- Record: found
- Abstract: found
- Article: found
9th Trends in Medical Mycology Held on 11–14 October 2019, Nice, France, Organized under the Auspices of EORTC-IDG and ECMM
meeting-report
08 October 2019
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Introduction
Dear Friends and Colleagues,
It is a great honor and pleasure for us to invite you cordially to participate in
the 9th Congress on Trends in Medical Mycology (TIMM-9), which will be held in 11–14
October 2019 at Nice-Acropolis Convention Center, Nice, France. TIMM-9 is the 9th
in the series of TIMM mycological international meetings organized jointly by the
European Confederation of Medical Mycology (ECMM) and the Infectious Diseases Group
of the European Organisation for Research and Treatment of Cancer (EORTC-IDG).
TIMM has become an important and essential meeting in the field of fungal infections,
a forum in which researchers and clinicians from all over the world present the most
important advances and research findings in clinical mycology. TIMM-9 will cover all
aspects of mycology, with special focus on evidence-based and personalized approach
to medical mycology, as well as diagnostic–therapeutic integrative efforts in the
quest to improve the present knowledge of epidemiology, diagnosis, clinical course,
and pathophysiological mechanisms of fungal diseases. It would be the place to present
recent innovations in medical mycology.
The meeting is designed for medical microbiologists, medical mycologists, hematologists,
oncologists, transplant physicians, intensivists, immunologists, and all those with
interest in medical mycology. We expect TIMM-9 to be at least as successful as previous
TIMM Congresses, which brought together around 1000 international delegates from all
over the world. Therefore, we would like to invite you to TIMM-9 in Nice to enjoy
with us excellent science in a stimulating environment.
We look forward to greeting you in Nice!
Jean-Pierre Gangneux
Olivier Lortholary
Oliver A. Cornely
Livio Pagano
TIMM-9 Executive Committee
COMMITTEES
Executive Committee
Jean-Pierre Gangneux, ECMM, France
Olivier Lortholary, EORTC, France
Livio Pagano, EORTC, Italy
Oliver A. Cornely, ECMM, Germany
Local Scientific Committee
Hamdi Akan, EORTC, Turkey
Valentina Arsić Arsenijević, ECMM, Serbia
Sharon Chen, ECMM, Australia
Raoul Herbrecht, EORTC, France
Martin Hoenigl, ECMM, Austria
Nikolai Klimko, ECMM, Rusland
Katrien Lagrou, ECMM, Belgium
Johan Maertens, EORTC, Belgium
Jacques F. Meis, ECMM, the Netherlands
Tony Pagliuca, EORTC, UK
Zděnek Racil, EORTC, Czech Republic
Esther Segal, ECMM, Israel
Don Sheppard, ECMM, Canada
Paul Verweij, EORTC, the Netherlands
Thomas Walsh, ECMM, USA
International Scientific Committee
Jean-Pierre Gangneux, Rennes
Olivier Lortholary, Paris
Alexandre Alanio, Paris
Jean-Philippe Bouchara, Angers
Marie-Elizabeth Bougnoux, Paris
Stephane Bretagne, Paris
Christophe D’Enfert, Paris
Jean-Paul Latgé, Paris
Laurence Millon, Besançon
CONTENT
Welcome Address
1
Introduction to the Scientific Programme
5
Scientific Programme
6
Honorary Lectures
16
Plenary Sessions
17
Meet the Expert Sessions
29
Symposia
31
Poster Presentations
97
Introduction to the Scientific Programme
Plenary Sessions:
No session will be held in parallel to these sessions.
Plenary sessions are indicated by the prefix: PS
Symposia:
Each day, symposia will convene renowned speakers from several continents, who will
cover a wide range of recent developments in their fields.
Symposia are indicated by the prefix: S
A part of the symposia includes a selected submitted abstract. These abstracts are
marked with an asterisk (*).
Meet the Expert Sessions:
The audience will actively participate in these small sessions.
Meet the expert sessions are indicated by the prefix: M
Poster Sessions:
All poster boards are situated on the exhibition floor of the congress centre. The
poster exhibition is open to all participants during the entire congress. The numbers
on the poster boards correspond with the abstract numbers in this abstract supplement.
All authors of odd poster numbers must be present at their poster on Saturday 12 October,
from 11:30 to 12:30. All authors of even poster numbers must be present at their poster
on Sunday 13 October, from 11:30 to 12:30.
All posters are indicated by the prefix: P
SCIENTIFIC PROGRAMME
FRIDAY 11 OCTOBER 2019
09:00
ECMM Council Meeting
12:25
Welcome Address
12:45–13:45
Plenary Session 1—Tropical
Chairs: Jean-Pierre Gangneux, FECMM, France & Olivier Lortholary, France
12:45
PS1.1
Histoplasmosis
Matthieu Nacher, France
13:15
PS 1.2
Emergomycosis
Nelesh Govender, FECMM, South Africa
13:45–15:15
Parallel Symposia 1–4
Symposium 1
Water Quality (Mis-)Management—An Opportunity for Fungal Contamination
Chairs: João Brandão, Portugal & Esther Segal, FECMM, Israel
13:45
S01.1
Fungal Contaminants in Drinking Water Regulation? A Tale of Ecology, Exposure, Purification,
and Clinical Relevance
Monica Novak Babič, Slovenia
14:05
S01.2
Hospital Environment: Water Supply and Containment of Aerosolised Fungal Particles
How Far Must We Go in Times of Antimicrobial Resistance?
Raquel Sabino, FECMM, Portugal
14:25
S01.3
Potential Transmission Pathways of Clinically Relevant Fungi in Indoor Swimming Pool
Facilities
Ciska Schets, the Netherlands
14:45
S01.4*
Study of Fungal Environmental Contamination in Nests of the Penguin Enclosure of a
Large French Animal Zoo Park
Guillaume Desoubeaux, France
14:55
S01.5*
Spectrum of Indoor Fungi Isolated from Indoor Environments in Busia—Kenya
Olga Mashedi, Kenya
Symposium 2
Pneumocystis jirovecii, an Airborne Transmissible and Human-Derived Ascomycete Showing
Strong Pulmonary Tropism
Chairs: Philippe Hauser, Switzerland & Gilles Nevez, France
13:45
S02.1
Pneumocystis jirovecii: An Obligate Parasite of Human Lungs with Unique Camouflage
and Sex Strategies
Philippe Hauser, Switzerland
14:10
S02.2
High-Throughput Methodologies in Molecular Epidemiology of Pneumocystis
Jirovecii Olga Matos, Portugal
14:35
S02.3
Airborne Acquisition and Transmission of Pneumocystis jirovecii: An Update
Gilles Nevez, France
15:00
S02.5*
Does Pneumocystis jirovecii Infection Aggravate the Prognosis of Invasive Pulmonary
Aspergillosis? Data from the RESSIF Network in France (2012–2016)
Florence Robert-Gangneux, France
Symposium 3
Mucormycosis
Chairs: Fanny Lanternier, FECMM, France & Arunaloke Chakrabarti, FECMM, India
13:45
S03.1
Hospital-Related Mucormycosis
Anna Skiada, Greece
14:00
S03.3
The New Treatment of Mucormycosis
Livio Pagano, FECMM, Italy
14:15
S03.4
Clinical Features Associated to Fungal Species
Dea Garcia Hermoso, France
14:30
S03.5
Molecular diagnosis
Laurence Millon, France
14:45
S03.6
Management of Mucormycosis in Low- and Middle-Income Countries
Arunaloke Chakrabarti, FECMM, India
15:00
Discussion
Symposium 4
MSG Symposium (Trial Design in Clinical Mycology: Innovative Approaches)
Chairs: Peter Pappas, USA & Sharon Chen, FECMM, Australia
13:45
S04.1
Candidiasis
Bart-Jan Kullberg, The Netherlands
14:05
S04.2
Endemic Mycoses
George Thompson, USA
14:25
S04.3
Cryptococcosis
David Boulware, USA
14:45
S04.4
Aspergillosis and other molds
Tom Patterson, USA
15:05
S04.5*
The Lung Transplant Community Is Interested in a Clinical Trial to Determine the Optimal
Strategy to Prevent Invasive Mold Disease
Ricardo La Hoz, USA
18:30
EDL
E. Drouhet Lecture (ECMM)
Chair: Martin Hoenigl, FECMM, Austria
How to Convince European institutions that Medical Mycology is a Major Science
Jean-Paul Latgé, France
SATURDAY 12 OCTOBER 2019
08:00–08:45
Meet the Expert Sessions
08:00
M01
Hematology
Malgorzata Mikulska, FECMM, Italy & Alessandro Busca, Italy
08:00
M02
Peadiatrics
Simone Cesaro, Italy & Adillia Warris, FECMM, UK
08:00
M03
Candida in the ICU
Matteo Bassetti, Italy & Jean-François Timsit, France
08:00
M04
Diagnostics/Laboratory
Alida Talento, Ireland & Michaela Lackner, FECMM, Austria
08:00
M05
Tropical
Arunaloke Chakrabarti, FECMM, India & Rita Oladele, FECMM, Nigeria
09:00–10:00
Plenary Session 2—Highlights on Fungal Biology
Chairs: Vishukumar Aimanianda, France & Don Sheppard, FECMM, Canada
08:50
PS 2.1
Candida
Christophe D’Enfert, France
09:20
PS 2.2
Aspergillus
Agostinho Carvalho, FECMM, Portugal
09:50
PS 2.3*
Lineage-Specific Behavioural Differences in Isolates of Candida auris
Andrew Borman, UK
10:30–11:30
Plenary Session 3—One World One Guideline ECMM MSG-ERC (EFISG) ISHAM Guidelines Initiative
Chairs: Martin Hoenigl, FECMM, Austria & John Perfect, USA
10:30
PS3.1
Mucormycosis
Oliver Cornely, FECMM, Germany
10:45
PS3.2
Endemic
George Thompson, USA
11:00
PS3.3
Rare Moulds
Martin Hoenigl, FECMM, Austria
11:15
PS3.4
Rare Yeasts
Sharon Chen, FECMM, Australia
11:30-12:30
Poster Session
14:15-15:45
Parallel Symposia 5–9
Symposium 5
Lung Transplantation
Chairs: Shahid Husain, FECMM, Canada & Paolo Grossi, Italy
14:15
S05.1
Pretransplant Assessment
Blandine Rammaert, France
14:35
S05.2
Pathophysiology and Epidemiology of Invasive Aspergillosis in Lung Transplant Recipients
Claire Aguilar, France
14:55
S05.3
Current Guidelines
Shahid Husain, FECMM, Canada
15:15
S05.4
Prophylaxis
John Perfect, USA
15:35
S05.5*
From the Lung to the Heart: Fatal Dissemination of Azole-Resistant Aspergillus Fumigatus
in a Lung Transplant Patient
Rose-Anne Lavergne, France
Symposium 6
Prophylaxis during Hematology Malignancies
Chairs: Livio Pagano, FEMCC, Italy & Oliver Cornely, FECMM, Germany
14:15
S06.1
AML—in the Era of FLT3 Inhibitors
Russel Lewis, Italy
14:35
S06.2
ALL—There Is a Role for Prophylaxis
Daniel Teschner, Germany
14:55
S06.3
Personalized Medicine/Approach by Genetic Risk Factors
Pierre-Yves Bochud, Switzerland
15:15
S06.4
Baseline CT upon Diagnosis of Acute Leukemia
Stefan Schwartz, Germany
15:35
S06.5*
Investigating the Impact of Posaconazole Prophylaxis on Systematic Fungal Screening
Using Galactomannan Antigen, Aspergillus qPCR and Mucorales qPCR
Anne-Pauline Bellanger, France
Symposium 7
Paediatric Mycology (EPMyN)
Chairs: Emmanuel Roilides, FECMM, Greece & Roger Brüggemann, FECMM, The Netherlands
14:15
S07.1
Fluconazole and Micafungin Dosing in Neonates
Roger Brüggemann, The Netherlands
14:35
S07.2
Antifungal Susceptibility of Pediatric Candidemia
Zoi-Dorothea Pana, Greece
14:55
S07.3
Primary Immunodeficiencies Characterized by Fungal Infections
Fanny Lanternier, FECMM, France
15:25
S07.5*
Isavuconazole use in pediatric hematoncologic patients: the Italian Association of
Pediatric Hematology Oncology (AIEOP) experience
Nunzia Decembrino, Italy
Symposium 8
Immunologic Markers for Diagnosis and Treatment Stratification in Invasive Mold Infection
Chairs: Martin Hoenigl, FECMM, Austria & Agostinho Carvalho, FECMM, Portugal
14:15
S08.1
Antimold Immune Response: the Impact of the Host, the Pathogen, and Translational
Implications
Agostinho Carvalho, FECMM, Portugal
14:35
S08.2
Immunologic Markers for Diagnosis of Invasive Aspergillosis and Other Invasive Mold
Infections
Carol Garcia-Vidal, Spain
14:55
S08.3
Immunologic Markers For Treatment Stratification in Invasive Mold Infection
Philipp Köhler, FECMM, Germany
15:15
S08.4
Fungal Translocation: From Persistent Inflammation to Non-AIDS Events
Martin Hoenigl, FECMM, Austria
15:35
S08.5*
Immunological Characteristics of Broncho Alveolar Lavage in Neutropenic Patients with
Invasive Aspergillosis
Claire Aguilar, France
Symposium 9
Chronic Pulmonary Aspergillosis
Chairs: David Denning, FECMM, United Kingdom & Aleksandra Barac, FECMM, Serbia
14:15
S09.1
Effect of Patient Immunodeficiencies on the Diagnostic Performance of Serological
Assays to Detect Aspergillus-Specific Antibodies in Chronic Pulmonary Aspergillosis
Elizabeth Hunter, UK
14:35
S09.2
Diagnosis of CPA. Where Do We Stand?
Aleksandra Barac, FECMM, Serbia
14:55
S09.3
Current Treatment Options for CPA
David Denning, FECMM, UK
15:15
S09.4
Future Directions
David Denning, FECMM, UK
15:25
S09.5*
Raised Amphotericin B MIC in Aspergillus fumigatus Isolates from Patients with Chronic
Pulmonary Aspergillosis
Fiona Lynch, UK
16:15–17:45
Parallel Symposia 10–14
Symposium 10
Sensing the Host
Chairs: Muriel Cornet, France & Mihai Mares, FECMM, Romania
16:15
S10.1
S10.1 Cell wall of Aspergillus fumigatus in murine lung tissue
Thierry Fontaine, France
16:45
S10.2
Hybrid Histidine Kinases: Major Sensing Proteins in Pathogenic Fungi
Nicolas Papon, France
17:15
S10.3
Adapting to the Host: How Candida Causes Bloodstream Infection
Oliver Kurzai, Germany
Symposium 11
The Antifungal Pipeline
Chairs: David Denning, FECMM, United Kingdom & Oliver Cornely, FECMM, Germany
16:15
S11.1
Olorofim—A Novel Mould-Active Antifungal (F2G)
John Rex, FECMM, UK
16:40
S11.2
Fosmanogepix: A Novel, Broad Spectrum Antifungal Therapy in Clinical Development (Amplyx)
Michael Hodges, USA
17:05
S11.3
Ibrexafungerp (formerly SCY-078) (Scynexis)
David Angulo, USA
Symposium 12
Environment and Fungal Outbreaks
Chairs: Jean-Pierre Gangneux, FECMM, France & Tom Chiller, USA
16:15
S12.1
Frogs
Matthew Fisher, UK
16:35
S12.2
Cryptococcus gattii
Ferry Hagen, FECMM, The Netherlands
16:55
S12.3
Natural Disasters
Tom Chiller, US
17:15
S12.4
Genomic Sequencing
Marie Desnos, France
17:35
S12.5*
Outbreak of Fluconazole-Resistant Candida Parapsilosis in a Hospital Ward: Arguments
for Clonal Transmission and Environmental Persistence
Arnaud Fekkar, France
Symposium 13
Fungal Respiratory Infections in Cystic Fibrosis (the ECMM/ISHAM Working Group Fri-CF)
Chairs: Jean-Philippe Bouchara, France & Petr Hamal, Czech Republic
16:15
S13.1
Rasamsonia Species and Other Emerging Fungi in CF
Solène Le Gal, France
16:35
S13.2
Immunodiagnosis of Scedosporium/Lomentospora Infection—Lessons from Immunoproteomic
Studies
Andoni Ramirez-Garcia, Spain
16:55
S13.3
Bacterial/Fungal Interactions in the CF Mucus
Françoise Botterel, France
17:15
S13.4*
Morphology, Growth, and Biofilm Formation of the Black Yeast-Like Fungus Exophiala
dermatitidis is Influenced by Pseudomonas aeruginosa under in Vitro Cystic Fibrosis
Conditions
Lisa Kirchhoff, Germany
17:25
S13.6*
Study of Antigenic Markers for Serological Detection of Scedosporium spp. in Cystic
Fibrosis Patients
Leire Martin-Souto, Spain
17:35
Discussion
Symposium 14
Teaching Medical Mycology: What, How, to Whom and When
Chairs: Patricia Muñoz, Spain & Esther Segal, FECMM, Israel
16:15
S14.1
Mycology Teaching in Medical School—How Much, What to Choose, at What Level
Peter Rath, FECMM, Germany
16:35
S14.2
Mycology Teaching in Medical School—Is Student Demography Important to What Should
Be Taught?
