- Record: found
- Abstract: found
- Article: found
Multimodal Evaluation of TMS - Induced Somatosensory Plasticity and Behavioral Recovery in Rats With Contusion Spinal Cord Injury
Read this article at
Abstract
Introduction: Spinal cord injury (SCI) causes partial or complete damage to sensory and motor pathways and induces immediate changes in cortical function. Current rehabilitative strategies do not address this early alteration, therefore impacting the degree of neuroplasticity and subsequent recovery. The following study aims to test if a non-invasive brain stimulation technique such as repetitive transcranial magnetic stimulation (rTMS) is effective in promoting plasticity and rehabilitation, and can be used as an early intervention strategy in a rat model of SCI.
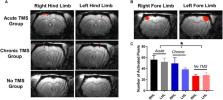
Methods: A contusion SCI was induced at segment T9 in adult rats. An rTMS coil was positioned over the brain to deliver high frequency stimulation. Behavior, motor and sensory functions were tested in three groups: SCI rats that received high-frequency (20 Hz) rTMS within 10 min post-injury (acute-TMS; n = 7); SCI rats that received TMS starting 2 weeks post-injury (chronic-TMS; n = 5), and SCI rats that received sham TMS (no-TMS, n = 5). Locomotion was evaluated by the Basso, Beattie, and Bresnahan (BBB) and gridwalk tests. Motor evoked potentials (MEP) were recorded from the forepaw across all groups to measure integrity of motor pathways. Functional MRI (fMRI) responses to contralateral tactile hindlimb stimulation were measured in an 11.7T horizontal bore small-animal scanner.
Results: The acute-TMS group demonstrated the fastest improvements in locomotor performance in both the BBB and gridwalk tests compared to chronic and no-TMS groups. MEP responses from forepaw showed significantly greater difference in the inter-peak latency between acute-TMS and no-TMS groups, suggesting increases in motor function. Finally, the acute-TMS group showed increased fMRI-evoked responses to hindlimb stimulation over the right and left hindlimb (LHL) primary somatosensory representations (S1), respectively; the chronic-TMS group showed moderate sensory responses in comparison, and the no-TMS group exhibited the lowest sensory responses to both hindlimbs.
Conclusion: The results suggest that rTMS therapy beginning in the acute phase after SCI promotes neuroplasticity and is an effective rehabilitative approach in a rat model of SCI.
Related collections
Most cited references52
- Record: found
- Abstract: found
- Article: not found
Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial.

- Record: found
- Abstract: found
- Article: found
Global prevalence and incidence of traumatic spinal cord injury
- Record: found
- Abstract: found
- Article: not found