- Record: found
- Abstract: found
- Article: found
Quantification of Anti-Aggregation Activity of Chaperones: A Test-System Based on Dithiothreitol-Induced Aggregation of Bovine Serum Albumin
Read this article at
Abstract
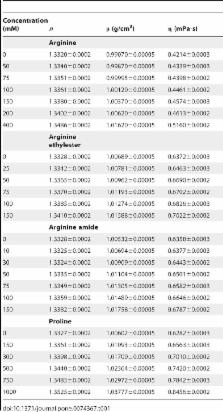
The methodology for quantification of the anti-aggregation activity of protein and chemical chaperones has been elaborated. The applicability of this methodology was demonstrated using a test-system based on dithiothreitol-induced aggregation of bovine serum albumin at 45°C as an example. Methods for calculating the initial rate of bovine serum albumin aggregation ( v agg) have been discussed. The comparison of the dependences of v agg on concentrations of intact and cross-linked α-crystallin allowed us to make a conclusion that a non-linear character of the dependence of v agg on concentration of intact α-crystallin was due to the dynamic mobility of the quaternary structure of α-crystallin and polydispersity of the α-crystallin–target protein complexes. To characterize the anti-aggregation activity of the chemical chaperones (arginine, arginine ethyl ester, arginine amide and proline), the semi-saturation concentration [L] 0.5 was used. Among the chemical chaperones studied, arginine ethyl ester and arginine amide reveal the highest anti-aggregation activity ([L] 0.5 = 53 and 58 mM, respectively).
Related collections
Most cited references95
- Record: found
- Abstract: found
- Article: not found
Alpha-crystallin can function as a molecular chaperone.
- Record: found
- Abstract: found
- Article: not found
Ageing and vision: structure, stability and function of lens crystallins.
- Record: found
- Abstract: found
- Article: not found