3-3γ, and this interaction stimulates the catalytic activity of Plk1. Knockdown of 14-3-3γ or replacement of wild-type (WT) Plk1 by a Ser99-phospho-blocking mutant leads to a prometaphase/metaphase-like arrest due to the activation of the spindle assembly checkpoint. Inhibition of phosphatidylinositol 3-kinase (PI3K) and Akt significantly reduces the level of Plk1–Ser99 phosphorylation and delays metaphase to anaphase transition. Plk1–Ser99 phosphorylation requires not only Akt activity but also protein(s) associated with Plk1 in a mitosis-specific manner. Therefore, mitotic Plk1 activity is regulated not only by Plk1–Thr210 phosphorylation, but also by Plk1 binding to 14-3-3γ following Plk1–Ser99 phosphorylation downstream of the PI3K–Akt signalling pathway. This novel Plk1 activation pathway controls proper progression from metaphase to anaphase.
Abstract
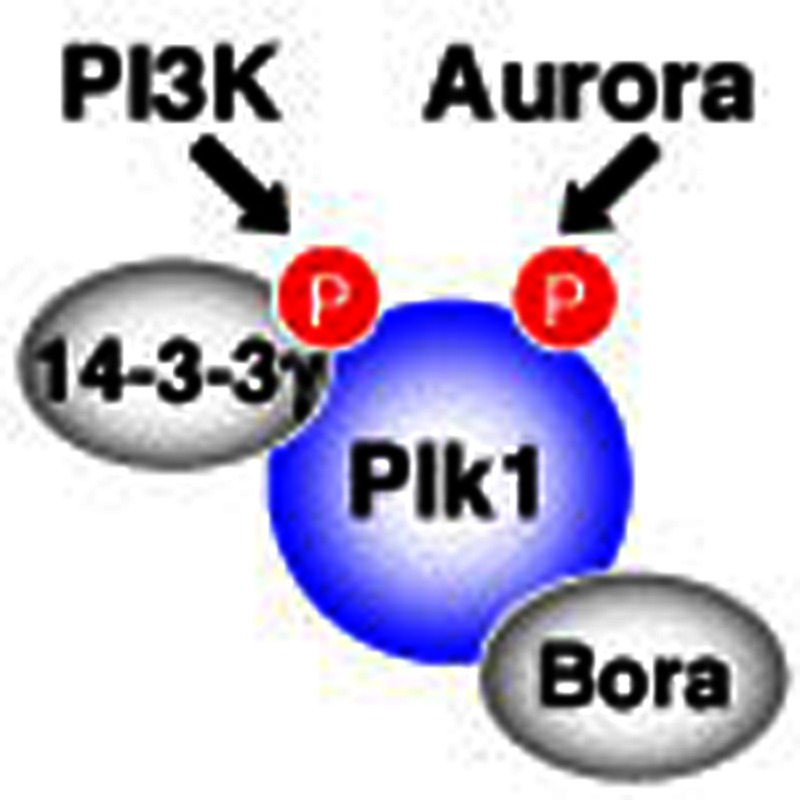 Polo-like kinase 1 (Plk1) controls the transition between metaphase and anaphase during
mitosis. Kasahara
et al. show that Plk1 activity is regulated by phosphatidylinositide 3-kinase signalling
through phosphorylation at a previously uncharacterized site.
Polo-like kinase 1 (Plk1) controls the transition between metaphase and anaphase during
mitosis. Kasahara
et al. show that Plk1 activity is regulated by phosphatidylinositide 3-kinase signalling
through phosphorylation at a previously uncharacterized site.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
The spindle-assembly checkpoint in space and time.
- Record: found
- Abstract: not found
- Article: not found
Polo-like kinases and the orchestration of cell division.
- Record: found
- Abstract: found
- Article: not found