Ester Segal, FECMM, Israel
16:55
S14.3
Specific Courses Outside the European Continent—The Indian Example
Ruth Ashbee, United Kingdom
17:15
S14.4
Teaching Clinical Laboratory Mycology—How and Who Is the Audience?
Sevtap Arikan-Akdagli, FECMM, Turkey
17:35
S14.5
The African Example
Aude Sturny, France
SUNDAY 13 OCTOBER 2019
08:00–08:45
Meet the Expert Sessions 6–10
08:00
M06
Hematology—hematopoietic stem cell transplantation
Nikolai Klimko, FECMM, Russia & Tony Pagliuca, UK
08:00
M07
Molecular Diagnostics
Maurizio Sanguinetti, Italy & Dieter Buchheidt, FECMM, Germany
08:00
M08
Surgery/Transplantation
Patricia Munoz, Spain & Paolo Grossi, Italy
08:00
M09
Neonates
Thomas Walsh, FECMM, USA & Emmanuel Roilides, FECMM, Greece
08:00
M10
Meet Mr. and Mrs. Fungus
Cornelia Lass-Flörl, FECMM, Austria & Neil Gow, FECMM, UK
08:50–09:50
Plenary Session 4—Top 10 papers in Mycology
Chairs: Maiken Arendrup, Denmark & Malcolm Richardson, FECMM, UK
08:50
PS4.1
The Clinical and Translational Perspective
Don Sheppard, FECMM, Canada
09:20
PS4.2
The Microbiology Perspective
Katrien Lagrou, FECMM, Belgium
ECMM Academy and Excellence Centers: A Story of Success
Chair: Martin Hoenigl, FECMM, Austria
09:50
ECMM Academy
Katrien Lagrou, FECMM, Belgium
09:55
ECMM Excellence Centers
Cornelia Lass-Flörl, FECMM, Austria
10:30–11:30
Plenary Session 5—ICU—Candida Infections/Breaking News from Hematology and ICU
Chairs: Johan Maertens, FECMM, Belgium / Maricela Valerio, Spain
10:30
PS5.1
Abdominal Candidiasis in ICU Patients
Philipp Köhler, FECMM, Germany
10:45
PS5.2
Are the New Management Strategies Useful?
Arnaldo Colombo, FECMM, Brazil
11:00
PS5.3
CNS Infections
Anna Candoni, Italy
11:15
PS5.4
CMV and Aspergillosis in HSCT Patients
Johan Maertens, FECMM, Belgium
11:30–12:30
Poster Session 2
11:30
Video Session
11:30
V01
Subcutaneous Nodule Caused by Phaeoacremonium fuscum in a Non-Immunocompromised Patient
Sofie Colman, Belgium
11:40
V02
Trichosporon Diagnosis—The Right Path
Thayanidhi Premamalini, India
11:50
V03
Disseminated Rhinosporidiosis with Different Morphological Lesions Involving Various
Anatomical Sites
Jagdish Chander, India
14:15–15:45
Parallel Symposia 15–19
Symposium 15
Aspergillus in the ICU
Chairs: Katrien Lagrou, FECMM, Belgium & Alessandro Pasqualotto, FECMM, Brazil
14:15
S15.1
Influenza
Joost Wauters, Belgium
14:35
S15.2
Invasive Aspergillosis in Patients with Underlying Liver Cirrhosis
Juergen Prattes, Austria
14:55
S15.3
Renal Failure
Riina Richardson Rautema, FECMM, UK
15:15
S15.4*
Fungal Pneumonia in Critically Ill Cirrhotics: Spectrum, Outcomes, Comparison of Diagnostic
Methods and Biomarkers
Pratibha Kale, India
15:25
S15.5*
IPAFLU Survey: Invasive Aspergillosis among Patients with Severe Influenza in Intensive
Care Units
Joost Wauters, Belgium
15:35
S15.6*
Trend of Candidemia with Bloodstream Infection in Intensive Care Units from 2006 to
2017: Results from the Korean National Healthcare-Associated Infections Surveillance
System
Young Hwa Choi, Korea
Symposium 16
Dermatology
Chairs: Valentina Arsic Arsenijevic, ECMM, Serbia & Sevtap Arıkan Akdagli, FECMM,
Turkey
14:15
S16.1
Cutaneous Aspergillosis, Is It So Rare?
Sevtap Arıkan Akdagli, FECMM, Turkey
14:35
S16.2
Is Resistance a Problem for Dermatophytosis?
Pietro Nenoff, Germany
14:55
S16.3
Phaeohyphomycosis
Teresa Martin, Spain
15:15
S16.4
Blastomycosis
Ilan Schwarz, Canada
15:35
S16.5*
Galleria mellonella as a Novelty Model to Study Host—Pathogen Interaction in Malassezia
furfur CBS 1878
Adriana Marcela Celis Ramírez, Colombia
Symposium 17
HIV-Associated Cryptococcal Meningitis
Chairs: John Perfect, USA & Olivier Lortholary, France
14:15
S17.1
Screening in Low-Resource Areas
Elvis Temfack, Cameroon
14:35
S17.2
Current Therapeutic Strategies
Olivier Lortholary, France
14:55
S17.3
Towards Having Antifungal Drugs in Low-Resource Areas
Ida Kolte, UK
15:15
S17.4
Early versus Delayed Antiretroviral Treatment in HIV-Positive People with Cryptococcal
Meningitis
Tihana Bicanic, FECMM, UK
15:35
S17.5
Current and Future Clinical Trials on HIV-Associated Cryptococcal Meningitis
Tom Harrison, UK
Symposium 18
Antifungal Stewardship in the Era of Resistance
Chairs: Paul Verweij, FECMM, The Netherlands & Souha Kanj, FECMM Lebanon
14:15
S18.1
Antifungal Stewardship: A Practical Experience in a Tertiary Care Institution
Maricela Valerio, Spain
14:35
S18.2
Stewardship and Azole-Resistant Aspergillosis: A Challenge for Farmer or Physician?
Paul Verweij, FECMM, The Netherlands
14:55
S18.3
Rapid Diagnosis of Fungal Infections: Impact on Stewardship
Souha Kanj, FECMM, Lebanon
15:15
S18.4
Mycology Laboratory Diagnostic Capabilities in Different Areas of the World
Alessandro Pasqualotto, FECMM, Brazil
15:35
S18.5*
Optimising Antifungal Stewardship: An Evaluation of Candidaemia Guideline Compliance
and Clinical Outcome
Laura Cottom, UK
Symposium 19
Pneumocystis
Chairs: Enrique Calderon, Spain & Stephane Bretagne, FECMM, France
14:15
S19.1
Diagnosis
Alexandre Alanio, FECMM, France
14:40
S19.2
Pneumocystosis in HIV and Non-HIV Patients
Joseph Kovacs, USA
15:05
S19.4
Pneumocystosis in Neonates
Enrique Calderon, Spain
15:30
S19.5*
Evaluation of a Novel Commercial Loop-Mediated Isothermal Amplification (LAMP) Assay
for Rapid Detection of Pneumocystis jirovecii
Dirk Schmidt, Germany
16:15–17:45
Parallel Symposia 20–24
Symposium 20
Candida auris
Chairs: Nelesh Govender, FECMM, South Africa & Anuradha Chowdhary, FECMM, India
16:15
S20.1
Schizophrenic Gram-Negative Yeast Conquering the World
Jacques Meis, FECMM, The Netherlands
16:35
S20.2
In the US
Tom Chiller, USA
16:55
S20.3
Outbreak Control
Alba Ruiz, Spain
17:15
S20.4
In Resource-Limited Countries
Anuradha Chowdhary, FECMM, India
17:35
S20.5*
Understanding Echinocandin Activity towards Candida auris
Milena Kordalewska, USA
Symposium 21
Immunotherapy for Opportunistic Fungal Infections
Chairs: Carol Garcia-Vidal, Spain & Dimitrios Kontoyiannis, FECMM, USA
16:15
S21.1
CAR-T Cells, NK Cells
Dimitrios Kontoyiannis, FECMM, USA
16:35
S21.2
WBC Transfusions
Livio Pagano, FECMM, Italy
16:55
S21.3
Cytokines
George Chamilos, Greece
17:15
S21.4*
Novel Chimeric Antigen Receptor T Cells for Invasive Aspergillosis Immunotherapy
Michelle Seif, Germany
17:25
S21.5*
Comparison of Circulating Lymphocyte Populations CD4+ and CD8+ T Cells, B and NK lymphocytes
According to the Favorable or Worsening Evolution of Patients with Pneumocystosis
Eléna Charpentier, France
17:35
S21.6*
Glucosylceramides from Lomentospora prolificans Induce Cytokines Production and Increase
the Microbicidal Activity of Macrophages
Mariana Ingrid Dutra Silva Xisto, Brazil
Symposium 22
NGS and Mycobiota
Chairs: Laurence Delhaes, France & Jean-Pierre Gangneux, FECMM, France
16:15
S22.1
Bacterial–Fungal Interactions at the Airway Mucosa and Implications for Chronic Rhinosinusitis
Emily Cope, USA
16:35
S22.2
Respiratory Mycobiome—A Clinical Perspective
Robert Krause, FECMM, Austria
16:55
S22.3
Characterization of Indoor Dust Microbiota in Homes of Asthma and Non-Asthma Patients
Jean-Pierre Gangneux, FECMM, France
17:15
S22.4
Understanding the Role of the Gut Mycobiome in Health and Disease
Maria Vehreschild, FECMM, Germany
17:35
S22.5*
An ECMM-ESCMID Survey on Goals and Practices for Mycobiota Characterization Using
Next-Generation Sequencing in European Laboratories
Jean-Pierre Gangneux, FECMM, France
Symposium 23
The Funny Life of C. albicans in the Oral Tract
Chairs: Marie-Elisabeth Bougnoux, France & Anna Maria Tortorano, Italy
16:15
S23.1
Within-Host Genomic Diversity of Candida albicans in Healthy Carriers
Marie-Elisabeth Bougnoux, France
16:35
S23.2
Activation of Oral Immune Responses by C. albicans
Julian Naglik, UK
16:55
S23.3
Modulation of the Fungal–Host Interaction by the Intraspecies Diversity of C. albicans
Dominique Sanglard, Switzerland
17:15
S23.4
Intestinal Th17 Drives Airway Inflammation in ABPA
Petra Bacher, Germany
17:35
S23.5*
Persistence of Clonal Azole-Resistant Isolates of Candida albicans from a Patient
with Chronic Mucocutaneous Candidiasis in Colombia
Andres Ceballos-Garzon, Colombia
Symposium 24
Fungal Neglected Tropical Diseases
Chairs: Flavio Queiroz-Telles, Brazil & Leila Lopes Bezerra, Brazil
16:15
S24.1
Mycetoma
Wendy van de Sande, FECMM, The Netherlands
16:35
S24.2
Chromoblastomycosis
Flavio de Queiroz-Telles, FECMM, Brazil
16:55
S24.3
Sporotrichosis
Leila Lopes Bezerra, Brazil
17:15
S24.4
Novel Diagnostic and Treatment Strategies for the Southeast Asian Fungus Talaromyces
marneffei
Thuy Le, USA
17:25
Discussion
MONDAY 14 OCTOBER 2019
08:00–8:45
Meet the Expert Sessions 11–15
08:00
M11
HIV
Blandine Denis, France & Nelesh Govender (FECMM), South Africa
08:00
M12
Environment
Ester Segal, FECMM, Israel & Raquel Sabino, FECMM, Portugal
08:00
M13
Diagnostics/Imaging
Frédéric Lamoth, FECMM, France & Christopher Thornton, UK
08:00
M14
Resistance
Maiken Arendrup, Denmark & Lewis White, FECMM, UK
08:00
M15
Registries
Danila Seidel, Germany & Oscar Marchetti, Switzerland
08:50–10:00
Plenary Session 6—Management of IFD in Pediatrics (EPMyN)
Chairs: Adilia Warris, FECMM, United Kingdom & Andreas Groll, FECMM, Germany
08:50
PS6.1
Biomarker-Based Diagnostic Work-Up of IFD in Immunocompromised Pediatric Patients
Thomas Lehrnbecher, Germany
09:20
PS6.2
Pre-Emptive Versus Empiric Antifungal Therapy in Children with Cancer
Maria Elena Santolaya, Chile
09:50
PS6.3*
Invasive Mucormycosis in Children with Hematological Malignancies and Solid Tumors:
Report from the Infection Working Group of the Hellenic Society of Pediatric Hematology
Oncology (2008–2017).
Emmanuel Roilides, Greece
10:30–11:15
Plenary Session 7—Superficial and Dermatology
Chairs: Arnaldo Colombo, FECMM, Brazil & Yee-Chun Chen, Taiwan, Province of China
10:30
PS7.1
When the Skin Is the Portal of Invasive Infections
Yee-Chun Chen, Taiwan, Province of China
11:00
PS7.2
When the Skin Talks for Systemic Infections
Fanny Lanternier, FECMM, France
11:15–12:30
Plenary Session 8—New (Antineoplastic) Drugs, New Risks
Chairs: Livio Pagano, FECMM, Italy & Johan Maertens, FECMM Belgium
11:30
PS8.1
In Hematology
Alessandro Busca, Italy
12:00
PS8.2
In Oncology
Lubos Drgona, FECMM, Slovakia
12:30
Closing TIMM-9
Honorary Lectures
Latgé
J.-P.
University of crete, Heraklion, Greece
E. Drouhet lecture EDL. How to convince European institutions that Medical Mycology
is a major science
How to convince Europe that Medical Mycology is a major science
Biochemistry; Molecular biology; Immunology
There are many reasons for flying the flag of Medical Mycology in medicine but also
in science in Europe. First, morbidity and mortality due to fungi is much higher than
the one due to tuberculosis and malaria in Europe. Second, essential advances in immunology
such as TLR or C-type lectins, had their root in the analysis of the host response
against fungal infections. Fungi continue to be models of choice for deciphering new
immunological pathways. Cell wall which is a unique feature of these eukaryotic microorganisms,
has been instrumental on this medical theme but also on the finding of new essential
enzymes in glycobiology. Third, Mycobiota is a majority underrecognized and understudied
component of the microbiota which is more and more recognized as an important constituent
of human health. Fourth, analysis of fungal biochemical and molecular pathways can
help understanding human diseases or metabolic dysregulations or stem cell dormancy.
Finally, more than other microbes, fungi have to be loved for their natural beauty.
All these cases will be illustrated with examples taken from the study of Aspergillus
fumigatus which has been my “baby” for the last 30 years.
Plenary session 1—Tropical
Govender
N.
National Institute for Communicable Diseases, Johannesburg, South Africa
PS1.2. Emergomycosis
Five species of thermally-dimorphic fungi within a new genus Emergomyces cause a disseminated
mycosis among immunocompromised persons. Distinct from the closely-related Emmonsia
and Blastomyces genera, Emergomyces strains have only been isolated from human infections
and all species produce yeast cells (usually <5 µm diameter and with narrow-based
budding) in the thermotolerant phase. The type species, Emergomyces pasteurianus was
first described from a case in 1992 and has an apparently cosmopolitan distribution
with cases diagnosed in Europe, Africa and Asia. The other four species were described
to have emerged over the last decade, coinciding with increasing use of molecular
diagnostic techniques in clinical and research laboratories, and may be geographically
restricted. Overall, Emergomyces africanus has been implicated in the largest number
of reported cases of emergomycosis. Restricted to southern Africa and first described
by Kenyon et al in 2013, E. africanus causes a multi-system disease among persons
living with advanced HIV disease. Systemic infection is presumed to occur following
inhalation of air-borne conidia from a soil reservoir, with a subsequent temperature-mediated
phase transition to a yeast form and dissemination through the reticuloendothelial
system among immunocompromised individuals. Most cases are diagnosed by conventional
culture of blood, tissues and fluids and/or histopathological tissue examination,
both of which require technical expertise. Limited pulmonary disease is probably under-diagnosed
in resource-limited settings; this has only been described to occur in the single
case of Emergomyces europaeus infection. The full spectrum of clinical infection and
prevalence in different populations could potentially be determined by better use
of non-culture-based methods, including antigen and PCR assays, in clinical settings
and for epidemiological surveillance. For instance, E. africanus is known to cross-react
with a commercially-available Histoplasma galactomannan antigen assay and Emergomyces
canadensis with a commercial DNA probe for Blastomyces dermatitidis. Although the
attributable mortality has not been defined, the crude mortality in a South African
case series was approximately 50%. Screening for emergomycosis among high-risk patients
in endemic areas could detect active disease earlier and thus reduce mortality associated
with late presentation. Treatment recommendations for emergomycosis are the same as
for patients with disseminated histoplasmosis and are based only on observational
data.
Plenary session 2—Highlights on fungal biology
D’Enfert
C.
Fungal Biology and Pathogenicity, Institut Pasteur, INRA, Paris, France
PS2.1. Candida albicans genome diversity: mechanisms and consequences
The fungal pathogen Candida albicans shows significant diversity at the genetic and
phenotypic levels. Here, I will review our current knowledge of the C. albicans diploid
genome and its variability, the genetic structure of the C. albicans population and
the mechanisms that are involved in C. albicans genome dynamics, with a focus on the
parasexual cycle and loss-of-heterozygosity events. I will further explore the impact
of genetic diversity and genome dynamics on C. albicans phenotypic diversity. Finally,
I will discuss how our current knowledge of C. albicans genetic diversity could be
leveraged in the future in order to get insights in the mechanisms underlying important
biological attributes that are subject to variations across C. albicans isolates.
