- Record: found
- Abstract: found
- Article: found
Is Irritable Bowel Syndrome Considered in Clinical Trials on Physical Therapy Applied to Patients with Temporo-Mandibular Disorders? A Scoping Review
Read this article at
Abstract
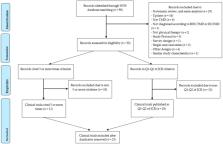
The aim of the current scoping review was to identify if the presence of irritable bowel syndrome was included as eligibility criteria of participants included in clinical trials investigating the effects of physical therapy in individuals with temporomandibular pain disorders (TMDs). A systematic electronic literature search in the Web of Science database was conducted. Scientifically relevant, randomized clinical trials (those cited in other studies at least 5 times, or clinical trials published in high-impact journals, i.e., first and second quartiles (Q1-Q2) of any category of the Journal Citation Report (JCR)) evaluating the effects of any physical therapy intervention in patients with TMDs were included. The Physiotherapy Evidence Database (PEDro) scale was used to evaluate the methodological quality of the selected trials. Authors affiliated to a clinical or non-clinical institution, total number of citations, objective, sex/gender, age, and eligibility criteria in each article were extracted and analyzed independently by two authors. From a total of 98 identified articles, 12 and 19 clinical trials were included according to the journal citation criterion or JCR criterion, respectively. After removing duplicates, a total of 23 trials were included. The PEDro score ranged from 4 to 8 (mean: 6.26, SD: 1.48). Based on the eligibility criteria of the trials systematically reviewed, none considered the presence of comorbid irritable bowel syndrome in patients with TMDs. The comorbidity between TMDs and irritable bowel syndrome is not considered within the eligibility criteria of participants in highly cited clinical trials, or published in a high-impact journal, investigating the effects of physical therapy in TMDs.
Related collections
Most cited references72
- Record: found
- Abstract: found
- Article: not found
PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation
- Record: found
- Abstract: not found
- Article: not found