- Record: found
- Abstract: found
- Article: not found
Management of bone metastasis and cancer treatment-induced bone loss during the COVID-19 pandemic: An international perspective and recommendations
Read this article at
Abstract
Optimum management of patients with cancer during the COVID-19 pandemic has proved extremely challenging. Patients, clinicians and hospital authorities have had to balance the risks to patients of attending hospital, many of whom are especially vulnerable, with the risks of delaying or modifying cancer treatment. Those whose care has been significantly impacted include patients suffering from the effects of cancer on bone, where delivering the usual standard of care for bone support has often not been possible and clinicians have been forced to seek alternative options for adequate management.
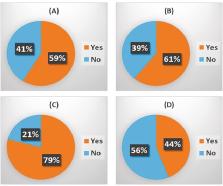
At a virtual meeting of the Cancer and Bone Society in July 2020, an expert group shared experiences and solutions to this challenge, following which a questionnaire was sent internationally to the symposium’s participants, to explore the issues faced and solutions offered. 70 respondents, from 9 countries (majority USA, 39%, followed by UK, 19%) included 50 clinicians, spread across a diverse range of specialties (but with a high proportion, 64%, of medical oncologists) and 20 who classified themselves as non-clinical (solely lab-based). Spread of clinician specialty across tumour types was breast (65%), prostate (27%), followed by renal, myeloma and melanoma.
Analysis showed that management of metastatic bone disease in all solid tumour types and myeloma, adjuvant bisphosphonate breast cancer therapy and cancer treatment induced bone loss, was substantially impacted. Respondents reported delays to routine CT scans (58%), standard bone scans (48%) and MRI scans (46%), though emergency scans were less affected. Delays in palliative radiotherapy for bone pain were reported by 31% of respondents with treatments often involving only a single dose without fractionation. Delays to, or cancellation of, prophylactic surgery for bone pain were reported by 35% of respondents. Access to treatments with intravenous bisphosphonates and subcutaneous denosumab was a major problem, mitigated by provision of drug administration at home or in a local clinic, reduced frequency of administration or switching to oral bisphosphonates taken at home. The questionnaire also revealed damaging delays or complete stopping of both clinical and laboratory research.
In addition to an analysis of the questionnaire, this paper presents a rationale and recommendations for adaptation of the normal guidelines for protection of bone health during the pandemic.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Bone metastases
- Record: found
- Abstract: found
- Article: not found
Use of Adjuvant Bisphosphonates and Other Bone-Modifying Agents in Breast Cancer: A Cancer Care Ontario and American Society of Clinical Oncology Clinical Practice Guideline.
- Record: found
- Abstract: found
- Article: not found
