- Record: found
- Abstract: found
- Article: found
Metformin requires 4E-BPs to induce apoptosis and repress translation of Mcl-1 in hepatocellular carcinoma cells
Read this article at
Abstract
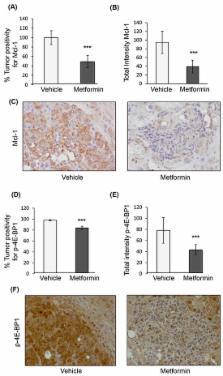
Metformin inhibits the mammalian target of rapamycin complex 1 (mTORC1) signaling pathway, which is frequently upregulated in hepatocellular carcinoma (HCC). Metformin has also been shown to induce apoptosis in this cancer. Here, we investigate whether metformin-induced apoptosis in HCC is mediated by the downstream mTORC1 effectors eukaryotic initiation factor 4E and (eIF4E)-binding proteins (4E-BPs). Further, we ask whether changes in 4E-BPs activity during metformin treatment negatively regulate translation of the anti-apoptotic myeloid cell leukemia 1 ( Mcl-1) mRNA. A genetic HCC mouse model was employed to assess the ability of metformin to reduce tumor formation, induce apoptosis, and control 4E-BP1 activation and Mcl-1 protein expression. In parallel, the HCC cell line Huh7 was transduced with scrambled shRNA (control) or shRNAs targeting 4E-BP1 and 4E-BP2 (4E-BP knock-down (KD)) to measure differences in mRNA translation, apoptosis, and Mcl-1 protein expression after metformin treatment. In addition, immunohistochemical staining of eIF4E and 4E-BP1 protein levels was addressed in a HCC patient tissue microarray. We found that metformin decreased HCC tumor burden, and tumor tissues showed elevated apoptosis with reduced Mcl-1 and phosphorylated 4E-BP1 protein levels. In control but not 4E-BP KD Huh7 cells, metformin induced apoptosis and repressed Mcl-1 mRNA translation and protein levels. Immunostaining of HCC patient tumor tissues revealed a varying ratio of eIF4E/4E-BP1 expression. Our results propose that metformin induces apoptosis in mouse and cellular models of HCC through activation of 4E-BPs, thus tumors with elevated expression of 4E-BPs may display improved clinical chemopreventive benefit of metformin.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
The insulin and insulin-like growth factor receptor family in neoplasia: an update.
- Record: found
- Abstract: found
- Article: found
Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells.
- Record: found
- Abstract: found
- Article: not found