- Record: found
- Abstract: found
- Article: found
Crosstalk of Brain and Bone—Clinical Observations and Their Molecular Bases
Read this article at
Abstract
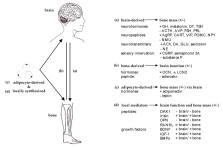
As brain and bone disorders represent major health issues worldwide, substantial clinical investigations demonstrated a bidirectional crosstalk on several levels, mechanistically linking both apparently unrelated organs. While multiple stress, mood and neurodegenerative brain disorders are associated with osteoporosis, rare genetic skeletal diseases display impaired brain development and function. Along with brain and bone pathologies, particularly trauma events highlight the strong interaction of both organs. This review summarizes clinical and experimental observations reported for the crosstalk of brain and bone, followed by a detailed overview of their molecular bases. While brain-derived molecules affecting bone include central regulators, transmitters of the sympathetic, parasympathetic and sensory nervous system, bone-derived mediators altering brain function are released from bone cells and the bone marrow. Although the main pathways of the brain-bone crosstalk remain ‘efferent’, signaling from brain to bone, this review emphasizes the emergence of bone as a crucial ‘afferent’ regulator of cerebral development, function and pathophysiology. Therefore, unraveling the physiological and pathological bases of brain-bone interactions revealed promising pharmacologic targets and novel treatment strategies promoting concurrent brain and bone recovery.
Related collections
Most cited references364
- Record: found
- Abstract: found
- Article: not found
Molecular characterization of a peripheral receptor for cannabinoids.
- Record: found
- Abstract: found
- Article: not found
WNT signaling in bone homeostasis and disease: from human mutations to treatments.
- Record: found
- Abstract: found
- Article: not found