- Record: found
- Abstract: found
- Article: found
Immune responses to oral poliovirus vaccine in HIV-exposed uninfected Zimbabwean infants
Read this article at
ABSTRACT
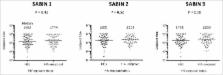
It remains uncertain whether HIV-exposed uninfected (HEU) infants have impaired responses to oral vaccines. We performed a cross-sectional study of 6-month-old infants recruited at birth to the ZVITAMBO trial in Zimbabwe between 1997–2001, before introduction of prevention of mother-to-child transmission interventions. We measured poliovirus-specific IgA to type 1–3 polio strains by semi-quantitative capture ELISA in cryopreserved serum samples collected from 85 HEU and 101 HIV-unexposed infants at 6 months of age, one month after their last immunisation with trivalent OPV. Almost all infants were breastfed, with the majority in both groups mixed breastfed (70.6% HEU versus 71.3% HIV-unexposed). Median (IQR) vaccine titers for HEU and HIV-unexposed infants were 1592 (618–4896) vs. 1774 (711–5431) for Sabin 1 ( P = 0.46); 1895 (810–4398) vs. 2308 (1081–4283) for Sabin 2 ( P = 0.52); and 1798 (774–4192) vs. 2260 (996–5723) for Sabin 3 ( P = 0.18). There were no significant differences in vaccine titers between HEU and HIV-unexposed infants, suggesting that vertical HIV exposure does not impact oral poliovirus vaccine immunogenicity.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Child mortality according to maternal and infant HIV status in Zimbabwe.
- Record: found
- Abstract: found
- Article: not found
Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival.
- Record: found
- Abstract: not found
- Article: not found