- Record: found
- Abstract: found
- Article: found
Structural diversity of ABC transporters
Read this article at
Abstract
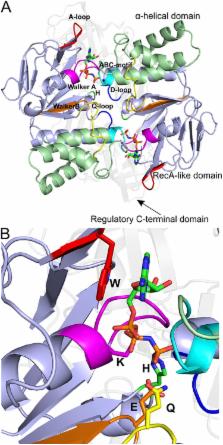
ATP-binding cassette (ABC) transporters form a large superfamily of ATP-dependent protein complexes that mediate transport of a vast array of substrates across membranes. The 14 currently available structures of ABC transporters have greatly advanced insight into the transport mechanism and revealed a tremendous structural diversity. Whereas the domains that hydrolyze ATP are structurally related in all ABC transporters, the membrane-embedded domains, where the substrates are translocated, adopt four different unrelated folds. Here, we review the structural characteristics of ABC transporters and discuss the implications of this structural diversity for mechanistic diversity.
Related collections
Most cited references78
- Record: found
- Abstract: not found
- Article: not found
Simple allosteric model for membrane pumps.
- Record: found
- Abstract: found
- Article: not found
P-glycoprotein: from genomics to mechanism.

- Record: found
- Abstract: found
- Article: found