- Record: found
- Abstract: found
- Article: found
Inhaled beta-2 agonist salbutamol and acute lung injury: an association with improvement in acute lung injury
Read this article at
Abstract
Introduction
β2 agonists have several properties that could be beneficial in acute lung injury (ALI). We therefore chose to study the effect of inhaled β2 agonist use (salbutamol) on duration and severity of ALI.
Methods
We undertook a retrospective chart review of 86 consecutive mechanically ventilated patients with ALI, who had varying exposure to inhaled salbutamol. The cohort was divided into two groups according to the average daily dose of inhaled salbutamol they received ('high dose' ≥ 2.2 mg/day and 'low dose' <2.2 mg/day). Severity of ALI and non-pulmonary organ dysfunction was compared between the groups by calculating the days alive and free of ALI and other organ dysfunctions.
Results
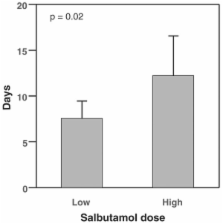
The high dose and low dose groups received a mean of 3.72 mg and 0.64 mg salbutamol per day, respectively. The high dose salbutamol group had significantly more days alive and free of ALI than the low dose group (12.2 ± 4.4 days versus 7.6 ± 1.9 days, p = 0.02). There were no associations between dose of β agonist and non-pulmonary organ dysfunctions. High dose salbutamol ( p = 0.04), APACHE II score ( p = 0.02), and cause of ALI ( p = 0.02) were independent variables associated with number of days alive and free of ALI in a multivariate linear regression model.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults.
- Record: found
- Abstract: found
- Article: not found
Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee.
- Record: found
- Abstract: found
- Article: not found