- Record: found
- Abstract: found
- Article: not found
Cellular Senescence in Type 2 Diabetes: A Therapeutic Opportunity
Read this article at
Abstract
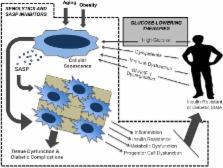
Cellular senescence is a fundamental aging mechanism that has been implicated in many age-related diseases and is a significant cause of tissue dysfunction. Accumulation of senescent cells occurs during aging and is also seen in the context of obesity and diabetes. Senescent cells may play a role in type 2 diabetes pathogenesis through direct impact on pancreatic β-cell function, senescence-associated secretory phenotype (SASP)-mediated tissue damage, and involvement in adipose tissue dysfunction. In turn, metabolic and signaling changes seen in diabetes, such as high circulating glucose, altered lipid metabolism, and growth hormone axis perturbations, can promote senescent cell formation. Thus, senescent cells might be part of a pathogenic loop in diabetes, as both a cause and consequence of metabolic changes and tissue damage. Therapeutic targeting of a basic aging mechanism such as cellular senescence may have a large impact on disease pathogenesis and could be more effective in preventing the progression of diabetes complications than currently available therapies that have limited impact on already existing tissue damage. Therefore, senescent cells and the SASP represent significant opportunities for advancement in the prevention and treatment of type 2 diabetes and its complications.
Related collections
Most cited references67
- Record: found
- Abstract: found
- Article: found
The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs
- Record: found
- Abstract: found
- Article: not found
Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas.
- Record: found
- Abstract: found
- Article: not found