Gonçalves
S.
Cunha
C.
Carvalho
A.
Life and Health Sciences Research Institute (icvs), University of Minho, Braga, Portugal
PS2.2. Metabolic regulation of innate immunity to Aspergillus fumigatus
The reprogramming of cellular metabolism was recently recognized as a fundamental
mechanism through which innate immune cells meet the energetic and anabolic needs
during host defense against invading pathogens. Sensing of microbial ligands by macrophages
drives the upregulation of glycolysis, which delivers a rapid source of energy to
support antimicrobial functions and the production of cytokines and other inflammatory
mediators. The stimulation of immune cells with structural components of the fungal
cell wall, such as β-1,3-glucan, has been demonstrated to promote the metabolic and
functional reprogramming of immune cells during infection with the yeast Candida albicans.
However, how the immune response to other fungi, such as Aspergillus fumigatus, is
also regulated at the metabolic level and whether other cell wall constituents also
participate in these signaling events remains unknow. Fungal melanin is a major virulence
factor, endowing A. fumigatus with the ability to survive killing by phagocytes. By
resorting to in vitro and in vivo models of infection and patients suffering from
invasive pulmonary aspergillosis (IPA), and using different pharmacological and genetic
tools to manipulate both the host and the pathogen, we reveal a novel mechanism whereby
fungal melanin is perceived by the host to regulate adequate immunometabolic responses
and susceptibility to infection. Specifically, we demonstrate that the host counters
the immune inhibitory mechanisms deployed by fungal melanin, by “sensing” its removal
during intracellular swelling inside the phagosome, and using these signals to rewire
cellular metabolism towards enhanced glycolysis and promote antifungal immune responses.
The contribution of glucose homeostasis to human antifungal immunity is further supported
by genetic variation in glycolytic regulators that act as cytokine quantitative trait
loci and predispose patients at-risk to IPA. Given the current limitations in diagnosis
and therapy of fungal diseases as well as concerns over the emergence of antifungal
resistance, these results may contribute towards the design of innovative therapeutic
approaches or metabolic adjuncts to reorient host cells towards immune protection
against IPA.
Borman
A.
1
Szekely
A.
1
Majoros
L.
2
Lockhart
S.
3
Hoog
G.S. De
4
Ben-Ami
R.
5
Johnson
E.
1
1Public Health England UK National Mycology Reference Laboratory, Bristol, United
Kingdom
2Department of Medical Microbiology, University of Debrecen, Debrecen, Hungary
3Centres For Disease Control and Prevention, Atlanta, United States of America
4Westerdijk Institute, Utrecht, Netherlands
5Tel Aviv Sourasky Medical Centre, Tel Aviv, Israel
PS2.3. Lineage-specific behavioural differences in isolates of Candida auris
Objectives:
Candida auris is a serious nosocomial health risk, with widespread outbreaks in hospitals
worldwide. Sequence analyses of outbreak isolates revealed that C. auris has simultaneously
emerged as multiple distinct, geographically restricted clonal lineages. We previously
reported multiple independent introductions of C. auris isolates from at least three
of these lineages (S. Asia, S. Africa and Japan/Korea) into hospitals across the UK,
and that isolates circulating in the UK displayed two different cell phenotypes which
correlated with differences in virulence in Galleria mellonella. The present study
aimed to more fully characterise clade-specific differences in the behaviour of Candida
auris isolates. Methods: Multiple independent Candida auris isolates corresponding
to 4 of the known geographically-restricted clonal lineages (S. Asia, S. Africa, S.
America/Israeli and Japanese/Korean) were subjected to extensive phenotypic characterisation
(cellular morphology, actidione tolerance, virulence in insect [Galleria mellonella]
and mammalian [mouse] infection models, antifungal susceptibility profiles, antifungal
drug-mediated morphological changes) using well-established methodologies. Results:
Significant clade-dependent differences in C. auris isolate behaviour were noted with
respect to all of the biological features examined, including cellular morphology,
resistance to actidione, virulence, antifungal susceptibility and morphological responses
to antifungal drug exposure. Several of these differences may be correlated with previously
described differences in cellular aggregation capacity, ERG11 resistance mutations
and gene copy numbers, biofilm formation and efflux pump activity. Conclusion: The
present data demonstrate that “one size does not fit all for Candida auris” and that
the behaviour of individual isolates is at least in part dependent on the clonal lineages
from which they originate. Further studies will aim to elucidate whether such differences
have clinical significance, and to attempt to establish why isolates of three of the
principal clonal lineages (S. Asian, S. African, S. American/Israeli) have been reported
from large scale nosocomial outbreaks but none to date have been attributed to isolates
from the Japanese/Korean clade.
Plenary session 3—One World One Guideline ECMM MSG-ERC (EFISG) ISHAM Guidelines initiative
Cornely
O.A.
1
2
3
4
Alastruey-Izquierdo
A.
5
Arenz
D.
1
3
Chen
S.C.-A.
6
Dannaoui
E.
7
Hochhegger
B.
8
9
Hoenigl
M.
10
11
Jensen
H.E.
12
Lagrou
K.
13
Lewis
R.
14
Mellinghoff
S.
1
3
Mer
M.
15
Pana
Z.
16
Seidel
D.
1
3
Sheppard
D.C.
17
Whaba
R.
18
Chakrabarti
A.
19
Akova
M.
20
Alanio
A.
21
Al-Hatmi
A.
22
Arikan-Akdagli
S.
23
Badali
H.
24
Ben-Ami
R.
25
Bonifaz
A.
26
Bretagne
S.
21
Castagnola
E.
27
Chayakulkeeree
M.
28
Colombo
A.
29
Corzo-León
D.E.
30
Drgona
L.
31
Groll
A.H.
32
Guinea
J.
33
Heussel
C.-P.
34
Ibrahim
A.S.
35
Kanj
S.
36
Klimko
N.
37
Lackner
M.
38
Lamoth
F.
39
Lanternier
F.
40
Lass-Flörl
C.
38
Lee
D.-G.
41
Lehrnbecher
T.
42
Lmimouni
B.E.
43
Mares
M.
44
Maschmeyer
G.
45
Meis
J.
46
Meletiadis
J.
47
Morrissey
O.
48
Nucci
M.
49
Oladele
R.
50
Pagano
L.
51
Pasqualotto
A.
52
Patel
A.
53
Racil
Z.
54
Richardson
M.D.
55
Roilides
E.
16
Ruhnke
M.
56
Seyedmousavi
S.
24
57
58
Sidharthan
N.
59
Singh
N.
60
Sinko
J.
61
Skiada
A.
62
Slavin
M.
63
64
Soman
R.
65
Spellberg
B.
66
Steinbach
W.
67
Tan
B.H.
68
Ullmann
A.J.
69
Vehreschild
J.-J.
1
2
Vehreschild
M.J.G.T.
1
2
70
Walsh
T.
71
White
P.L.
72
Wiederhold
N.
73
Zaoutis
T.
74
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany
2German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany
3Cecad Cluster of Excellence, University of Cologne, Cologne, Germany
4Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany
5Mycology Reference Laboratory, National Centre For Microbiology, Instituto de Salud
Carlos III, Madrid, Spain
6Centre For Infectious Diseases and Microbiology Laboratory Services, New South Wales
Health Pathology, and The Department of Infectious Diseases, Westmead Hospital, School
of Medicine, University of Sydney, Sydney, Australia
7Unité De Parasitologie-mycologie, Service De Microbiologie, Université Paris-Descartes,
Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Paris, France
8Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do
Sul (PUCRS), Porto Alegre, Brazil
9Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto
Alegre, Brazil
10Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology,
Medical University of Graz, Graz, Austria
11Division of Infectious Diseases, Department of Medicine, University of California
San Diego, San Diego, United States of America
12Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
13Department of Microbiology, Immunology and Transplantation, Clinical Department
of Laboratory Medicine and National Reference Center For Mycosis, KU Leuven, University
Hospitals Leuven, Leuven, Belgium
14Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical
and Surgical Sciences, University of Bologna, Bologna, Italy
15Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke
Johannesburg Academic Hospital, Johannesburg, South Africa
16Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle
University School of Health Sciences, Hippokration General Hospital, Thessaloniki,
Greece
17Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology,
McGill University, Montreal, Canada
18Department of General, Visceral and Cancer Surgery, University Hospital of Cologne,
Cologne, Germany
19Department of Medical Microbiology, Postgraduate Institute of Medical Education
& Research, Chandigarh, India
20Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara,
Turkey
21Institut Pasteur, National Reference Center For Invasive Mycoses and Antifungals,
Department of Mycology, Cnrs Umr2000, Parasitology-mycology Laboratory, Fernand Widal
Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris,
France
22Directorate General of Health Services, Ministry of Health, Ibri, Oman
23Department of Medical Microbiology, Hacettepe University School of Medicine, Ankara,
Turkey
24Department of Medical Mycology/invasive Fungi Research Center (ifrc), School of
Medicine, Mazandaran University of Medical Sciences, Sari, Iran
25Infectious Diseases Unit, Tel Aviv Medical Center, Sackler Faculty of Medicine,
Tel Aviv University, Tel Aviv, Israel
26Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo
Liceaga”, Mexico City, Mexico
27Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genova,
Italy
28Department of Medicine, Faculty of Medicine, Siriraj Hospital, Mahidol University,
Bangkok, Thailand
29Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine,
Universidade Federal de São Paulo (UNIFESP), Sao Paulo, Brazil
30Department of Epidemiology and Infectious Diseases, Hospital General Dr. Manuel
Gea González, Mexico City, Mexico
31Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer
Institute, Bratislava, Slovak Republic
32Infectious Disease Research Program, Department of Paediatric Hematology/oncology
and Center For Bone Marrow Transplantation, University Children’s Hospital Münster,
Münster, Germany
33Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio
Marañón, Madrid, Spain
34Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg,
Heidelberg, Germany
35Division of Infectious Diseases, Los Angeles Biomedical Research Institute, Harbor-University
of California, Torrance, United States of America
36Department of Internal Medicine, Division of Infectious Diseases, American University
of Beirut Medical Center, Beirut, Lebanon
37Department of Clinical Mycology, Allergology and Immunology, North Western State
Medical University, St. Petersburg, Russian Federation
38Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology
and Public Health, Medical University Innsbruck, Innsbruck, Austria
39Infectious Diseases Service, Department of Medicine and Institute of Microbiology,
Lausanne University Hospital, Lausanne, Switzerland
40Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals,
Department of Mycology, CNRS UMR2000, Paris Descartes University, Necker-Enfants Malades
University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre,
Paris, France
41Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology
Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea, Democratic
People’s Republic of
42Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents,
Johann Wolfgang Goethe-University, Frankfurt, Germany
43School of Medicine and Pharmacy, University Mohammed the fifth, Rabat, Morocco
44Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iasi,
Romania
45Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann,
Potsdam, Germany
46Department of Medical Microbiology and Infectious Diseases, Centre of Expertise
In Mycology, Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands
47Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian
University of Athens, Department of Medical Microbiology and Infectious Diseases,
Erasmus Medical Center, Athens, Greece
48Department of Infectious Diseases, Alfred Health & Monash University, Melbourne,
Australia
49Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de
Janeiro, Brazil
50Department of Medical Microbiology & Parasitology, College of Medicine, University
of Lagos, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester,
United Kingdom
51Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –irccs–,
Universita Cattolica del Sacro Cuore, Rome, Italy
52Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer,
Porto Alegre, Brazil
53Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Ahmeddabad, India
54Department of Internal Medicine, Hematology and Oncology, Masaryk University and
University Hospital Brno, Brno, Czech Republic
55UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester,
United Kingdom
56Department Haematology / Oncology, Paracelsus Klinik, Osnabrück, Germany
57Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology,
Tehran, Iran
58Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology,
National Institute of Allergy and Infectious Diseases, National Institutes of Health,
Bethesda, United States of America
59Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa
Vidyapeetham University, Kochi, India
60Division of Infecatious Diseases, University of Pittsburgh Medical Center and VA
Pittsburgh Helthcare System, Infectious Diseases Section, University of Pittsburgh,
Pittsburgh, United States of America
61Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary
62Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian
University of Athens, Athens, Greece
63University of Melbourne, Melbourne, Australia
64The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Melbourne,
Australia
65Dept. of Medicine, P. D. Hinduja Hospital & Medical Research Centre, Mumbai, India
66Los Angeles County + University of Southern California (LAC+USC) Medical Center,
Los Angeles, United States of America
67Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University
Medical Center, Durham, United States of America
68Department of Infectious Diseases, Singapore General Hospital, Singapore, Singapore
69Department For Internal Medicine Ii, University Hospital Würzburg, Würzburg, Germany
70Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt,
Frankfurt, Germany
71Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine,
and New York Presbyterian Hospital, New York, United States of America
72Public Health Wales Microbiology Cardiff, UHW, Cardiff, United Kingdom
73Fungus Testing Laboratory, The University of Texas Health Science Center at San
Antonio, San Antonio, United States of America
74Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia,
United States of America
Background Mucormycosis is a difficult to diagnose rare disease with high morbidity
and mortality. Diagnosis is often delayed, and disease tends to progress rapidly.
Urgent surgical and medical intervention is lifesaving. Guidance on the complex multidisciplinary
management has potential to improve prognosis, but approaches differ between health
care settings. Methods from January 2018, authors from 33 countries in all United
Nations regions analysed the published evidence on mucormycosis management and provided
consensus recommendations addressing differences between the regions of the world
as part of the “One World One Guideline” initiative of the European Confederation
of Medical Mycology (ECMM). The author group based in 17 time zones, relied on electronic
media including video tutorial on methodology, and central document repository with
several daily updates. Results Signs and symptoms of mucormycosis depend on organ
patterns and underlying conditions. Diagnostic management does not differ greatly
between world regions. Upon suspicion of mucormycosis appropriate imaging is strongly
recommended to document extent of disease and is followed by strongly recommended
surgical intervention. First-line treatment with high-dose liposomal amphotericin
B is strongly recommended, while intravenous isavuconazole and intravenous or delayed
release tablet posaconazole are recommended with moderate strength. Both triazoles
are strongly recommended salvage treatments. Amphotericin B deoxycholate is recommended
against, because of substantial toxicity, but may be the only option in resource limited
settings. Conclusion Management of mucormycosis depends on recognising disease patterns
and on early diagnosis. Limited availability of contemporary treatments burdens patients
in low and middle income settings. Areas of uncertainty were identified and future
research directions specified.
PS3.1. Global guideline for the diagnosis and management of mucormycosis: An initiative
of the European Confederation of Medical Mycology in cooperation with the Mycoses
Study Group Education and Research Consortium
Hoenigl
M.
1
2
1Division of Infectious Diseases, University of California, San Diego, United States
of America
2Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology,
Medical University of Graz, Graz, Austria
In the context of a growing population of immunocompromised patients at risk for opportunistic
infections, prevalence of invasive mould infections, including moulds other than Aspergillus
and Mucorales, are on the rise. While new diagnostic and therapeutic options are now
available to tackle rare invasive mould infections, up to date guidance for the correct
utilization in the clinical setting is urgently needed. On that background, ECMM together
with MSG-ERC set out an unprecedented orphan diseases guidance initiative involving
all disciplines involved in diagnosis and treatment of rare mould infections. Utilizing
the global network of the ECMM Academy and the ECMM Excellence Centers, clinicians,
microbiologists and other medical professionals from around the world will be invited
by ECMM to contribute their expertise to the project "One World-One Guideline". The
rare mould guideline covers diagnosis and treatment of infections caused by Fusarium,
Lomentospora, Phaeohyphomycetes/dematiaceous fungi/black fungi, Rasamsonia, Scedosporium,
Schizophyllum and other basidiomycetes, Scopulariopsis, Penicillium, and Talaromyces
other than marneffei. The guideline will include taxonomy and epidemiology and give
detailed recommendations regarding diagnosis and clinical management of these rare
mould infections for adults and the pediatric population. The guideline follows the
structure and definitions of the ESCMID/ECMM guidelines on invasive fungal infection,
and the ECMM MSG-ERC guideline on mucormycosis, which are in accord with the GRADE
and AGREE systems.
PS3.3. Rare moulds: Clinical Practice Guideline of the ECMM and the MSG-ERC
Plenary session 5—ICU—candida infections/Breaking news from Hematology and ICU
Köhler
P.
Department I for Internal Medicine, European Excellence Center for Medical Mycology
(ecmm), Germany and Cecad Cluster of Excellence, University of Cologne, Cologne, Germany
With increased admission rates to intensive care unit and high morbidity and mortality
in the presence of severe sepsis or septic shock abdominal candidiasis is a frequent
complication in surgical patients. In the majority of cases Candida species are part
of polymicrobial infections after upper gastrointestinal tract perforation, anastomotic
leaks after bowel surgery, acute necrotizing pancreatitis or peritoneal dialysis.
