- Record: found
- Abstract: found
- Article: found
Enhancing Electrochemical Water-Splitting Kinetics by Polarization-Driven Formation of Near-Surface Iron(0): An In Situ XPS Study on Perovskite-Type Electrodes**
Read this article at
Abstract
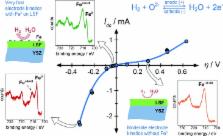
In the search for optimized cathode materials for high-temperature electrolysis, mixed conducting oxides are highly promising candidates. This study deals with fundamentally novel insights into the relation between surface chemistry and electrocatalytic activity of lanthanum ferrite based electrolysis cathodes. For this means, near-ambient-pressure X-ray photoelectron spectroscopy (NAP-XPS) and impedance spectroscopy experiments were performed simultaneously on electrochemically polarized La 0.6Sr 0.4FeO 3− δ (LSF) thin film electrodes. Under cathodic polarization the formation of Fe 0 on the LSF surface could be observed, which was accompanied by a strong improvement of the electrochemical water splitting activity of the electrodes. This correlation suggests a fundamentally different water splitting mechanism in presence of the metallic iron species and may open novel paths in the search for electrodes with increased water splitting activity.
Related collections
Most cited references45
- Record: found
- Abstract: not found
- Article: not found
Factors governing oxygen reduction in solid oxide fuel cell cathodes.
- Record: found
- Abstract: found
- Article: not found
In situ growth of nanoparticles through control of non-stoichiometry.
- Record: found
- Abstract: found
- Article: not found