- Record: found
- Abstract: found
- Article: found
Real-World Outcomes in First-Line Treatment of Metastatic Castration-Resistant Prostate Cancer: The Prostate Cancer Registry
Read this article at
Abstract
Background
Metastatic prostate cancer has a 30% 5-year survival rate despite recent therapeutic advances. There is a need to improve the clinical understanding and treatment of this disease, particularly in the real-world setting and among patients who are under-represented in clinical trials.
Objective
We aimed to evaluate the characteristics and clinical outcomes of patients who received their first treatment for metastatic castration-resistant prostate cancer (mCRPC) in routine clinical practice, independent of treatment used, including subgroups with baseline cardiac disease, diabetes mellitus, or visceral metastases.
Patients and methods
Prospective, noninterventional analysis of patient record data in the multicenter Prostate Cancer Registry (PCR) of men with mCRPC. The data were collected in 16 countries with the aim of recruiting more than 3000 patients between 2013 and 2016. The study end date was 9 July 2018. Data evaluated included baseline characteristics, treatment exposure, and efficacy outcomes [overall survival (OS) and time to progression (TTP)] of patients treated with abiraterone acetate plus prednisone or prednisolone (collectively, “abiraterone”), enzalutamide, or docetaxel. Descriptive outcomes are reported from the overall patient population and subgroups of patients with baseline cardiovascular disease, diabetes mellitus, or visceral metastases. The treatment effects for time to progression were compared for the overall patient population.
Results
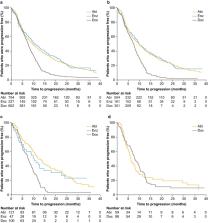
The study enrollment period lasted 2.5 years, and each patient was followed for a maximum of 3 years. A total of 1874 patients in the PCR had not received previous mCRPC treatment at baseline, although they had received androgen-deprivation therapy. Prevalent co-morbidities included cardiovascular disease in 65.4% and diabetes mellitus in 17.4% of patients. Baseline characteristics suggested that patients with more advanced disease received docetaxel treatment. In the overall patient population, the median time to progression with abiraterone, enzalutamide, and docetaxel as first-line mCRPC therapy was 9.6, 10.3, and 7.6 months, respectively, and median OS was 27.1, 27.1, and 27.9 months, respectively. Outcomes in the subgroups of patients with cardiovascular disease or diabetes mellitus were similar to those of the whole population in the analysis. As expected, patients with visceral metastases had shorter TTP and OS than patients in the overall population.
Conclusions
This analysis shows, for the first time, the effectiveness in parallel of first-line abiraterone, enzalutamide, and docetaxel in mCRPC, including in patients with co-morbidities such as cardiovascular disease or diabetes mellitus or in patients with visceral metastases. These real-world findings from the PCR provide meaningful information to help manage mCRPC, particularly in patients under-represented in clinical studies.
Related collections
Most cited references4
- Record: found
- Abstract: found
- Article: not found
Update on Systemic Prostate Cancer Therapies: Management of Metastatic Castration-resistant Prostate Cancer in the Era of Precision Oncology
- Record: found
- Abstract: found
- Article: found
Management of Patients with Advanced Prostate Cancer: The Report of the Advanced Prostate Cancer Consensus Conference APCCC 2017
- Record: found
- Abstract: found
- Article: not found