- Record: found
- Abstract: found
- Article: found
Therapeutic Potential of ω-3 Polyunsaturated Fatty Acids in Human Autoimmune Diseases
Read this article at
Abstract
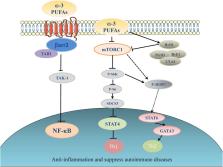
The recognition of ω-3 polyunsaturated acids (PUFAs) as essential fatty acids to normal growth and health was realized more than 80 years ago. However, the awareness of the long-term nutritional intake of ω-3 PUFAs in lowering the risk of a variety of chronic human diseases has grown exponentially only since the 1980s ( 1, 2). Despite the overwhelming epidemiological evidence, many attempts of using fish-oil supplementation to intervene human diseases have generated conflicting and often ambiguous outcomes; null or weak supporting conclusions were sometimes derived in the subsequent META analysis. Different dosages, as well as the sources of fish-oil, may have contributed to the conflicting outcomes of intervention carried out at different clinics. However, over the past decade, mounting evidence generated from genetic mouse models and clinical studies has shed new light on the functions and the underlying mechanisms of ω-3 PUFAs and their metabolites in the prevention and treatment of rheumatoid arthritis, systemic lupus erythematosus (SLE), multiple sclerosis, and type 1 diabetes. In this review, we have summarized the current understanding of the effects as well as the underlying mechanisms of ω-3 PUFAs on autoimmune diseases.
Related collections
Most cited references112
- Record: found
- Abstract: found
- Article: found
The relation between inflammation and neurodegeneration in multiple sclerosis brains
- Record: found
- Abstract: not found
- Article: not found