- Record: found
- Abstract: found
- Article: found
m6A-dependent up-regulation of DRG1 by METTL3 and ELAVL1 promotes growth, migration, and colony formation in osteosarcoma
Read this article at
Abstract
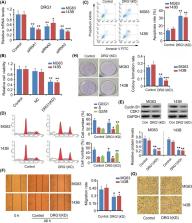
Osteosarcoma (OS) is a malignant tumor commonly observed in children and adolescents. Developmentally regulated GTP-binding protein (DRG) 1 plays an important role in embryonic development; aberrantly expressed DRG1 has been associated with pathological processes in cancer. The present study aimed to explore the role of DRG1 in OS and the mechanisms underlying DRG1 overexpression in OS. Clinical studies were performed to evaluate Drg1 expression in OS tissues and to identify a correlation between Drg1 expression and the clinicopathological features in patients with OS. Drg1 was knocked down in OS cells to determine its effects on cell viability, cell cycle distribution, apoptosis, migration, invasion, and colony formation rate. METTL3 and ELAVL1 were also silenced to determine their effects on the levels of N6-methyladenosine (m6A), RNA stability, and Drg1 expression. Higher levels of Drg1 mRNA and protein were observed in OS tissues than those in paracancerous tissues. High expression of DRG1 was associated with large tumor size and advanced clinical stages in OS. Silencing of Drg1 resulted in decreased viability and inhibition of the migration and colony formation abilities of OS cells; it also resulted in cell cycle arrest in the G 2/M stage and induced apoptosis. Knockdown of METTL3 led to decreased m6A and Drg1 mRNA levels. ELAVL1 knockdown impaired the stability of DRG1 mRNA, thereby reducing both the mRNA and protein levels of DRG1. In all, DRG1 exerted tumorigenic effects in OS, and the up-regulation of DRG1 in OS was induced by METTL3 and ELAVL1 in an m6A-dependent manner.
Related collections
Most cited references24

- Record: found
- Abstract: found
- Article: found
METTL3 facilitates tumor progression via an m 6 A-IGF2BP2-dependent mechanism in colorectal carcinoma
- Record: found
- Abstract: found
- Article: not found