Further risk factors comprise premorbid conditions, total parenteral nutrition, and
previous antibiotic therapy among others. Blood cultures are often negative and the
serum beta-d-glucan assay can support diagnosis in patients with suspected intraabdominal
candidiasis. The diagnosis is achieved by aspiration of fluid under ultrasound or
CT guidance or at the time of surgery. Current treatment strategies comprise both
surgical intervention and antifungal therapy, preferably with an echinocandin. Therapy
should continue for at least two weeks and often longer, until the abscess and all
signs and symptoms of peritonitis are resolved.
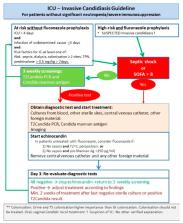
PS5.1. Abdominal Candidiasis in ICU patients
Colombo
A.
Medicine, Federal University of São Paulo, São Paulo, Brazil
Along the last decades, we faced a substantial progress in the clinical validation
of new diagnostic and therapeutic tools for treating patients with invasive Candidiasis
(IC). However, we still have several gaps in knowledge that may mitigate our results
in the clinical management of complex patients with invasive candidiasis. We selected
3 main topics to be addressed along this presentation: (1) How Candida Biomarkers
might be utilized in the clinics, (2) Should we continuous to order universal screening
of eye infection and endocarditis for candidemic patients? (3) Concerns in the clinical
management of neutropenic patients with candidemia.
PS5.2. Are the new management strategies useful?
Candoni
A.
University Hospital, Asuiud, Division of Hematology and Stem Cells Transplantation,
Udine, Italy
Fungal Infections of the Central Nervous System (IFI-CNS) are rare life-threatening
infections in hematologic patients and their management remains a challenge despite
availability of new diagnostic techniques and novel antifungal agents (1–4). In addition,
analyses of large cohort of patients focusing on these rare IFIs, are still lacking.
The most important causative agent remains Aspergillus species and a concomitant extra-CNS
involvement (mainly lung) is common. Recent reports highlight that the fungal biomarkers
in CSF could be highly informative reinforcing that diagnostic lumbar puncture should
be performed when IFI-CNS is suspected, enabling diagnosis confirmation and prompt
initiation of targeted therapy (2). Surgical approach of this complications is feasible
only in a minority of cases (those with focal, cortical-subcortical lesions and no
severe thrombocytopenia), and, even with a broad spectrum of antifungal drugs, overall
response rate remains poor (less than 40%) with a 12-months survival probability less
than 30% (1–4). Availability of new drugs, with better CNS permeability and less interaction
with immunosuppressive agents and chemotherapy (such as Isavuconazole), and prompt
diagnostic work-up (high diagnostic value of CSF biomarkers) can guide a rapid, specific
and aggressive therapy (including combination antifungal therapy ± surgery) to further
improve outcomes of IFI-CNS in hematologic patients (1–4). (1) Pagano L, Caira M,
Falcucci P, Fianchi L. Fungal CNS infections in patients with hematologic malignancy.
Expert Rev Anti Infect Ther. 2005;3:775–85. (2) Candoni A, Klimko N, Busca A, et al.
Fungal infections of the central nervous system and paranasal sinuses in onco-haematologic
patients. Epidemiological study reporting the diagnostic-therapeutic approach and
outcome in 89 cases. Mycoses. 2019;62(3):252–260. (3) Schwartz S, Kontoyiannis DP,
Harrison T, Runke M. Advances in the diagnosis and treatment of fungal infections
of the CNS. Lancet Neurology. 2018;17:362–372. (4) McCarthy M, Rosengart A, Schuetz
AN, et al. Mold Infections of the Central Nervous System. N Engl J Med. 2014; 10(371-2):
150–160.
PS5.3. Breaking news from Hematology and ICU: CNS infections
Plenary session 6—Management of IFD in paediatrics (EPMyN)
Lehrnbecher
T.
Pediatric Hematology and Oncology, University of Frankfurt, Frankfurt, Germany
Immunocompromised pediatric patients such as children undergoing therapy for hematological
malignancies or receiving allogeneic hematopoietic stem cell transplantation are at
high risk for invasive fungal diseases, which are a major cause of morbidity and mortality
in these patient populations. Unfortunately, clinical signs and symptoms of invasive
fungal disease are mostly unspecific, which makes early diagnosis difficult. However,
early diagnosis and prompt treatment of invasive fungal disease is associated with
better outcome. Non-invasive antigen-based assays such as galactomannan (GM) or β-d-glucan
and molecular biomarkers in peripheral blood have the potential to indicate invasive
fungal disease prior to the development of clinical symptoms, and may therefore be
helpful in the decision to institute and choose antifungal compounds and also to guide
duration of therapy. When GM in blood is used as a diagnostic test in neutropenic
children with prolonged febrile neutropenia unresponsive to broad-spectrum antibiotics
or in children with a pulmonary infiltrate in the CT scan, sensitivity and specificity
of the assay do not significantly differ between children and adults. However, the
utility of the assay is clearly limited by the poor positive predictive value and
the fact that despite the high negative predictive value of the test, a negative GM
assay does not rule out non-Aspergillus molds. Data on β-d-glucan in immunocompromised
children are too limited to give a final recommendation on its use, and similarly,
the results of PCR testing in blood are variable and do not allow a firm conclusion
on its utility. However, despite these limitations, biomarkers in blood may help to
plan invasive diagnostic procedures such as broncho-alveolar lavage (BAL) or bioptic
procedures. As compared to blood, both galactomannan and PCR testing in BAL lavage
seem to be superior, but again, further studies in the pediatric population are warranted.
Regarding the value of biomarker in CNS samples such as cerebrospinal fluid, little
is known in both pediatric and adult patients. Further pediatric studies are warranted
to evaluate whether the combination of diagnostic methods can improve sensitivity
and specificity, but in addition, new diagnostic tools are needed that will improve
the early and reliable diagnosis of invasive fungal disease in children.
PS6.1. Biomarker based diagnostic work-up of IFD in immunocompromised paediatric patients
Santolaya
M.E.
Hospital de niños Dr. Luis Calvo Mackenna, Santiago, Chile
Invasive fungal disease (IFD) causes significant morbidity and mortality in paediatric
cancer patients with high-risk febrile neutropenia (HRFN), along with high utilization
of resources for prevention, diagnosis and treatment. Early diagnosis of IFD and prompt
implementation of aggressive antifungal treatment have proven to be critical for patient
survival. Nevertheless, early identification of the causal pathogen of an IFD continues
to be difficult. The classic approach is currently based on clinical, imaging, microbiological
(cultures from sterile sites) and histological studies. Major advances for early diagnosis
of IFD have been made by the development of non-culture assays such as detection of
galactomannan (GM) antigen, (1-3)-b-d-glucan antigen detection and nucleic acid detection,
by PCR techniques. Despite these advances, IFD diagnosis continues to be a challenge
and current recommendations propose to initiate empirical antifungal therapy in IFD
high-risk paediatric patients with persistent (96 h) fever and neutropenia that are
unresponsive to broad-spectrum antibacterial agents. The downside of this approach
is the overtreatment of patients meeting the above criteria but who do not have an
IFD, leading to an increase in adverse events, prolonged hospitalizations and elevated
costs associated with the use of antifungal drugs. A more reasonable approach in cancer
subjects would be to consider early identification of patients at high risk of IFD,
application of a complete screening diagnosis strategy followed by a rational approach
to antifungal therapy based on results of this early and extensive diagnostic workup,
adopting a more selective preemptive treatment strategy in patients with persistent
fever and neutropenia. We performed a prospective, multicenter study in children with
cancer and persistent HRFN, aimed to determine the efficacy of pre-emptive treatment
compared with current standard empirical antifungal treatment. In our study, pre-emptive
antifungal therapy was as effective as empirical antifungal therapy, with a significant
reduction in antifungal use. A reduction of antifungal use, based on stringent diagnostic
criteria, could favor the adoption of evidence-based management strategies in this
population. This approach requires optimal laboratory support with rapid turnaround
response. Introduction of non-culture-based diagnostic techniques into clinical practice
could contribute to better management of these patients, favoring the possibility
of patient-based individualized therapy. Active monitoring and early diagnostic workup
is a necessary step prior to proposing an evidence-based management strategy. In our
experience, 58% of patients of the pre-emptive group did not receive antifungal therapy,
with similar clinical outcome to the empirical group, with the aim of reserving antifungal
therapy for the subset of patients who have early evidence of IFD by careful clinical,
laboratory, imaging and microbiological assessment. The main findings of our study
lead us to propose a step forward in the rational approach to treating children with
cancer focusing on one yet-unresolved issue in the management of the patients: adoption
of a more selective pre-emptive antifungal treatment strategy in children with prolonged
fever and neutropenia.
PS6.2. Pre-emptive versus empiric antifungal therapy in children with cancer
Antoniadi
C.
1
Lialias
I.
2
Tsipou
H.
3
Kazakou
N.
4
Iosifidis
E.
2
Papakonstantinou
E.
5
Polychronopoulou
S.
1
Kattamis
A.
3
Roilides
E.
2
Tragiannidis
A.
4
1Department of Pediatric Hematology-oncology, "Agia Sofia" Children’s Hospital, Athens,
ATHENS, Greece
23rd Pediatric Department, Aristotle University of Thessaloniki, Hippokration Hospital,
THESSALONIKI, Greece
3Pediatric Hematology-oncology Unit, 1st Pediatric Department, National and Kapodistrian
University of Athens, "Agia Sofia" Children’s Hospital, ATHENS, Greece
4Haematology-oncology Unit, 2nd Pediatric Department, Aristotle University of Thessaloniki,
AHEPA Hospital, Thessaloniki, Greece
5Department of Pediatric Hematology-oncology, Hippokration Hospital, THESSALONIKI,
Greece
PS6.3. Invasive mucormycosis in children with hematological malignancies and solid
tumors: Report from the Infection Working Group of the Hellenic Society of Pediatric
Hematology Oncology (2008-2017)
Objectives: Mucormycosis is an invasive, life-threatening fungal infection that mainly
affects immunocompromised hosts. Zygomycetes can invade virtually any tissue or organ,
resulting in a variety of clinical presentations. The Infection Working Group (IWG)
of the Hellenic Society of Pediatric Hematology-Oncology (HELSPHO) collected and analyzed
data of pediatric mucormycosis cases from 7 Hematology-Oncology Departments within
2008–2017.
Methods: Six cases of proven invasive mucormycosis were reported retrospectively (female/male:
2/4, median age: 9.6 years, range: 2–15 years) during chemotherapy for hematologic
malignancies and solid tumors within 2008–2017.
Results: Among the 6 children with invasive mucormycosis, three presented with acute
lymphoblastic leukaemia (ALL), one with acute myeloid leukemia (AML), one with neuroblastoma
and one child with brain tumor, as the primary underlying malignancy. In 4/6 children
(66%), mucormycosis occurred within the first 20 days from the diagnosis of the underlying
disease. One child with ALL developed mucormycosis 4 months since the initial diagnosis,
with prior invasive aspergillosis two months earlier treated with voriconazole. Four
out of 6 patients had received prolonged corticosteroid treatment and 3/6 had severe
neutropenia with a neutrophil count <500 μL at the time of diagnosis. Four out of
6 patients (66%) had received intensive chemotherapy within the last 4 weeks and all
patients had been hospitalized for at least 20 days. All patients presented with fever
(100%), 4/6 with pain (66%), 3/6 with palpable mass (50%) (one at the left forearm
and two at the orbit) and 3 patients presented with central nervous system symptoms
(seizures, headache, and blur of vision) (50%). Primary infection sites were combined
paranasal sinus with orbital involvement (2), central nervous system (1), occipital
area (1), orbital involvement (1) and forearm (1). The diagnosis of mucormycosis was
documented by histology and mycology (PCR or culture) in all patients (2 Rhizopus
arrhizus,1 Mucor spp., 1 Absidia spp., 2 unidentified Mucorales). Dissemination was
defined by clinical signs, imaging studies and histology in 4/6 (66%) patients. Four
(4) out of 6 patients had received prophylactic treatment with antifungal agents (2/4
patients received micafungin and 2/4 l-Amphotericin B). Therapeutically, all patients
received l-Amphotericin B (l-AmB) (median dose: 7.5mg/kg, range: 3-10 mg/kg). Two
(2) patients received combined antifungal treatment with l-AmB and caspofungin, one
(1) l-AmB and voriconazole, one (1) l-AmB and posaconazole and one (1) l-AmB, voriconazole
and caspofungin. Three (3) patients received maintenance treatment with posaconazole.
Surgical excision was performed in 4/6 cases (66%). Two out of 6 patients (33%) died,
after 6 and 12 months, respectively.
Conclusion: Pediatric invasive mucormycosis mainly presents as paranasal sinus and
orbital disease and is still related to high mortality rates. Our data demonstrated
that the disease occurs early after initiation of intensive chemotherapy and febrile
neutropenia and prolonged corticosteroids are the main predisposing factors. Early
recognition and prompt intervention with combined prolonged antifungal and surgical
treatment may rescue the patients and improve overall survival.
Plenary session 8—New (antineoplastic) drugs, new risks
Busca
A.
Division of Hematology, AOU Citta della Salute e della Scienza, Torino, Turin, Italy
Over the last 10 to 15 years, therapeutic options for patients with hematologic malignancies
have largely increased with the growth of new immunotherapeutic agents. At least two
issues regarding invasive fungal infections (IFI) and the new immunotherapeutic agents
should be addressed. First, there are relevant pharmacokinetic interactions of the
new targeted therapies with coadministered mold active azoles, namely posaconazole
and voriconazole that are strong inhibitors of CYP3A4. Awareness of these interactions
is of paramount importance for optimization of the treatment of patients, since we
are asked to monitor for potential toxicity, or to modulate the dose of the drugs
or rather to avoid the combination and consider alternative therapy. Second, is the
risk of IFI increased with the use of the new agents? For many of these, for instance
monoclonal antibodies (MoAb), bcl-2 inhibitors (i.e. venetoclax) and tyrosine kinase
inhibitors (TKI) (i.e. sorafenib and midostaurin) when used for the treatment of patients
with acute leukemia, it is hard to realize whether they have an additive risk to the
underlying disease: indeed, there are very few information respect the incidence of
IFI based on the clinical trials published so far. By contrast, several studies raised
concern that the Bruton’s tyrosine kinase inhibitor Ibrutinib may increase the risk
for IFI in patients with lymphomas. In this respect, different mechanisms by which
Ibrunitib may favor the occurrence of IFI have been described, including alveolar
macrophage, neutrophil, T-cell, and platelet function as well as alterations of cytokine
environment. A recent study of Ibrutinib treatment for primary CNS lymphoma reported
a 39% rate of invasive aspergillosis in patients concurrently receiving steroids even
in absence of neutropenia, while in other reports the incidence appears to be much
lower in the 3–4% range. Conversely, the risk of IFI with the use of Idelalisib, another
B-cell receptor inhibitor, seems to be restricted to Pneumocystis jirovecii pneumonia
only. Until more detailed epidemiological data will be available, anti-mold prophylaxis
is not recommended for patients receiving Ibrutinib or Idelalisib. Chimeric antigen
receptor (CAR) T cell therapy is an innovative strategy to harness the immune system
to treat patients with diffuse large B cell lymphoma (DLBCL). CAR-T cells are T lymphocytes
genetically engineered to express a tumor-targeting receptor, directing T cells to
bind to a tumor-associated antigen, namely the CD19 in the case of DLBCL. A recent
study evaluated infectious complications in 133 patients with ALL and lymphomas treated
with CD19 CAR-T cells: IFI occurred in 5% of the patients during the first 28 days
after CAR-T cell infusion, all of whom had severe cytokine release syndrome (CRS)
or neurotoxicity requiring tocilizumab and/or corticosteroids. CRS severity was the
only factor after CAR-T cell infusion associated with infection in a multivariable
analysis. In summary, the risk of IFI in patients receiving targeted therapies needs
to be defined by further experience in clinical practice and ongoing research trials.
Preventive strategies of IFI in patients managed long term with immunotherapy remain
at the present a challenge.
PS8.1. New antineoplastic drugs, new risks in hematology
Drgona
L.
Department of Oncohematology, Comenius University and National Cancer Institute, Bratislava,
Slovak Republic
The field of new antineoplastic therapeutical agents is increasing rapidly over the
last decade. New drugs in oncology are basically considered as targeted ones due to
their mechanism of action. These drugs directly target the cells or pathways involved
in pathophysiology of neoplastic disease. Cell surface receptors, cytokines, immunoglobulines,
intracellular enzymes are the most common targets involved in the therapy. Other drugs
are focusing on the patient’s own immune system with the aim to boost the responsible
cells to fight against the disease. The new drugs have different mechanisms of action
resulted in less “classical“ toxicity known from chemotherapy agents. The adverse
events after the targeted therapy are mainly caused by interaction with the immune
system—targeted sites are often key elements of physiological processes like cell
cycle control. This may have an influence on responses to acute infection or on control
of latent/chronic infection. Theoretically, the potential of the new drugs to predispose
to infection depends on their site of action. But this is simplification of the whole
problem, because cancer patients are treated for months or years before the installation
of the innovative therapy. Thus, an old, traditional risk factors for infectious complications
should be added to the overall risk for the given patient. Invasive fungal diseases
(IFDs) are associated with the treatment of cancer, patients with solid tumors have
lower risk than majority of patients with hematological malignancies. The obvious
risk factors for IFDs in patients with solid tumors are neutropenia, steroids, central
venous catheter, progressive or refractory malignancy, gastrointestinal surgery with
leak of anastomosis among others. Invasive candidiasis followed by invasive aspergillosis
are the most common IFDs in this setting, other pathogens are less involved. With
the advent of the new therapeutic approaches in oncology patients and physicians are
challenged with the new spectrum of adverse events including infections. Generally,
monoclonal antibodies, tyrosine kinase inhibitors, check point inhibitors and other
targeted and biological agents are not associated with the increasing risk of IFDs
in solid tumor patients. In special circumstancies patient undergoing such antineoplastic
therapy may have an additional risk for IFDs. An example are the patients with melanoma
or lung cancer treated with check point inhibitor developing an immune related adverse
event. High dose steroids with or without anti -TNF blocker are recommended in the
management of the serious toxicity; these patients are potentially at risk for IFD
development due the supportive treatment of AE. Risk of IFD will be discussed in the
patients with solid tumors reflecting the advances of antineoplastic treatment. Despite
the relatively low risk for the development of IFDs and slowly growing experience
some recommendations could be made. Anticipation, prediction and alertness are the
pillars of action in this setting of cancer patients.
