- Record: found
- Abstract: found
- Article: found
Mimicking Natural Photosynthesis: Solar to Renewable H 2 Fuel Synthesis by Z-Scheme Water Splitting Systems

Read this article at
Abstract
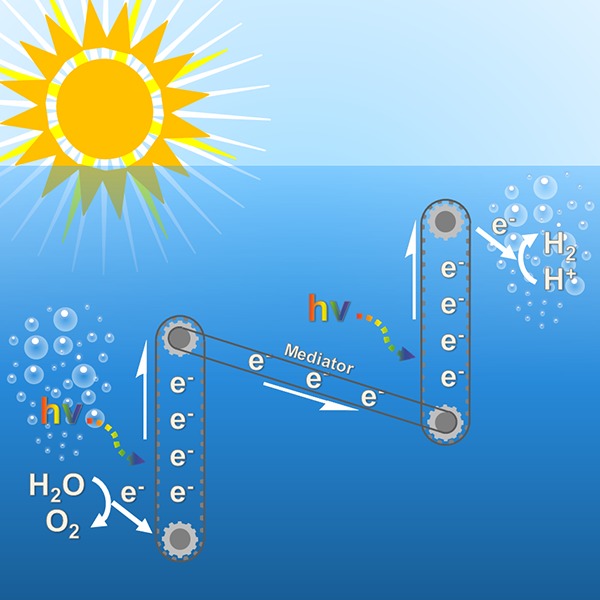
Visible light-driven water splitting using cheap and robust photocatalysts is one of the most exciting ways to produce clean and renewable energy for future generations. Cutting edge research within the field focuses on so-called “Z-scheme” systems, which are inspired by the photosystem II–photosystem I (PSII/PSI) coupling from natural photosynthesis. A Z-scheme system comprises two photocatalysts and generates two sets of charge carriers, splitting water into its constituent parts, hydrogen and oxygen, at separate locations. This is not only more efficient than using a single photocatalyst, but practically it could also be safer. Researchers within the field are constantly aiming to bring systems toward industrial level efficiencies by maximizing light absorption of the materials, engineering more stable redox couples, and also searching for new hydrogen and oxygen evolution cocatalysts. This review provides an in-depth survey of relevant Z-schemes from past to present, with particular focus on mechanistic breakthroughs, and highlights current state of the art systems which are at the forefront of the field.
Related collections
Most cited references136
- Record: found
- Abstract: not found
- Article: not found
Electrochemical Photolysis of Water at a Semiconductor Electrode
- Record: found
- Abstract: not found
- Article: not found
Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides
- Record: found
- Abstract: found
- Article: not found
