- Record: found
- Abstract: found
- Article: found
Myostatin Is Elevated in Congenital Heart Disease and After Mechanical Unloading
Read this article at
Abstract
Background
Myostatin is a negative regulator of skeletal muscle mass whose activity is upregulated in adult heart failure (HF); however, its role in congenital heart disease (CHD) is unknown.
Methods
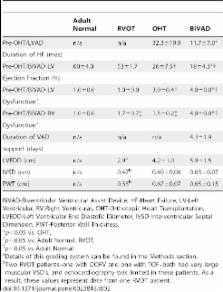
We studied myostatin and IGF-1 expression via Western blot in cardiac tissue at varying degrees of myocardial dysfunction and after biventricular support in CHD by collecting myocardial biopsies from four patient cohorts: A) adult subjects with no known cardiopulmonary disease (left ventricle, LV), (Adult Normal), (n = 5); B) pediatric subjects undergoing congenital cardiac surgery with normal RV size and function (right ventricular outflow tract, RVOT), (n = 3); C) pediatric subjects with worsening but hemodynamically stable LV failure [LV and right ventricle (LV, RV,)] with biopsy collected at the time of orthotopic heart transplant (OHT), (n = 7); and D) pediatric subjects with decompensated bi-ventricular failure on BiVAD support with biopsy collected at OHT (LV, RV, BiVAD), (n = 3).
Results
The duration of HF was longest in OHT patients compared to BIVAD. The duration of BiVAD support was 4.3±1.9 days. Myostatin expression was significantly increased in LV-OHT compared to RV-OHT and RVOT, and was increased more than double in decompensated biventricular HF (BiVAD) compared to both OHT and RVOT. An increased myostatin/IGF-1 ratio was associated with ventricular dysfunction.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Myostatin decreases with aerobic exercise and associates with insulin resistance.
- Record: found
- Abstract: found
- Article: not found
Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure.
- Record: found
- Abstract: found
- Article: not found