PS8.2. In oncology
Meet the expert sessions
Lackner
M.
Institute of Hygiene and Medical Microbiology, Medical University of Innsbruck, Innsbruck,
Austria
M04. Novelties in the molecular diagnostics of fungal infections
The aim of this meet the expert session is to provide an overview on the most recent
developed diagnostic kits in the field of medical mycology. We aim to present and
compare novel diagnostic tools with established assay based on our personal experience
and available literature. The presentation will be based on a literature review of
the past 18 months and a web search for commercially available products. A mix of
currently developed assays of research groups will be presented, as well as CE-IVD
labelled commercial tests. The most promising innovative diagnostic assays will be
presented and discussed. The audience is invited to share their experience with novel
assays to enable a lively discussion and exchange of opinions.
Sabino
R.
1
Segal
E.
2
1Reference Unit for Parasitic and Fungal Infections, Infectious Diseases, National
Health Institute Dr. Ricardo Jorge, Lisbon, Portugal
2Sackler School of Medicine Tel-Aviv University, Tel-Aviv, Israel
Exposure to environmental fungi, whether this occurs indoors or outdoors, in leisure
activities, in the workplace or in the home, is an everyday occurrence. Different
types of environment may contain variable levels of fungal particles and include viable
and non-viable yeasts, conidia, hyphal fragments, as well as fragments derived from
the fungal cell wall. Mycotoxins and other volatile organic compounds should also
be considered as environmental potential hazards. Recognition of the importance of
the environment as a source of human infection has come about, at least in part, as
result of the emergence of an unprecedented number of ubiquitous environmental fungi
as major causes of disease. Exposure to environmental fungi is associated with high
number of hospital admissions, and asthma related ailments. Allergic bronchopulmonary
mycosis, rhinosinositis, asthma with fungal sensitization and hypersensivity pneumonitis
are among the diseases more frequently associated with fungal exposure. In addition,
immunocompromised patients are at higher risk for the development of invasive infections.
Changes in environmental factors, including changing land-use patterns, use of antifungals
in agriculture, and climate changes have led to epidemiological shifts. Therefore,
special attention should be paid in regard to the isolated fungal species. Environmental
fungal species such as Aspergillus spp., Mucorales, Candida spp., dermatophytes and
dimorphic fungi will be discussed during this session; emphasizing their importance
as etiological agents of fungal infections.
M12. Environment
Thornton
C.
Biosciences, University of Exeter and ISCA Diagnostics Ltd., Exeter, United Kingdom
Bench-to-Bedside Diagnosis of Invasive Pulmonary Aspergillosis. Invasive pulmonary
aspergillosis (IPA) is a life-threatening lung disease of haematological malignancy
and bone marrow transplant patients caused by the air-borne mould Aspergillus. Current
diagnostic tests for the disease lack sensitivity or specificity, and culture of the
fungus from invasive lung biopsy, considered the gold standard for IPA detection,
is slow and often not possible in critically ill patients. While Computed Tomography
(CT) is a non-invasive diagnostic procedure, it has limited clinical utility for IPA
diagnosis, but is nevertheless used as a trigger for commencing antifungal treatment
in a number of centres. Innovative approaches to the diagnosis of IPA are needed to
enable diagnostic-driven treatment with mould active drugs. In this talk, I will discuss
the development of diagnostic technologies for IPA detection based on the Aspergillus-specific
mouse monoclonal antibody JF5, including the CE-marked Aspergillus lateral-flow device
(OLM Diagnostics), and CE-marked Aspergillus-ELISA (Euroimmun AG). In addition, I
will describe the humanisation of mAb JF5 for PET/MR imaging of Aspergillus lung infections
in vivo, and translation of the imaging technology to the clinical setting. I will
finish by describing the recent development of a mAb, PD7, specific to A. fumigatus,
and its use to detect a novel protein biomarker of IPA in urine.
M13. Diagnostics/Imaging
White
P.
Microbiology, Public Health Wales Microbiology, Cardiff, United Kingdom
Until recently the identification of antifungal resistance has been based on the availability
of a cultured organism to perform susceptibility testing to determine a MIC against
a particular antifungal agent. The development of molecular techniques has provided
an alternative option, by identifying genetic markers potentially associated with
resistance or identifying species that may by inherently resistant, or have an increased
likelihood of resistance to particular drugs. Indeed, molecular assays offer the only
alternative to culture dependent procedures, that themselves can suffer from limited
sensitivity and delayed time to result. However, molecular approaches are not without
limitations. PCR based methods targeting specific mutations (for example: single nucleotide
polymorphisms and/or tandem repeats), by design have a limited detection range, missing
existing, likely less prevalent mutations, but also the presentation of novel genetic
mechanisms. PCR methods that identify species that are usually resistant to certain
antifungal drugs will be reliant on the knowledge of local epidemiology and the subsequent
susceptibility testing of relevant cultures to guide antifungal prescribing. The use
of PCR-sequencing, but more so next generation sequencing, will help identify a wider
range of polymorphisms in genes encoding the proteins targeted by antifungal therapy
and also in promoter regions that may enhance up-regulation, but the detection of
novel mutations may lack the necessary phenotypic evidence confirming associated resistance.
In addition, PCR-sequencing is associated with a significant processing time and has
only had limited evaluation of direct specimen testing, where sensitivity may be lacking
when targeting single copy genes. As of yet none of these methods can be confidently
associated with a specific MIC range, and the presence of well defined polymorphisms
have been associated with typically susceptible MIC values. This presentation will
describe the benefits and limitations of using molecular methods for determining antifungal
resistance, while providing data on the clinical performance of assays, including
those that are commercially manufactured.
M14. Resistance—Utilizing molecular methods
Symposium 1 Water quality (mis-)management—an opportunity for fungal contamination
Babič
M. Novak
1
Gunde-Cimerman
N.
1
Brandão
J.
2
1Department of Biology, University of Ljubljana, Biotechnical Faculty, Ljubljana,
Slovenia
2Department of Environmental Health, National Institute of Health Doutor Ricardo Jorge,
Lisbon, Portugal
S01.1. Fungal Contaminants in Drinking Water Regulation? A Tale of Ecology, Exposure,
Purification and Clinical Relevance
Safe drinking water is one of the fundamental human rights according to the Resolution
64/292 issued by the United Nations Human Rights Council [1]. Although the parameters
for safe drinking water depend on the living conditions and vary between continents
and countries, they strive for a common goal: drinking water is suitable for users
when it does not contain dangerous chemical substances and microorganisms [2]. To
achieve this, raw water from an underground or surface source is subject to various
purification techniques before entering the distribution system. The processes usually
include aeration, coagulation, flocculation, sedimentation, and ultrafiltration, depending
on the location and quality of raw water [3]. Finally, water quality in Europe is
controlled based on the parameters listed in Drinking Water Directive (98/83/CE).
Water not reaching the required microbiological minimum is additionally subject to
chemical treatment, mostly chlorination. Fungi are not listed in the current directive
and are therefore not specifically monitored. Yet, their presence in fresh water is
well documented and has been studied in at least 19 countries of Europe over the past
30 years [3]. More than 400 different species of fungi have been reported from water
sources intended for human consumption, with prevailing presence of ascomycetous fungi
of the genera Alternaria, Acremonium, Aspergillus, Aureobasidium, Candida, Cladosporium,
Exophiala, Fusarium, Penicillium, Phoma, Sarocladium, Scopulariopsis, Sporothrix,
Stachybotrys and Trichoderma, followed by Absidia, Mortierella, Mucor and Rhizopus
from subphylum Mucoromycotina. Most commonly encountered yeasts were basidiomycetous
genera Cystobasidium, Naganishia (formerly Cryptococcus) and Rhodotorula [3]. While
water purification procedures remove 8–90% of fungal propagules, the remaining will
ultimately form mixed biofilms inside the tap water distribution systems, later affecting
the taste and odour of drinking water. Also, several water-related fungal species
were recognised as opportunistic or emerging pathogens, although their transmission
and effect on the health of drinking water consumers remains poorly investigated.
Besides drinking, consumers use potable water also for daily activities such as cooking,
washing and personal hygiene. During these activities they are in constant contact
with fungi and their metabolites directly with the skin while washing, the digestive
system while drinking, and through the respiratory system by inhaling aqueous aerosols
[3,4]. With increasing transitory and serious immune alterations among patients as
well as an increase of azole-resistant fungal strains in recent years also a need
for monitoring of fungi increased, not only in drinking water but also in relation
to materials in contact with drinking water.
References:
[1]
UN. Resolution adopted by the General Assembly on 28 July 2010 64/292. The human right
to water and sanitation. United Nations: New York, USA, 2010; p. 3.
[2]
WHO. Guidelines for Drinking Water Quality, 4th ed.; World Health Organization: Geneva,
Switzerland, 2011; p. 564.
[3]
Novak Babič, M., et al. Fungal Contaminants in DrinkingWater Regulation? A Tale of
Ecology, Exposure, Purification and Clinical Relevance. Int. J. Environ. Res. Public
Health 2017, 14, 636.
[4]
DEFRA. A Review of Fungi in Drinking Water and the Implications for Human Health,
1st ed.; BIO Intelligence Service: Paris, France, 2011; p. 107.
Sabino
R.
Reference Unit for Parasitic and Fungal Infections, Infectious Diseases, National
Health Institute Dr. Ricardo Jorge, Lisbon, Portugal
S01.2. Hospital environment: water supply and containment of aerosolised fungal particles.
How far must we go in times of antimicrobial resistance?
Invasive fungal infections depend on the interplay between host susceptibility and
environmental exposure. Therefore, hospital environment is one of the major concerns
in the management of nosocomial fungal infections, especially in wards bearing immunocompromised
patients. Particular attention should be paid to the environmental risks associated
with water since fungi can be aerosolized at water taps and showers. This may lead
to fungal exposure by inhaled and ingested droplets, or even by direct contact with
mucosae. Studies report that filamentous fungi and yeasts are commonly found on water-pipe
inner surfaces, even in the presence of free chlorine. Air levels of Fusarium and
Aspergillus conidia were found to increase in hospital environments after running
showers multiple times. Species of these two genera are described as the most frequently
found in this setting due to their conidial dispersion mode, as well as their ability
to form biofilms. Despite the intrinsic resistance found in some species of these
two genera, fungal exposure to antifungal agents via medical or agricultural use of
these compounds, appears to have a major impact on acquisition of resistance to azoles;
namely in Aspergillus fumigatus. The isolation of this species in hospital water and
water reservoirs is therefore an even bigger matter of concern. More recently, several
reports on nosocomial outbreaks caused by Candida auris have been described. This
species is resistant to several classes of antifungals and is associated with high
mortality. Contamination of hospital environment or transient colonization of medical
devices and equipment may display an important role in the transmission of this species.
C. auris was already found in water samples and therefore this reservoir should not
be excluded as possible source of infection. In conclusion, fungal counts and detection
of potential pathogenic species in water were, until a few years ago, the major concern
of clinical and scientific community towards the reduction of nosocomial fungal infections
originating in water devices. The emergence of infections caused by fungal isolates
with intrinsic or acquired antifungal resistance triggered new levels of alert in
this field.
Mashedi
O.
1
Amukoye
E.
2
Bii
C.
3
Kimani
B.
3
Chepchirchir
A.
4
Okoth
S.
5
1Centre For Respiratory Dise Ases Research, Kenya Medical Research Institute, Nairobi,
Kenya
2Centre For Respiratory Diseases Research, Kenya Medical Research Institute, Nairobi,
Kenya
3Centre For Microbiology Research, Kenya Medical Research Institute, Nairobi, Kenya
4School of Health Sciences, University of Nairobi, Nairobi, Kenya
5School of Biological Sciences, University of Nairobi, Nairobi, Kenya
S01.5. Spectrum of indoor fungi isolated from indoor environments in Busia-Kenya
Objectives: The unplanned growth, rapid evolution of industrialization has deteriorated
the ambient air quality. Anthropogenic indoor air pollution continues to be seen as
an environmental and public health problem. Its seriousness lies in exposure to fungi
that can trigger allergic reactions, hypersensitivity pneumonitis, allergic rhinitis,
and some types of asthma. Very few studies in Kenya have been conducted to determine
the possible types of indoor fungi allergens in indoor environment and to evaluate
the relationship between allergen exposure in residential environments and respiratory
morbidity. The aim of this study is to determine the prevalence of fungal indoor pollution
in residential environments and to establish allergens which are associated with fungal
pathogens.
Methods: This was a cross-sectional study carried out in 60 households in Busia County.
Sedimentary and Volumetric sample collection method was used on water, air, swabs
of dust and surfaces from homes. Mycological procedures in identification and analysis
were used in fungal characterization. Comprehensive questionnaires were administered
to determine the risk factors associated with indoor fungi infestation.
Results: 60 study participants were interviewed from Busia urban setting with mean
age of 35.2 ± 7.7 (SD) years where most 60.3% of them were aged between 40–49 years;
while 19.0% were aged 20–29 years. 68.4% of the respondents were females. As regard
to education background, 61.4% of the respondents had primary education as the highest
education level whereas 3.5% had tertiary level. 43.1% of the households were occupied
by more than 5 people. Filamentous and yeast fungi were both isolated. Candida albicans
and Rhodotorula accounted for the 39% of the isolated yeast species. Among the filamentous
fungi Fusarium had the highest prevalence of 79.7%, followed by Cladosporium spp,
Alternaria spp, Acremonium Apergillus and Penicillium respectively. There was a significant
association between Fusarium and Acremonium moulds and respiratory conditions. Acremonium
species was significantly associated with itchy eyes allergies, p = 0.023.
Conclusion: There is a need for the identification and monitoring of fungi in our
home environment. Residential environment could potentially be considered an effective
target for interventions aimed at reducing inciting fungi exposures to prevent upper
respiratory allergies and infection in urban populations.
Symposium 2 Pneumocystis jirovecii, an airborne transmissible and human derived ascomycete
showing strong pulmonary tropism
Hauser
P.
Institute of Microbiology, CHUV Hospital, Lausanne, Switzerland
Pneumocystis jirovecii is a fungus colonizing the human lungs. Should the immune system
weaken, it can turn into an opportunistic pathogen causing severe pneumonia that can
be fatal if not treated. A method of in vitro long-term culture for this pathogen
is not available yet. Nevertheless, the advent of the high throughput DNA sequencing
methods allowed sequencing the P. jirovecii genome out of the lung microbiome. The
analysis of this genome revealed the loss of several synthesis pathways, demonstrating
that this fungus is an obligate parasite. The presence of a fusion of the minus and
plus mating-type loci suggested that its mode of sexual reproduction is primary homothallism
(self-fertility). The sexuality of P. jirovecii proved to be obligatory with human
lungs, which is compatible with asci and/or ascospores being the particles responsible
for the transmission between humans by the air route. Long reads sequencing permitted
characterizing six hypervariable gene families encoding surface antigens within the
subtelomeres of all chromosomes. These gene families are responsible for surface antigenic
variation allowing presumably escape from the human immune system, as well as for
adhesion to host cells. The most abundant family is subject to mutually exclusive
expression of a single gene, whereas each gene of the other families possesses its
own promoter, suggesting independent expression. Recombinations between members of
each family is thought to generate gene mosaicism that contribute to antigenic variation.
The mechanisms involved in P. jirovecii antigenic variation suggest that the infecting
populations are antigenically heterogeneous, i.e., made of subpopulations displaying
each a unique antigen of the most abundant family, as well as a minority of antigens
of the other families.
S02.1. Pneumocystis jirovecii: an obligate parasite of human lungs with unique camouflage
and sex strategies
Matos
O.
Medical Parasitology Unit, Group of Opportunistic Protozoa/hiv and Other Protozoa,
Global Health and Tropical Medicine, Instituto de Higiene e Medicina Tropical, Universidade
Nova de Lisboa, Lisboa, Portugal
The microscopic fungus Pneumocystis jirovecii is a pathogen that can cause fatal pneumonia
(PcP) in immunocompromised patients (AIDS, transplant recipients, patients with haematologic
and solid malignancies, patients with rheumatoid conditions and connective tissue
disorders, patients receiving immuno-modulatory therapies or with pre-existing chronic
lung conditions) worldwide. In the absence of an in vitro culture system to isolate
and maintain live organisms, previous efforts to understand transmission patterns
to develop methods of intervention and management for PcP relied on PCR-based approaches.
