- Record: found
- Abstract: found
- Article: not found
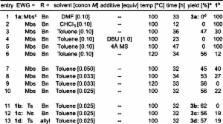
Torquoselective Ring Opening of Fused Cyclobutenamides: Evidence for a Cis,Trans-Cyclooctadienone Intermediate
rapid-communication
Xiao-Na Wang
† ,
Elizabeth H. Krenske
‡
,
,
Ryne C. Johnston
‡ ,
K. N. Houk
¶
,
,
Richard P. Hsung
†
,
03 July 2014
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
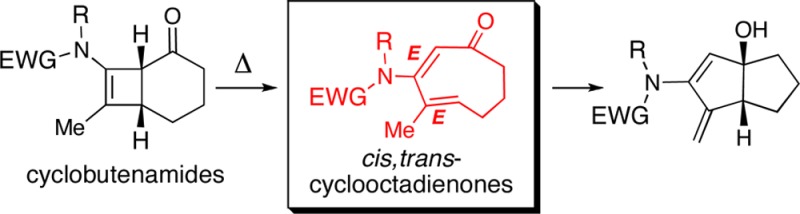
Electrocyclic ring opening of 4,6-fused cyclobutenamides 1 under thermal conditions leads to cis,trans-cyclooctadienones 2- E, E as transient intermediates, en route to 5,5-bicyclic products 3. Theoretical calculations predict that 4,5-fused cyclobutenamides should likewise undergo thermal ring opening, giving cis,trans-cycloheptadienones, but in this case conversion to 5,4-bicyclic products is thermodynamically disfavored, and these cyclobutenamides instead rearrange to vinyl cyclopentenones.
Related collections
Most cited references9
- Record: found
- Abstract: not found
- Article: not found
Ynamides: a modern functional group for the new millennium.
Kyle A DeKorver, Hongyan Li, Andrew G Lohse … (2010)
