- Record: found
- Abstract: found
- Article: found
Divergent calcium signaling in RBCs from Tropidurus torquatus (Squamata – Tropiduridae) strengthen classification in lizard evolution
Read this article at
Abstract
Background
We have previously reported that a Teiid lizard red blood cells (RBCs) such as Ameiva ameiva and Tupinambis merianae controls intracellular calcium levels by displaying multiple mechanisms. In these cells, calcium stores could be discharged not only by: thapsigargin, but also by the Na +/H + ionophore monensin, K +/H + ionophore nigericin and the H + pump inhibitor bafilomycin as well as ionomycin. Moreover, these lizards possess a P2Y-type purinoceptors that mobilize Ca 2+ from intracellular stores upon ATP addition.
Results
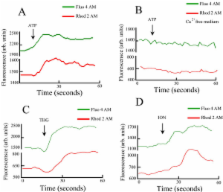
Here we report, that RBCs from the tropidurid lizard Tropidurus torquatus store Ca 2+ in endoplasmic reticulum (ER) pool but unlike in the referred Teiidae, these cells do not store calcium in monensin-nigericin sensitive pools. Moreover, mitochondria from T. torquatus RBCs accumulate Ca 2+. Addition of ATP to a calcium-free medium does not increase the [Ca 2+] c levels, however in a calcium medium we observe an increase in cytosolic calcium. This is an indication that purinergic receptors in these cells are P2X-like.
Conclusion
T. torquatus RBCs present different mechanisms from Teiid lizard red blood cells (RBCs), for controlling its intracellular calcium levels. At T. torquatus the ion is only stored at endoplasmic reticulum and mitochondria. Moreover activation of purinergic receptor, P2X type, was able to induce an influx of calcium from extracelullar medium. These studies contribute to the understanding of the evolution of calcium homeostasis and signaling in nucleated RBCs.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria.
- Record: found
- Abstract: found
- Article: not found
Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin.
- Record: found
- Abstract: found
- Article: not found