Several genotyping methods have been used so far to address the genetic heterogeneity
of P. jirovecii, PCR of multiple loci followed by direct DNA sequencing (Sanger),
restriction fragment length polymorphism (RFLP), single-strand conformation polymorphism
(SSCP), short tandem repeat (STR) or single-base extension (SBE). These methodologies
were followed by the introduction of next generation sequencing (NGS), which is considered
a revolution in the field of DNA analysis. NGS has been applied in the assembly of
P. jirovecii whole genome and in amplicon analysis, especially for outbreaks investigation.
Here we will explore the advantages and disadvantages of these approaches applied
in the expansion of knowledge of the epidemiology of P. jirovecii.
S02.2. High-throughput methodologies in molecular epidemiology of Pneumocystis jirovecii
Nevez
G.
1
2
Laurence
P.
1
2
Gal
S. Le
1
2
1University of Brest, Brest, France
2Host-Pathogen Interaction Study Group, SFR ICAT 4208, Univ Angers, Univ Brest, Institute
of Biology in Health, IRIS, University Hospital Center, Angers, France, Angers Cedex,
France
Airborne transmission of Pneumocystis sp. from host to host has been demonstrated
in rodent models and several observations suggest that interindividual transmission
occurs in humans. Moreover, it is accepted that the Pneumocystis organisms infecting
each mammalian species are host specific. Thus, the hypothesis of an animal reservoir
for Pneumocystis jirovecii (P. jirovecii), the human-specific Pneumocystis species,
can be excluded. An exosaprophytic form of the fungus cannot be strictly ruled out.
Nonetheless, these data point out that: (i) the specific host may serve as its own
reservoir (ii) Pneumocystis infection in humans is an anthroponosis with humans as
a reservoir for P. jirovecii. This review highlights the main data on host-to-host
transmission of Pneumocystis sp. in rodent models and in humans by the airborne route.
Specifically, recent data on P. jirovecii exhalation by patients developing PCP or
with pulmonary colonization in their surrounding environment are described. Taken
together, these recent findings render it possible to provide a rationale for prevention
of P. jirovecii acquisition and transmission within the hospitals.
S02.3. Airborne acquisition and transmission of Pneumocystis jirovecii: an update
Robert-Gangneux
F.
1
Bouzille
G.
2
Dromer
F.
3
Gangneux
J.P.
4
T.H.E. French Mycoses Study Group
5
1Univ Rennes, CHU Rennes, Inserm, EHESP, IRSET UMR S_U1085, RENNES, France
2Univ Rennes, CHU Rennes, Inserm, LTSI – UMR 1099, RENNES, France
3National Reference Center For Invasive Mycoses & Antifungals, Molecular Mycology
Unit, Cnrs Umr2000, Institut Pasteur, Paris, France
4Laboratoire De Parasitologie-mycologie, CHU de Rennes, Rennes, France
5National Reference Center For Invasive Mycoses & Antifungals, Molecular Mycology
Unit, Cnrs Umr2000, Institut Pasteur, RENNES, France
S02.5. Does Pneumocystis jiroveci infection aggravate the prognosis of invasive pulmonary
aspergillosis? Data from the RESSIF network in France (2012–2016)
Objectives:
Pneumocystis jiroveci pneumonia (PjP) is a frequent opportunistic infection in immunocompromised
patients with various immune deficiency backgrounds, whereas invasive aspergillosis
(IA) mostly occurs in patients with neutropenia or neutrophil impairment. Occasionally
both fungi can be detected simultaneously. In that instance, Pj detection is sometimes
considered as simple colonization, particularly when microscopic examination is negative
and PCR is positive. In this study, we addressed the question of the presence of Pj
as an aggravating factor during IA.
Methods: We took advantage of the surveillance of invasive fungal infections (IFIs)
implemented by the National Reference Center for Invasive Mycoses and Antifungals
through the RESSIF network. All cases of IA and PjP recorded during a 5-year period
(2012–2016) were analyzed. Multivariate analysis compared cases of IA and cases of
IA+PjP co-infection, using Cox regression after multiple imputation of missing data.
All cases with a follow-up of 90 days were taken into account, extra-pulmonary aspergillosis
was excluded. The following variable were included for analysis: age, sex, clinical
background (hematological malignancy, allogeneic stem cell transplantation (allo-HSCT),
auto-HSCT, graft versus host (GVH) disease, solid organ transplantation (SOT), cancer),
corticotherapy or biotherapy, delay of AI onset, AI classification (proven, probable),
and Aspergillus species.
Results: 730 patients were included, of whom 701 had IA and 29 had IA+PjP. The mean
age of patients with IA and IA+PjP was 58.5 and 61.3, respectively (p = 0.08). There
were no statistical differences in the sex ratio, clinical background, type of SOT,
corticotherapy or biotherapy exposure, time from transplantation to diagnosis, time
from hospitalization to IA diagnosis, time to death, nor classification of IA, between
the two groups. The mortality rate was higher in the group IA+PjP than in IA alone
(65% versus 39%, respectively, p = 0.009). Species from the Fumigati section accounted
for 58.6% and 43.1% of cases in IA+PjP and IA, respectively (ns). In the multivariate
analysis, the factos independently associated with death at 3 months were: age > 60
years (HR = 1.74 ; CI95% [1.4–2.2]), solid cancer (HR = 2.2 ; CI95% [1.1–4.6]), co-infection
AI+PjP (HR = 1.9 ; CI95% [1.2–3.1]) and section Fumigati IA (HR = 1.65 ; CI95% [1.3–2.1]).
Conclusion: Pj infection is an aggravating factor of IA, particularly in patients
with solid cancer. No other associated risk factor was uncovered probably due to the
limited number of co-infections recorded. We plan to extend the study period to increase
the statistical power of the analysis.
Symposium 3 Mucormycosis
Skiada
A.
1st Dpt. of Medicine, Laiko Hospital, Athens University, Athens, Greece
Mucormycosis is a rare invasive fungal infection due to fungi of the order Mucorales.
The usual underlying diseases are hematological malignancies and diabetes mellitus,
but a significant proportion of patients are immunocompetent. The main clinical presentations
are rhinocerebral, pulmonary, cutaneous and disseminated. The agents of mucormycosis
are ubiquitous in nature, and they are transmitted by inhalation, direct inoculation
or ingestion. There have been multiple reports of healthcare associated mucormycosis,
either as isolated cases or as outbreaks. In a publication from India, during an eighteen
month period, 75 cases of mucormycosis were reported, of which 9% were nosocomial.
Healthcare associated mucormycosis has been attributed to various exposures in the
hospital environment, including: (a) Use of non-sterile products. This is the most
commonly suspected cause of infection. Bandages, adhesives, nitroglycerin patches,
contaminated linen, wooden tongue depressors, ostomy bags, probiotics, have all been
implicated. There has even been a report of an outbreak due to allopurinol tablets
and pre-packaged food. (b) Various procedures and medical devices, such as catheters,
insulin pumps and finger sticks, as well as insertion of tubes, tooth extractions
and surgery. (c) Environmental factors may also be a source of infection. Molds may
be found in the air, dust, water or any surfaces in the hospital. Construction works
increase the risk of invasive fungal infections. Outbreaks have been linked to defective
ventilation systems and water leakage. The clinical presentation varies, depending
on the source of infection. Infections due to bandages, adhesives or contaminated
wound dressings are mostly cutaneous. Percutaneous exposure in immunocompromised patients
has led to disseminated disease. Inhalation leads to pulmonary and rhino-cerebral
infection, while ingestion of tablets or food, as well as the use of tongue depressors,
are responsible for gastrointestinal mucormycosis. Dialysis catheters have been linked
to peritonitis. It is not easy to investigate a possible mucormycosis outbreak. The
Centers of Disease Control of USA have recently published a guide to investigating
suspected outbreaks due to mucormycosis. Due to the rarity of the disease, investigation
is warranted even when it is unclear if there is a true outbreak. The first step is
to verify the diagnosis and define a case. Histopathology and culture are the mainstay
of diagnosis. In recent years, newer tools have been added to our diagnostic armamentarium.
These include PCR, whole-genome sequencing and matrix-assisted laser desorption/ionization
time-of-flight mass spectrometry (MALDI-TOF MS). The next step in the investigation
of an outbreak is to describe the epidemiologic features of the cases, scrutinize
patient care activities and determine environmental involvement. The management of
outbreaks is multi-disciplinary and should include treating physicians, hospital administration,
workers in the microbiology laboratory and health departments. Rapid identification
of cases and initiation of treatment is important, in order to decrease mortality
of existing cases and prevent the emergence of new ones.
S03.1. Hospital related mucormycosis
Pagano
L.
Fondazione Policlinico Universitario A. Gemelli—IRCCS—Universita Cattolica del Sacro
Cuore, Rome, Italy
S03.3. The new treatment of mucormycosis
The antifungal drug that showed the best in vivo activity in the past has been deoxycolate
Amphotericin B (d-AmB). Although this molecule, although extremely effective, has
been characterized by an important series of side effects (i.e. renal insufficiency,
hypokalemia) which have made its use disadvantageous. In more recent years, the introduction
of new formulations of AmB in lipid formulations (l-AmB, ABCD, ABLC) have mitigated
the unpleasant side effects without eliminating them completely but have made their
use more manageable. Nowadays lipid formulations, and especially l-AmB, are considered
by all the international guidelines as the first-choice drugs for the treatment of
mucormycosis. In recent years new azole formulations, first and second-generation
azoles (i.e. posaconazole, isavuconazole) have shown considerable efficacy in the
treatment of these molds even in highly immunocompromised patients such as those suffering
from acute leukemia or undergoing bone marrow transplantation, whereas voriconazole
and itraconazole show no effect on mucorales. Posaconazole was registered as a drug
to be used as an alternative to liposomal amphotericin and often the availability
of the oral solution alone has considerably limited its use. The oral-only route of
posaconazole is anyway a great limitation, especially in hematologic patients, often
inable to take drug per os or undergoing chemotherapy with subsequent high incidence
of vomiting. However the availability of posaconazole in intravenous formulation and
in coated tablets has favored a greater absorbability and, consequently, greater efficacy.
Isavuconazole in registrative trial has shown a good efficacy in the treatment of
this fungal complication and is now recognized as an excellent alternative. We must
also take into account the possibility, supported in recent years by multiple studies
in real life, to approach the most aggressive and disseminated forms of mucormycosis
with the combination of multiple drugs. l-Amb combined with caspofungin would appear
to be preferable due to the lack of negative interactions between the drugs used,
however there are multiple reports showing that l-Amb plus posaconazole may have a
positive role.
Garcia-Hermoso
D.
1
Sitbon
K.
1
Gautier
C.
1
Dromer
F.
2
T.H.E. French Mycoses Study Group
2
1National Reference Center for Invasive Mycoses & Antifungals, Molecular Mycology
Unit, Cnrs Umr2000, Institut Pasteur, PARIS, France
2National Reference Center for Invasive Mycoses & Antifungals, Molecular Mycology
Unit, Cnrs Umr2000, Institut Pasteur, Paris, France
S03.4. Clinical features associated to fungal species
Background Fungi of the order of Mucorales are ubiquitous saprobes that can be responsible
for life-threatening infections especially in immunocompromised patients but also
in immunocompetent individuals. An increased incidence of mucormycosis has been reported
mostly due to the rise of populations at risk and the use of better diagnostic tools.
There are more than 20 pathogenic species involved in this type of infections, with
some of them associated with specific body sites. The main localizations of mucormycosis
are rhino-orbito-cerebral and pulmonary infections, depending on the underlying conditions
of the patient. We present here a preliminary analysis aiming to dissect the association
between clinical manifestations and Mucorales species using the active surveillance
system (RESSIF) implemented in France at the NRCMA since 2012.
Methods Three-hundred cases of probable and proven mucormycosis were so far collected
through RESSIF. The isolates were characterized using phenotypic analysis by morphological
methods, and antifungal susceptibility testing of 8 antifungal drugs done using EUCAST
microdilution method. In addition, a multi-locus sequencing based on LSU, ITS, TEF-1
was performed.
Results Among those 300 cases of invasive mucormycosis, lung involvement was the main
presentation (52%) followed by rhinocerebral (18%) and cutaneous (15%) involvement.
Deep infections occurred most often in patients with hematological malignancies. Six
major species of Mucorales were identified with Rhizopus arrhizus being the most frequent
fungus. Our current data expand previous findings showing that distribution of these
6 major species depended on the body site localizations and underlying condition of
the patient.
Millon
L.
Parasitology—Mycology—Umr6249 Cnrs Chrono-environnement, University Hospital Besançon,
Besançon, France
S03.5. Molecular diagnosis of mucormycosis
Molecular techniques have provided a new understanding of the epidemiology of mucormycosis.
Several factors may explain the growing incidence of mucormycosis, especially the
increase in the number of susceptible people and the change in antifungal practices
(which may have altered the relative frequency of mucormycosis and aspergillosis among
patients at risk for both infection). In addition, the routine use of molecular techniques
has helped to accurately identify Mucorales fungi in tissue samples when cultures
are negative. As a result, the number of mucormycosis cases diagnosed in the last
10 years has increased. The use of molecular techniques has also improved the therapeutic
management of mucormycosis. Indeed, mucormycosis and aspergillosis have similar underlying
conditions, and similar radiological and clinical signs, but their antifungal treatments
are different. For each of these infections, early diagnosis and early initiation
of directed treatment are essential to improving patient outcome Molecular techniques
may help to provide fast, effective treatment by accurately identifying the causative
fungi. PCR amplification and sequencing were first applied to better identify isolates
grown from cultures of biopsies or bronchalveolar lavage samples collected in patients
with Mucorales infection. Molecular techniques have also been used to identify the
fungus directly from the tissue samples when cultures were negative. The first technical
approach for detection fungi in tissue samples consists of using panfungal primers,
targeting ITS regions, followed by sequencing. The second possible approach consists
of using Mucorales-specific primers. Diverse PCR techniques were tested and were successful,
and several studies confirmed that PCR results were better in fresh/ frozen samples
than in formalin-fixed paraffin-embedded samples. Molecular detection of fungi on
hyphal positive biopsy samples is now strongly recommended (AII) by the European ESMID
and ECMM joint clinical guidelines. Recent studies confirmed that Mucorales quantitative
PCR (qPCR) applied on bronchoalveolar lavage fluid could provide additional arguments
favoring earlier initiation of specific antifungal therapy, thus improving the outcome
of pulmonary mucormycosis patients. However, these tools require invasive sampling
(biopsy, bronchoalveolar lavage), which is not feasible in patients in poor condition
in Hematology or Intensive Care units. QPCR-based procedures to detect Mucorales DNA
in non-invasive samples such as plasma or serum have proved successful in diagnosing
mucormycosis early (as early as 8 days before mycological diagnosis and 3 days before
imaging in patients with hematological malignancies). The test can be performed in
all patients, even those who cannot endure a biopsy or bronchoalveolar lavage. The
Mucorales qPCR performed on serum or plasma is becoming essential to improve the management
of at-risk patients. The main problem at the moment is a lack of standardization,
because of the use of multiple in-house assays. Efforts from the Fungal PCR Initiative
(FPCRI)/Mucorales Lab working group of the International Society for Human and animal
Mycology (ISHAM) are currently ongoing to improve standardization and provide recommendations,
as was previously done for Aspergillus PCR assays by the European Aspergillus PCR
Initiative (EAPCRI) working group.
Chakrabarti
A.
Department of Medical Microbiology, Postgraduate Institute of Medical Education &
Research, Chandigarh, India
S03.6. Management of mucormycosis in low and middle income countries
The epidemiology of mucormycosis is different in low and middle income countries (LMIC)
from developed nations. In LMIC the number of mucormycosis cases is alarmingly high
in diabetic patients, the spectrum of causative agents is wide with lack of knowledge
on antifungal susceptibility of the new agents. Occasional unusual clinical presentation
baffles the clinicians about diagnosis, and delay in seeking healthcare by patients
leads to poor outcome of the disease. Additionally, the lack of awareness of the disease
among clinicians, the lack of availability of appropriate diagnostic facilities, poor
availability and affordability of antifungal agents pose serious challenge in the
management of the disease. In a recent descriptive study covering 465 mucormycosis
cases from 13 centres in India, it was noted that 24% patients could not complete
or start antifungal therapy due to affordability issue and nearly 20% patients were
treated with amphotericin B deoxycholate. In 4.2% patients the therapy was shifted
from liposomal to deoxycholate preparation of amphotericin B after certain period
due to financial constraints, and improper antifungal therapy was prescribed in 7%
patients. Due to those challenges, 90-day mortality was 52.0%, though majority (78.2%)
of the patients had rhino-cerebral or cutaneous mucormycosis, which were not difficult
for early diagnosis. Deferasirox of label use was also noted in certain number of
patients.
Symposium 4 MSG symposium (Trial Design in Clinical Mycology: Innovative Approaches)
Hoz
R. La
1
Jones
C.
2
Mcgwin
G.
3
Patterson
T.
4
Pappas
P.
3
Baddley
J.
3
Morrissey
O.
