- Record: found
- Abstract: found
- Article: found
Recessive nephrocerebellar syndrome on the Galloway-Mowat syndrome spectrum is caused by homozygous protein-truncating mutations of WDR73
Read this article at
Abstract
Galloway-Mowat syndrome (GMS) is a neurodevelopmental disorder characterized by microcephaly, cerebellar hypoplasia, nephrosis, and profound intellectual disability. Jinks et al. extend the GMS spectrum by identifying a novel nephrocerebellar syndrome with selective striatal cholinergic interneuron loss and complete lateral geniculate nucleus delamination, caused by a frameshift mutation in WDR73.
Abstract

Galloway-Mowat syndrome (GMS) is a neurodevelopmental disorder characterized by microcephaly, cerebellar hypoplasia, nephrosis, and profound intellectual disability. Jinks et al. extend the GMS spectrum by identifying a novel nephrocerebellar syndrome with selective striatal cholinergic interneuron loss and complete lateral geniculate nucleus delamination, caused by a frameshift mutation in WDR73.
Abstract
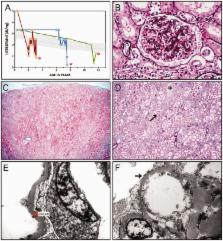
We describe a novel nephrocerebellar syndrome on the Galloway-Mowat syndrome spectrum among 30 children (ages 1.0 to 28 years) from diverse Amish demes. Children with nephrocerebellar syndrome had progressive microcephaly, visual impairment, stagnant psychomotor development, abnormal extrapyramidal movements and nephrosis. Fourteen died between ages 2.7 and 28 years, typically from renal failure. Post-mortem studies revealed (i) micrencephaly without polymicrogyria or heterotopia; (ii) atrophic cerebellar hemispheres with stunted folia, profound granule cell depletion, Bergmann gliosis, and signs of Purkinje cell deafferentation; (iii) selective striatal cholinergic interneuron loss; and (iv) optic atrophy with delamination of the lateral geniculate nuclei. Renal tissue showed focal and segmental glomerulosclerosis and extensive effacement and microvillus transformation of podocyte foot processes. Nephrocerebellar syndrome mapped to 700 kb on chromosome 15, which contained a single novel homozygous frameshift variant ( WDR73 c.888delT; p.Phe296Leufs*26). WDR73 protein is expressed in human cerebral cortex, hippocampus, and cultured embryonic kidney cells. It is concentrated at mitotic microtubules and interacts with α-, β-, and γ-tubulin, heat shock proteins 70 and 90 (HSP-70; HSP-90), and the carbamoyl phosphate synthetase 2/aspartate transcarbamylase/dihydroorotase multi-enzyme complex. Recombinant WDR73 p.Phe296Leufs*26 and p.Arg256Profs*18 proteins are truncated, unstable, and show increased interaction with α- and β-tubulin and HSP-70/HSP-90. Fibroblasts from patients homozygous for WDR73 p.Phe296Leufs*26 proliferate poorly in primary culture and senesce early. Our data suggest that in humans, WDR73 interacts with mitotic microtubules to regulate cell cycle progression, proliferation and survival in brain and kidney. We extend the Galloway-Mowat syndrome spectrum with the first description of diencephalic and striatal neuropathology.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: not found
Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1.
- Record: found
- Abstract: found
- Article: not found
Whole exome sequencing identifies recessive WDR62 mutations in severe brain malformations
- Record: found
- Abstract: found
- Article: not found