- Record: found
- Abstract: found
- Article: found
Optimal Route for Mesenchymal Stem Cells Transplantation after Severe Intraventricular Hemorrhage in Newborn Rats
Read this article at
Abstract
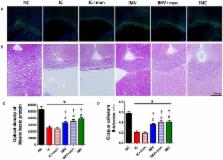
Recently, we showed that intracerebroventricular (IC) transplantation of human umbilical cord blood (UCB)-derived mesenchymal stem cells (MSCs) significantly attenuates posthemorrhagic hydrocephalus (PHH) and brain damage after severe IVH in newborn rats. This study was performed to determine the optimal route for transplanting MSCs for severe IVH by comparing IC transplantation, intravenous (IV) transplantation, and IV transplantation plus mannitol infusion. Severe IVH was induced by injecting 100 uL of blood into each ventricle of Sprague-Dawley rats on postnatal day 4 (P4). After confirming severe IVH with brain magnetic resonance imaging (MRI) at P5, human UCB-derived MSCs were transplanted at P6 by an IC route (1×10 5), an IV route (5×10 5), or an IV route with mannitol infused. Follow-up brain MRIs and rotarod tests were performed. At P32, brain tissue samples were obtained for biochemical and histological analyses. Although more MSCs localized to the brain after IC than after IV delivery, both methods were equally effective in preventing PHH; attenuating impaired rotarod test; increasing the number of TUNEL-positive cells, inflammatory cytokines, and astrogliosis; and reducing corpus callosal thickness and myelin basic protein expression after severe IVH regardless of mannitol co-infusion. Despite the superior delivery efficacy with IC than with the IV route, both IC and IV transplantation of MSCs had equal therapeutic efficacy in protecting against severe IVH. These findings suggest that the less invasive IV route might be a good alternative for clinically unstable, very preterm infants that cannot tolerate a more invasive IC delivery of MSCs.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells.
- Record: found
- Abstract: found
- Article: not found
Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993-1994.
- Record: found
- Abstract: found
- Article: not found