5
1Internal Medicine, UT Southwestern Medical Center, Dallas, United States of America
2The Ohio State University, Columbus, United States of America
3University of Alabama at Birmingham, Birmingham, United States of America
4University of Texas Health Science Center at San Antonio, San Antonio, United States
of America
5Monash University, Melbourne, Australia
S04.5. The Lung Transplant Community is Interested in a Clinical Trial to Determine
the Optimal Strategy to Prevent Invasive Mold Disease
Objectives: The objectives of this survey were to: (1) describe the strategies used
to prevent invasive mold disease in lung transplant recipients, (2) quantify the interest
in participating in a clinical trial to determine the optimal prophylactic strategy
for invasive mold disease in lung transplant recipients and (3) to describe the design
types and factors that influence willingness to participate in the clinical trial.
Methods: We invited transplant providers to participate in an anonymous cross-sectional
web-based survey via email between December 2018 and February 2019. The 139-item survey
was hosted on the University of Alabama at Birmingham Research Electronic Data Capture
(REDCap) server. The contact list was generated from a variety of sources. The study
was approved the University of Alabama at Birmingham Institutional Review Board.
Results: of the 238 practitioners contacted, 50 (21.0%) responded to the survey. Responses
were mainly from the United States 85.7% (36/42) and were transplant infectious diseases
physicians 65.2% (30/43). The number of transplants performed at the responder’s centers
during 2016 was less than 20 in 44.4% (16/36), 21 to 50 in 19.4% (7/36) and more than
50 in 36.1% (13/16). Most responders, 81.0% (30/37), had a mold prevention protocol
at their program. Universal prophylaxis was the most common prevention strategy 75.7%
(28/37), followed by pre-emptive prophylaxis 10.8% (4/37), and targeted prophylaxis
5.4% (2/37). A quarter of the responders (7/34) used prophylaxis for <3 months, 58.8%
(20/34) for 3 to 6 months, 11.8% (4/34) for 6 to 12 months and 8.8% (3/27) for more
than 12 months. The majority of the responders, 84.6% (22/26) were interested in participating
in a clinical trial to assess the optimal prophylactic strategy for mold infections
in lung transplant recipients. Almost a quarter of the participants were willing to
participate of the clinical trial with a universal prophylaxis study design of new
azole formulation to mold active azole (Figure 1). The study design arms and the anti-fungals
selected for the study arms were the factors that had the most influence in study
participation (Figure 2).
Conclusion: Universal prophylaxis was the most common strategy to prevent invasive
mold disease in lung transplant recipients. The majority of the responders were interested
in a clinical trial to determine the optimal strategy to prevent mold disease in lung
transplant recipients. The study design arms and the anti-fungals selected for the
study arms were the factors that had the most influence in study participation.
Symposium 5 Lung transplantation
Rammaert
B.
1
2
3
1Infectious Diseases, CHU Poitiers, Poitiers, France
2U1070, INSERM, Poitiers, France
3Faculté De Médecine Et Pharmacie, Université de Poitiers, Poitiers, France
S05.1. Pre-transplant assessment
All the three main chronic pulmonary diseases leading to lung transplantation are
at risk for fungal respiratory tract colonization: cystic fibrosis (CF), chronic obstructive
pulmonary disease (COPD), and interstitial lung diseases (ILD). Aspergillus and Scedosporium
species are the two main molds found in the lower respiratory tract of lung transplant
candidates. Colonization in CF patients could be explained by impairment of muco-ciliary
clearance, and in COPD and ILD patients by use of inhaled or systemic corticosteroids
that alter pulmonary immunity. Mortality due to invasive fungal diseases in post-transplantation
can reach 60% depending on the causal species. Therefore, fungal colonization of the
lower respiratory tract is on the list of relative contraindications for lung transplantation.
Although passage from colonization to infection is not well understood, fungal spores
could remain quiescent in the lungs, and secondarily reactivate once patients have
received immunosuppressive drugs. Donor-derived fungal infection is one of the best
examples of this reactivation. Pre-transplant colonization is not considered predictive
for fungal invasive disease in post-transplantation. However, the timing and bronchoalveolar
lavage protocols for fungal screening in respiratory tract before lung transplantation
are heterogeneous between centers. In addition, culture of lower respiratory tract
specimens has to be done on specific media such as Sabouraud glucose agar or yeast
extract-peptone-dextrose-agar (YPDA), which could miss out on some fungi. Utilization
of culture media supplemented with antibiotics has increased the number of CF patients
culture-positive for fungi in their respiratory specimens from 18% to 78%. Scedosporium
specific media such as Scesel+ increase the detection of Scedosporium spp. compared
to Sabouraud glucose agar. Active screening for fungal colonization during the pre-transplant
period is consequentley crucial not only in patients on a waiting list, but also in
donors. There is an urgent need for bronchoalveolar lavage and fungal culture standardization
in the different mycological laboratories dealing with lower respiratory tract specimens
before focusing on pre-transplant fungal colonization.
Lavergne
R.-A.
1
2
Lepoivre
T.
3
Groot
T. De
4
Jeddi
F.
2
Boutoille
D.
5
Danner-Boucher
I.
6
Morio
F.
1
2
Meis
J.
7
Pape
P. Le
1
2
1Ea1155 Parasitology and Mycology Department, Nantes University, Nantes, France
2Parasitology and Mycology Laboratory, Nantes University Hospital, Nantes, France
3Thoracic Transplantation Intensive Care Unit, Nantes University Hospital, Nantes,
France
4Medical Microbiology and Infectious Diseases, Canisius-Wilhelmina Hospital, Nijmegen,
Netherlands
5Tropical Infectious Diseases, University Hospital of Nantes, Nantes, France
6Pneumology Unit, Nantes University Hospital, Nantes, France
7Department of Medical Microbiology and Infectious Diseases, Excellence Center For
Medical Mycology (ECMM), Canisius Wilhelmina Hospital, Nijmegen, Netherlands
S05.5. From the lung to the heart: fatal dissemination of azole-resistant Aspergillus
fumigatus in a lung transplant patient
Azole resistance in Aspergillus fumigatus has emerged as a worrisome problem since
the last ten years. Patients suffering from cystic fibrosis (CF) are at risk of being
colonized with azole-resistant isolates. At our CF reference center hospital, prevalence
of azole resistance in A. fumigatus reached 6.8% in 2015. Though resistance is increasingly
described, its impact in CF patients is yet poorly known. Here, we describe the case
of a CF patient having long-term colonization of the respiratory tract with a non-cyp51A
mediated azole-resistant Aspergillus fumigatus isolate, prior to lung transplantation.
Then, proven azole-resistant invasive aspergillosis and mucormycosis were diagnosed
in the first days after transplantation and treated with liposomal amphotericin B.
Two years later, azole-resistant Aspergillus fumigatus mitral-aortic endocarditis
was diagnosed. Despite surgery and multiple antifungal regimens, the patient died
with intracerebral hematoma. Objectives: The aim of the study was to investigate the
genetic relationship between isolates collected at the time of transplantation and
those from the native valve. Methods: Five isolates were retrospectively investigated
for azole susceptibility to itraconazole (ITR), voriconazole (VOR), posaconazole (POS)
and isavuconazole (ISA) (EUCAST method). Identification of A. fumigatus sensu stricto
was confirmed using partial beta-tubulin sequencing. Molecular mechanisms leading
to resistance (nucleotide sequencing of cyp51A gene and its promotor) and STRAf genotypes
were determined. Results: Two isolates were collected at time of transplantation (bronchial
secretion (n = 1) and postoperative wound (n = 1)) and three isolates were collected
during endocarditis episode (respiratory tract (n = 2) and mitral valve (n = 1)).
All isolates were identified as Aspergillus fumigatus and were pan-azole resistant
(MIC ITR: > 8 mg/L, VOR: 4 to 16 mg/L, POS: 0.5 to 1 mg/L, ISA: 8 to >16 mg/L). Sequencing
of the cyp51A gene and its promotor (4 isolates) showed wildtype cyp51A and promotor.
All isolates shared the same STRAf genotype (M2: 26-20-8 M3: 35-9-7 M4: 9-10-20) confirming
dissemination of this isolate from the lung tissue to the heart. Conclusion: This
case report highlights the need for regular screening for azole resistance in CF patients
especially for those awaiting lung transplantation. Physicians should keep in mind
that azole-resistant colonizing isolates could be a possible source of life threatening
invasive infection after lung transplantation. Yet, antifungal treatment of Aspergillus
endocarditis is based on voriconazole or lipid formulation of amphotericin B but no
data are available in case of azole-resistant isolate.
Symposium 6 Prophylaxis during hematology malignancies
Lewis
R.
1
2
1Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical
and Surgical Sciences, University of Bologna, Bologna, Italy
2Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy
S06.1. AML—in the era of FLT3 inhibitors
FLT3 mutations are among the most common genetic mutations in acute myeloid leukemia
(AML) associated with increased risk of disease relapse and poorer survival. A number
of small molecule tyrosine kinase inhibitors (TKI) of FLT3 signalling have shown promise
for reducing relapse of FLT3+ AML. One TKI, midostaurin, received fast-track approval
in 2017 for the treatment of FLT3 positive AML on the basis of data demonstrating,
for the first time in over 45 years, improved survival compared to standard induction
7+3 (cytarabine/anthracycline) chemotherapy. The use of FLT3 inhibitors, however,
is problematic in patients who require antifungal prophylaxis or treatment with triazole
antifungals. In general FLT3 inhibitors are extensively metabolized by CYP3A4 to active
metabolites with high potency and potential off-target effects. When coadministered
with triazoles, FLT3 exposures may increase between 1.3 to 10 fold and alter production
of active metabolites essential for FLT3 inhibition or increase off-target effects.
Indeed, higher rates of pulmonary toxicity including fatal events, were observed among
patients receiving midostarin with azole antifungals. Currently, the midostaurin SmPC
does not recommend the co-administration of strong CYP3A4 inhibitors such as voriconazole
or posaconazole; although this recommendation is not practical for many patients especially
with active fungal disease. Unfortunately, second-generation FLT3 inhibitors currently
in clinical studies (e.g., quizartinib) also appear to have similar risks as midostaurin
as well as greater risk for dose-limiting myelotoxicity and QTC prolongation. It is
clear that striking the balance between optimal antifungal prophylaxis vs. optimal
FLT3 inhibition will become a major challenge in the management of AML in the coming
decade. This presentation will discuss the pros and cons of possible strategies for
managing drug-drug interactions with FLT3 inhibitors and antifungals in the absence
of clearly-defined guidance.
Schwartz
S.
Charité Berlin, Berlin, Germany
S06.4. Baseline CT upon diagnosis of acute leukemia
Computed tomography (CT) is a routine diagnostic procedure in immunocompromised patients
(i.e. patients with long-lasting neutropenia) with suspected fungal pneumonia. Particular
radiological appearances of lung infiltrates (e.g. halo-sign) have been associated
with lung infections caused by molds and findings have been incorporated into the
well acknowledged definitions of invasive fungal infections developed by the EORTC/MSG
(1). A conventional chest X-ray is usually performed in immunocompromised patients
with fever, but the value of a conventional chest X-ray has been questioned due to
its poor diagnostic yield and most recommendations advocate CT (2). A baseline chest
CT in asymptomatic patients scheduled for therapies resulting in a profound immunosuppression
(e.g. induction chemotherapy for acute leukemia) appears meaningful, but published
data about this are limited. In a prospective study, 198 adult patients scheduled
for hematopoietic stem cell transplantation or intensive chemotherapy were evaluated
with a baseline CT (3). Overall, 72 (36%) patients showed lung abnormalities. of these,
19 exhibited disease-defining lesions according to the EORTC/MSG definitions (nodules,
halo sign, cavitation) and 53 patients showed other abnormalities (most frequently
consolidations or ground-glass opacifications). Nine patients in each group were subsequently
categorized as having proven or probable invasive mold infections. The risk of developing
an invasive pulmonary mold infection was significantly higher among patients with
disease-defining lesions according to the EORTC/MSG definitions or, less pronounced,
with other abnormalities compared to patients with a normal baseline CT. In another
study, 145 baseline CT scans from patients with acute leukemia scheduled for their
first induction therapy were assessed retrospectively (4). Remarkably, the majority
of baseline CT scans disclosed abnormalities (127; 88%) with nodules in 71 (49%) patients.
Cavitations or lesions with a halo- or an air-crescent-sign were not present in this
patient series. However, an independent radiological review and results from a comprehensive
diagnostic evaluation were not available from this study. The high frequency of lung
lesions at baseline, including disease-defining lesions associated with invasive mold
infections, in patients scheduled for intensive chemotherapy or hematopoietic stem
cell transplantation is striking. A baseline CT scan in these patients appears reasonable,
but the overall benefit of this diagnostic approach in asymptomatic patients should
be explored further in future studies. It would be desirable to evaluate in more detail
other underlying, infectious/non-fungal and non-infectious, conditions (e.g. opacities
associated with hyperleukocytosis) associated with lung lesions (5). Literature 1.
de Pauw B, et al. Clin Infect Dis 2008;46(12): 1813–21. 2. Maschmeyer G. Curr Opin
Oncol 2001;13: 229-35. 3. Ceesay MM, et al. Br J Haematol 2018;182: 723–27. 4. Vallipuram
J, et al. Leuk Lymphoma 2017;58: 834–41. 5. Stefanski M, et al. Medicine 2016;95:
e5285.
Bellanger
A.-P.
1
Tatoyan
N.
1
Berceanu
A.
2
Gbaguidi-Haore
H.
3
Monnot
T.
4
Bichard
D.
4
Millon
L.
1
1Mycology, University Hospital Jean Minjoz, Besancon, France
2Hematology, University Hospital Jean Minjoz, BESANCON, France
3Infectious Risk Control, University Hospital Jean Minjoz, BESANCON, France
4Pharmacy, University Hospital Jean Minjoz, BESANCON, France
S06.5. Investigating the impact of posaconazole prophylaxis on systematic fungal screening
using galactomannan antigen, Aspergillus qPCR and Mucorales qPCR
Objectives: At the University Hospital of Besancon, a systematic panel of fungal biomarkers
is applied systematically to screen for invasive fungal infection (IFI) highly immunocompromised
patients of the Hematology Department. This panel of biomarkers does include the galactomannan
antigen (GM), Aspergillus qPCR and Mucorales qPCR. Most of the stem cell transplant
(SCT) recipients receive now posaconazole prophylaxis. The pertinence of systematic
fungal screening has been discussed in the literature for patients under active antifungal
prophylaxis, especially the realization of the GM. In this context, our main objective
was to investigate retrospectively if the posaconazole prophylaxis influenced the
numeric value of the GM. Our secondary aims were to assess the contribution of Aspergillus
qPCR and Mucorales qPCR as well as the prevalence of IFI under active antifungal prophylaxis.
Methods: The results of systematic fungal screening (GM, Aspergillus and Mucorales
qPCR) were collected for all the SCT recipients, treated with posaconazole as prophylaxis
treatment for more than 8 days, from January 2015 to December 2016. Similarly, results
of systematic fungal screening were collected for patients without posaconazole as
prophylaxis treatment (induction chemotherapy-January 2010 to December 2015). The
statistical analysis of data was performed using the Stata 2 software.
Results: For the group of patients receiving posaconazole prophylaxis: 60 patients
were included, with a mean period of prophylaxis of 107 days and a total 916 fungal
screenings. The mean value of the GM was 0.17 (±0.32), the frequency of positive GM
was 4.37%, the frequency of positive Aspergillus qPCR was 0.68% and the frequency
of positive Mucorales qPCR was 3.66%. The frequency of IFI in this group was estimated
at 5%. For the control group: 38 patients were included with a total of 392 fungal
screenings. The mean value of the GM was 0.22 (±0.64), the frequency of positive GM
was 5.61%, the frequency of positive Aspergillus qPCR was 12.35% and the frequency
of positive Mucorales qPCR was 5.33%. The frequency of IFI in this group was estimated
at 13%. The statistical analysis showed that posaconazole prophylaxis did not affect
significantly neither the numeric value nor the frequency of positivity of the GM.
A significant decrease of the frequency of positive Aspergillus qPCR was observed
(0.68 vs 12.35, p = 0.015). A tendency of decreased frequency of IFI was also observed
(5% vs 13%, p = 0.076).
Conclusion: Our study did not demonstrate any negative effect of the posaconazole
prophylaxis on the GM. This study highlights the high efficacy of the posaconazole
prophylaxis, especially when using the tablet form, which was supported by a significant
decreased frequency of positive Aspergillus qPCR and relatively lower frequency of
IFI. The current screening strategy combining GM and fungal qPCRs is of high interest,
even if the posaconazole prophylaxis is efficient, in a context of emerging infection
with azole resistant strains of Aspergillus fumigatus and risk of A. fumigatus-Mucorales
mixed infections.
Symposium 7 Paediatric Mycology (EPMyN)
Decembrino
N.
1
Perruccio
K.
2
Colombini
A.
3
Calore
E.
4
Muggeo
P.
5
Soncini
E.
6
Comelli
A.
7
Molinaro
M.
8
Goffredo
B.M.
9
Gregori
S. De
8
Giardini
I.
8
Cairoli
S.
9
Scudeller
L.
10
Zecca
M.
1
Cesaro
S.
