- Record: found
- Abstract: found
- Article: found
Functional dyspepsia: new insights into pathogenesis and therapy
Read this article at
Abstract
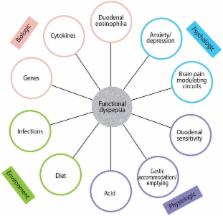
One in 10 people suffer from functional dyspepsia (FD), a clinical syndrome comprising chronic bothersome early satiety, or postprandial fullness, or epigastric pain or burning. Postprandial distress syndrome (PDS, comprising early satiety and/or postprandial fullness) and epigastric pain syndrome (EPS) are increasingly accepted as valid clinical entities, based on new insights into the pathophysiology and the results of clinical trials. Diagnosis is based on the clinical history, and exclusion of peptic ulcer and cancer by endoscopy. Evidence is accumulating FD and gastroesophageal ref lux disease are part of the same disease spectrum in a major subset. The causes of FD remain to be established, but accumulating data suggest infections and possibly food may play an important role in subsets. FD does not equate with no pathology; duodenal eosinophilia is now an accepted association, and Helicobacter pylori infection is considered to be causally linked to dyspepsia although only a minority will respond to eradication. In those with EPS, acid suppression therapy is a first line therapy; consider a H 2 blocker even if proton pump inhibitor fails. In PDS, a prokinetic is preferred. Second line therapy includes administration of a tricyclic antidepressant in low doses, or mirtazapine, but not a selective serotonin reuptake inhibitor.
Related collections
Most cited references96
- Record: found
- Abstract: found
- Article: not found
Immune activation in patients with irritable bowel syndrome.
- Record: found
- Abstract: found
- Article: not found
Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia.
- Record: found
- Abstract: found
- Article: not found