- Record: found
- Abstract: found
- Article: found
Roles of sarcoplasmic reticulum Ca 2+ ATPase pump in the impairments of lymphatic contractile activity in a metabolic syndrome rat model
Read this article at
Abstract
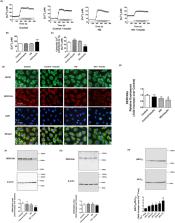
The intrinsic lymphatic contractile activity is necessary for proper lymph transport. Mesenteric lymphatic vessels from high-fructose diet-induced metabolic syndrome (MetSyn) rats exhibited impairments in its intrinsic phasic contractile activity; however, the molecular mechanisms responsible for the weaker lymphatic pumping activity in MetSyn conditions are unknown. Several metabolic disease models have shown that dysregulation of sarcoplasmic reticulum Ca 2+ ATPase (SERCA) pump is one of the key determinants of the phenotypes seen in various muscle tissues. Hence, we hypothesized that a decrease in SERCA pump expression and/or activity in lymphatic muscle influences the diminished lymphatic vessel contractions in MetSyn animals. Results demonstrated that SERCA inhibitor, thapsigargin, significantly reduced lymphatic phasic contractile frequency and amplitude in control vessels, whereas, the reduced MetSyn lymphatic contractile activity was not further diminished by thapsigargin. While SERCA2a expression was significantly decreased in MetSyn lymphatic vessels, myosin light chain 20, MLC 20 phosphorylation was increased in these vessels. Additionally, insulin resistant lymphatic muscle cells exhibited elevated intracellular calcium and decreased SERCA2a expression and activity. The SERCA activator, CDN 1163 partially restored lymphatic contractile activity in MetSyn lymphatic vessel by increasing phasic contractile frequency. Thus, our data provide the first evidence that SERCA2a modulates the lymphatic pumping activity by regulating phasic contractile amplitude and frequency, but not the lymphatic tone. Diminished lymphatic contractile activity in the vessels from the MetSyn animal is associated with the decreased SERCA2a expression and impaired SERCA2 activity in lymphatic muscle.
Related collections
Most cited references73
- Record: found
- Abstract: found
- Article: not found
Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase.
- Record: found
- Abstract: found
- Article: not found
NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart.
- Record: found
- Abstract: found
- Article: not found