- Record: found
- Abstract: found
- Article: found
Mini-Tablets versus Nanoparticles for Controlling the Release of Amoxicillin: In vitro/In vivo Study
Abstract
Introduction
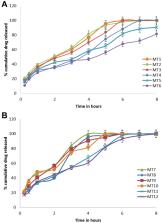
Controlling the drug release from the dosage form at a definite rate is the main challenge for a successful oral controlled-release drug delivery system. In this study, mini-tablets (MTs) and lipid/polymer nanoparticles (LPNs) of lipid polymer and chitosan in different ratios were designed to encapsulate and control the release time of Amoxicillin (AMX).
Methods
Physical characteristics and in vitro release profiles of both MT and LPN formulations were studied. Antimicrobial activity and oral pharmacokinetics of the optimum MT and LPN formulations in comparison to market tablet were studied in rats.
Results
All designed formulations of AMX as MTs and LPNs showed accepted characteristics. MT-6 (Compritol/Chitosan 1:1) showed the greatest retardation among all prepared minitablet preparations, releasing about 79.5% of AMX over 8 h. In contrast, LPN-11 (AMX: Cr 1:3/Chitosan 1 mg/mL) had the slowest drug release, revealing the sustained release of 80.9% within 8 h. The MIC of both optimized tablet formula (MT-6) and LPNs formula (LPN-11) was around two-fold lower than the control against H. pylori. The C max of MT-6 and LPN11 were non significantly different compared with the marketed AMX product. While the bioavailability experiment proved that the relative bioavailability of the AMX was 1.85 and 1.8 after the oral use of LPN11 and MT-6, respectively, compared to the market tablet.
Most cited references57
- Record: found
- Abstract: found
- Article: not found
Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release.
- Record: found
- Abstract: found
- Article: not found
Modeling and comparison of dissolution profiles.
- Record: found
- Abstract: found
- Article: not found