- Record: found
- Abstract: found
- Article: not found
T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections
Abstract
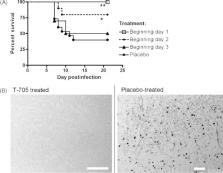
A series of pyrazinecarboxamide derivatives T-705 (favipiravir), T-1105 and T-1106 were discovered to be candidate antiviral drugs. These compounds have demonstrated good activity in treating viral infections in laboratory animals caused by various RNA viruses, including influenza virus, arenaviruses, bunyaviruses, West Nile virus (WNV), yellow fever virus (YFV), and foot-and-mouth disease virus (FMDV). Treatment has in some cases been effective when initiated up to 5–7 days after virus infection, when the animals already showed signs of illness. Studies on the mechanism of action of T-705 have shown that this compound is converted to the ribofuranosyltriphosphate derivative by host enzymes, and this metabolite selectively inhibits the influenza viral RNA-dependent RNA polymerase without cytotoxicity to mammalian cells. Interestingly, these compounds do not inhibit host DNA and RNA synthesis and inosine 5′-monophosphate dehydrogenase (IMPDH) activity. From in vivo studies using several animal models, the pyrazinecarboxamide derivatives were found to be effective in protecting animals from death, reducing viral burden, and limiting disease manifestations, even when treatment was initiated after virus inoculation. Importantly, T-705 imparts its beneficial antiviral effects without significant toxicity to the host. Prompt development of these compounds is expected to provide effective countermeasures against pandemic influenza virus and several bioweapon threats, all of which are of great global public health concern given the current paucity of highly effective broad-spectrum drugs.
Related collections
Most cited references32
- Record: found
- Abstract: not found
- Article: not found
Update on avian influenza A (H5N1) virus infection in humans.
- Record: found
- Abstract: found
- Article: not found
