- Record: found
- Abstract: found
- Article: found
Genetics of Childhood Steroid Sensitive Nephrotic Syndrome: An Update
Read this article at
Abstract
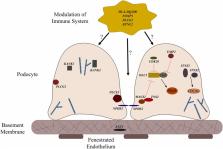
Advances in genome science in the last 20 years have led to the discovery of over 50 single gene causes and genetic risk loci for steroid resistant nephrotic syndrome (SRNS). Despite these advances, the genetic architecture of childhood steroid sensitive nephrotic syndrome (SSNS) remains poorly understood due in large part to the varying clinical course of SSNS over time. Recent exome and genome wide association studies from well-defined cohorts of children with SSNS identified variants in multiple MHC class II molecules such as HLA-DQA1 and HLA-DQB1 as risk factors for SSNS, thus stressing the central role of adaptive immunity in the pathogenesis of SSNS. However, evidence suggests that unknown second hit risk loci outside of the MHC locus and environmental factors also make significant contributions to disease. In this review, we examine what is currently known about the genetics of SSNS, the implications of recent findings on our understanding of pathogenesis of SSNS, and how we can utilize these results and findings from future studies to improve the management of children with nephrotic syndrome.
Related collections
Most cited references97
- Record: found
- Abstract: found
- Article: not found
Idiopathic nephrotic syndrome in children
- Record: found
- Abstract: found
- Article: not found
Modification of kidney barrier function by the urokinase receptor.
- Record: found
- Abstract: found
- Article: not found