11
1Pediatric Hematology/oncology, Fondazione IRCCS Policlinico San Matteo, Pavia, Pavia,
Italy
2Division of Pediatric Hematology, University Hospital of Perugia, Perugia, Italy
3Pediatric Hematology/oncology, Fondazione Monza e Brianza per il Bambino e la Mamma,
Monza, Monza, Italy
4Clinic of Pediatric Hemato- Oncology, Department of Women’s and Children’s Health,
University Hospital of Padova, Padova, Italy, Padova, Japan
5Department of Pediatric Oncology and Hematology, University Hospital of Policlinico,
Bari, Italy, Bari, Italy
6Pediatric Hematology/oncology, Spedali Civili, Brescia, Italy, Brescia, Italy
7Department of Infectious and Tropical Diseases, Spedali Civili, Brescia, Italy, Brescia,
Italy
8Clinical and Experimental Pharmacokinetics Lab, Fondazione IRCCS Policlinico San
Matteo, Pavia, Pavia, Italy
9Metabolic Pathology Lab, Ospedale Pediatrico Bambino Gesù, Roma, Roma, Italy
10Clinical Epidemiology and Biometric Unit, Scientific Direction, Fondazione IRCCS
Policlinico San Matteo, Pavia, Pavia, Italy
11Pediatric Hematology/oncology, Azienda Ospedaliera Universitaria Integrata, Verona,
Italy, Verona, Italy
S07.5. Isavuconazole use in pediatric hematoncologic patients: the Italian Association
of Pediatric Hematology Oncology (AIEOP) experience
Objectives: Isavuconazole (ISA) is a new triazole approved for invasive aspergillosis
and mucormycoses treatment in adults only. Advantages are activity against both moulds
and yeasts spp, excellent bioavailability after oral administration without relevant
food or gastric pH effect, a water-soluble prodrug without nephrotoxic excipients,
poor drug-drug interactions. We analyzed ISA use, safety and efficacy in immunocompromised
children.
Methods: Italian Association Paediatric Hematology Oncology (AIEOP) multicentric retrospective
analysis of pediatric hematoncologic patients who received ISA asinvasive fungal disease
(IFD) treatment or prophylaxis. Due to the lack of recommended dosing in children
and of PK/PD target value, therapeutic monitoring (TDM) was applied by a validated
liquid chromatography-tandem mass spectrometry (HLPC-MS/MS) assay technique. ISA trough
plasma concentrations (C0) and 3 hours after drug intake (C3h) were measured.
Results: Twenty-nine patients were included, (M/F 20/9); median age: 14.5 years (range
3–18), median bodyweight 47 kg (range 15–80). Ten patients only received chemotherapy,
19 patients received allogeneic hematopoietic stem cell transplantation (HSCT). Donors
were haploidentical in 9 patients, matched-sibling in 4, allogenic-unrelated in 4
cases. ISA was used as prophylaxis in 5 patients, as IFD treatment in 24 cases: 20
for previous therapeutic failure (7 l-AMB, 4 Voriconazole, 5 combination therapy,
1 Posaconazole, 3 Caspofungin), 4 as first line therapy. According to EORTC criteria,
IFD was proven in 5 patients (1 Aspergillus spp, 1 Aspergillus flavus, 1 Aspergillus
fumigatus, 1 Penicillium spp. and 1 Mucor spp), probable in 9 and possible in 10 patients.
Lungs were the main localization (18 cases), liver was involved in 2 cases, 3 patients
had sinus involvement (2 with central nervous system localizations), 1 had a disseminated
infection. Patients under 30 kg bodyweight received half ISA dose, the others received
adult schedule (200 mg TID loading dose on days 1 and 2, 200 mg/die maintenance);
only 13 patients received loading dose. Ten patients only received oral therapy, in
the others IV route was switched to oral during treatment. ISA was administered for
a median of 75.5 days (range 6–523 day), in combination with other antifungals in
7 patients (Caspofungin in 3 cases, l-AMB in 4). Overall response rate was 70.8%.
IFD complete remission was achieved in 12 cases, partial remission in 5. Treatment
failure was experienced by 5 patients, in 3 cases fungal lesions remained stable.
No breakthrough infections were registered in prophylaxis group. TDM was applied to
17 patients including 1with severe veno-occlusive disease and 1 with renal failure
secondary to thrombotic-microngiopathy. Overall median ISA Ctroughss concentration
observed was 4.91 mg/L (2.15–8.54); Conc/Dose (kg) ratio was 1.13 (0.47–3.42). In
6 cases a 12h-PK profile was carried out and median AUC0–12h of 153,16 mgxh/L (range
86.31–169.45) was obtained. CTAE grade II-III toxicity was observed in 6 patients,
with increased transaminase and/or creatinine levels which resolved after temporary
ISA withdrawal. We did not observe drug-drug interactions in the patients receiving
immunosuppressant as graft versus host disase prophylaxis (5 cyclosporine, 2 sirolimus,
1 tacrolimus).
Conclusion: Isavuconazole may be useful and safe in the pediatric population with
hematoncologic diseases, even in the HSCT setting. Prospective studies are warranted.
Symposium 8 Immunologic markers for diagnosis and treatment stratification in invasive
mold infection
Köhler
P.
Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany
S08.3. Immunologic Markers for Treatment Stratification in Invasive Mold Infection
The two major entities, invasive aspergillosis and mucormycosis account for the majority
of fungal mould infections in haematology and oncology. With increased hospital admission
rates and admission to intensive care unit and high morbidity and mortality the need
for prompt identification and outcome prediction is needed. Current in vitro diagnostic
approaches comprise direct observation of fungi in biopsy or bronchoalveolar lavage
samples, blood cultures and detection of diagnostic antigens such as galactomannan.
New immunologic markers for diagnosis and treatment stratification have been recently
published. Fungus-reactive CD4 T cells, interleukin profiling and inflammation parameters
will be discussed with regard to treatment stratification depending of response of
invasive mould infections. Possible developments could lead to algorithms incorporating
measurement of these biomarkers at admission and throughout initial treatment for
the purpose of identifying patients who warrant more aggressive treatment.
Hoenigl
M.
1
2
1Division of Infectious Diseases, Department of Medicine, UCSD, San Diego, United
States of America
2Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology,
Medical University of Graz, Graz, Austria
1,3-β-d-glucan is a fungal cell wall polysaccharide produced by ascomycete fungi including
Candida albicans and other yeast that colonize the GI tract. Recently we and others
have shown that in the absence of an invasive fungal infection blood 1,3-β-d-glucan
levels may serve as a marker of microbial translocation in patient populations with
hypoperfusion of the gut and those with persistent inflammation, including patients
with advanced liver cirrhosis, sepsis or HIV infection, where blood 1,3 β-d-glucan
have been shown to be elevated compared to HIV negative controls and to correlate
with other biomarkers of microbial translocation, with markers of immune activation
and with lower Lactobacillales proportion (i.e. less protective) in the gut microbiome.
In animal models 1,3-β-d-glucan has been shown to decrease Dectin-1 (innate pattern
recognition receptor essential for the control of fungal infections), and also NKp30
(NK receptor mediates direct fungal recognition and killing) on monocytes and NK cells
respectively, and induced pro-inflammatory cytokines secretion, indicating a potential
role in pathogenesis of clinical events. Going one step further, studies have shown
clear correlations between blood 1,3-β-d-glucan levels and non-AIDS events, including
cardiopulmonary morbidity, and neurocognitive impairment. Only few studies to date
have evaluated a potential benefit of antifungal treatment for tackling fungal translocation
and reducing blood 1,3-β-d-glucan levels. In vitro model of mixed rat neuronal cultures
that screened more than 2000 compounds, half of which were US Food and Drug Administration
approved drugs for putative neuroprotective effects against oxidative stress-mediated
neuronal injury not only paroxetine, a selective serotonin reuptake inhibitor, but
also fluconazole, protected hippocampal neurons. In another study, the combination
of paroxetine and fluconazole protected macaques from simian immunodeficiency virus–associated
neurodegeneration. However, a phase 1/2, randomized, double-blind, placebo-controlled
study for the treatment of HIV-associated neurocognitive impairment did not show an
improvement of neurocognitive outcomes among the 9 participants randomized to fluconazole
treatment alone, indicating that more research and larger studies are needed on how
to prevent/treat fungal translocation.
S08.4. Fungal Translocation: from persistent inflammation to non-AIDS events
Aguilar
C.
1
Moshkelgosha
S.
2
Gohir
W.
1
Yee
K.
3
Schimmer
A.
3
Minden
M.
3
Schuh
A.
3
Ryan
C.
4
Juvet
S.
2
Husain
S.
1
1Transplant Infectious Diseases, University Health Network, Toronto, Canada
2Lung Transplant Program, University Health Network, Toronto, Canada
3Hematology Department, University Health Network, Toronto, Canada
4Respirology, University Health Network, Toronto, Canada
S08.5. Immunological characteristics of Broncho alveolar lavage in neutropenic patients
with invasive aspergillosis
Objectives: Invasive aspergillosis (IA) is a major complication in patients with acute
leukemia receiving high dose chemotherapy. Diagnosis of IA classically relies on radiological
criteria associated with direct and/or indirect microbiological tests, which have
some limitations. some limitations. Antifungal immunity involves phagocytic cells
including neutrophils and macrophages, as well a dendritic cells presenting antigen
to T-CD4+ T cells. Antigen presentation leads to differentiation of CD4+ T cells into
Th1 and Th17 cells, under the influence of cytokinic environment. Those cells secrete
cytokines and amplify effector functions of phagocytic cells in order to kill the
fungus. The triggering of those mechanisms relies on the recognition of fungal patterns
by receptors of innate immunity, both soluble (such as PTX-3) and membrane bound (such
as Dectin-1 and Dectin-2). In this study we aimed to assess the immunological parameters
of broncho alveolar lavage (BAL) fluid of neutropenic patients with or without IA.
Methods: We included acute myeloid leukemia patients who were neutropenic after receiving
high dose chemotherapy and who underwent a bronchoscopy for diagnosis of pneumonia.
Ten milliliters of BAL fluid were collected for the research study. Supernatant and
cell pellet were separated and frozen until use. The level of 35 cytokines (EGF, Eotaxin,
FGF basic, G-CSF, GM-CSF, HGF, IFN-α, IFN-γ, IL1-ra, IL-1α, IL1-β, IL-2, IL-2r, IL-3,
IL-4, IL-5, IL-6, IL-7, IL-7, IL-8, IL-9, IL-10, IL-12 (p40/p70), IL-13, IL-15, IL-17A,
IL-17F, IL-22, IP-10, MCP-1/CCL2- MIG, MIP-α, RANTES, TNF-α, and VEGF) was measured
in BAL supernatant using Luminex technology. PTX-3 level in BAL supernatant was measured
by Enzyme-linked immunosorbent assy. The cell pellet was analyzed by flow cytometry
for expression of CD45, CD206, CD163, Dectin-1 and Dectin-2.
Results: Forty three patients were included in the study. Seven patients were diagnosed
with probable IA and 20 with possible IA. One patient had mucormycosis diagnosed on
lung biopsy and 1 patient had fusariosis. While PTX-3 level was not significantly
different in patients with probable IA compared to patients without IA, IL-6, IL-8
land GM-CSF levels were higher in patients with probable IA compared to patients without
invasive fungal infection. Flow cytometry could be performed on 31 BAL cell pellets.
The proportion of alveolar macrophages (CD163+ CD206+) among CD45 positive cells was
similar in all groups. Dectin-1 and Dectin-2 expression on alveolar macrophages (CD163+
CD206+ cells) was similar in patients with and without invasive fungal infection.
Conclusion: Neutropenic patients with probable IA exhibit higher IL-8, IL-6 and GM-CSF
levels in BAL supernatant compared to neutropenic patients without any fungal infection.
The cellular expression of membranar receptors Dectin-1 and Dectin-2, as well as the
level of soluble PTX-3 were not significantly modified in patients with IA. Further
studies are needed to assess the role of immunological parameters, especially cytokines
profile, in the panel of tests used for the diagnosis of IA in immunocompromised patients.
Symposium 9 Chronic Pulmonary Aspergillosis
Barac
A.
Clinic for Infectious and Tropical Diseases, Clinical Center of Serbia, Belgrade,
Serbia
Chronic pulmonary aspergillosis (CPA) is an uncommon, slowly destructive pulmonary
disease characterized by progressive cavitation, fibrosis, and pleural thickening.
CPA is usually seen in immunocompetent individuals with underlying respiratory disorders.
Estimates suggest that up to 3 million people are affected worldwide causing high
rates of morbidity and mortality. Diagnosis of CPA is often challenging and delayed
and should be based upon a combination of characteristics. Patients are present with
indolent, non-specific symptoms such as fatigue, weight loss, sweats, cough, dyspnea,
or hemoptysis. The disease often remains undiagnosed for years, resulting in progression.
A high index of suspicion is required in patients with predisposing factors such as
previous tuberculosis (TB) with residual cavities, severe chronic obstructive pulmonary
disease, previous treatment for lung cancer, or nontuberculous mycobacteria. The most
important diagnostic finding is changes on chest imaging, usually CT scan. The most
common presentation is one or more cavities, characteristically with an irregular
or thick wall, but occasionally thin-walled, with or without intracavitary material
or well-formed aspergilloma. Cavities tend to affect the upper lobes and often cause
para-cavitary or adjacent pleural fibrosis. In early stages, nodules may be present,
which may develop into cavitary disease over time. Differential diagnosis includes
mycobacterial infection, bacterial abscess, cancer, or vasculitis. Expertise of the
reporting radiologist is essential in highlighting the possibility of CPA. In addition
to compatible radiological findings, microbiological and/or serological evidence of
Aspergillus infection is required, in addition to ruling out alternative diagnoses.
Direct microscopy for hyphae, fungal culture of respiratory secretions and histology
are all recommended for diagnosis of CPA. Sensitivity of sputum or bronchoalveolar
lavage (BAL) culture remains moderate at best, even when specific fungal media are
used. Culturing a higher volume of sputum may lead to increased sensitivity. Sensitivity
is significantly higher with the high-volume culture, which in addition detected azole-resistant
isolates that could be missed with conventional culture. When microscopy and cultures
are negative, molecular markers like Aspergillus polymerase chain reaction (PCR),
as well as beta D-glucan, galactomannan (GM) testing in BAL samples can be used to
increase sensitivity. In addition, using these tests in combination may result in
better PPV. The usefulness of testing GM in sputum is not clear in a cohort of patients
with CPA and ABPA. Therefore, sputum GM cannot be recommended for the diagnosis of
CPA at present. Serological methods can contribute to the diagnosis of CPA. Aspergillus
precipitins were used previously but have been supplanted by more sensitive serologic
assays. Several assays detecting Aspergillus-specific immunoglobulin G (IgG) are commercially
available. In summary, clinical and radiological findings, along with microbiological
and serological tests are used in combination to diagnose CPA. There is no individual
reference-standard test as all tests are characterized by suboptimal sensitivity and
specificity. A high index of suspicion is needed, along with the need to rule out
alternative diagnoses like TB or lung cancer.
S09.2. Diagnosis of CPA. Where do we stand?
Lynch
F.
1
2
Moore
C.
2
3
Rautemaa-Richardson
R.
1
2
4
1Infectious Diseases, Manchester University NHS Foundation Trust, Manchester, United
Kingdom
2National Aspergillosis Centre, Manchester, United Kingdom
3Mycology Reference Centre Manchester, Manchester University NHS Foundation Trust,
Manchester, United Kingdom
4Division of Infection, Immunity and Respiratory Medicine, University of Manchester,
Manchester, United Kingdom
S09.5. Raised amphotericin B MIC in Aspergillus fumigatus isolates from patients with
Chronic Pulmonary Aspergillosis
Objectives: Chronic pulmonary aspergillosis (CPA) is a fungal infection of the lung
caused by Aspergillus species, which usually affects patients with an underlying respiratory
condition. The mainstay of treatment is prolonged antifungal therapy to improve symptoms
and prevent disease progression. Aspergillus fumigatus with elevated minimum inhibitory
concentrations (MIC) for amphotericin B (AMB) have been isolated. The aim of this
study is to investigate the emergence and clinical relevance of elevated AMB MICs
in A. fumigatus.
Methods: Patients with CPA reported with AMB resistant (MIC > 4 mg/L) A. fumigatus
were identified from the Mycology Reference Centre Manchester databases. All A. fumigatus
isolates reported from these patients were included in the analyses. The date of sampling
and resistance patterns of the isolates were analysed against the antifungal history
of each patient. EUCAST antifungal breakpoints were used to determine sensitivity.
Clinic letters and dispensing records were used to determine medical history, antifungal
therapy and clinical outcomes.
Results: Nine patients reported with one or more AMB resistant A. fumigatus isolates
and a total of 148 isolates from these patients were identified. of the isolates,
34 (22%) were resistant to AMB; 13 (38%) had an MIC of 4 mg/L, five (15%) had an MIC
of 8 mg/L and 16 (47%) had an MIC of >8 mg/L. In addition, 18 (12%) isolates were
reported as intermediate to AMB (MIC = 2 mg/L). Five of the nine patients had prior
exposure to AMB (IV AmBisome). Patients received 3-5 mg/kg once daily and different
treatment regimens were used; one patient had one 3-week course, two patients had
two 3-week courses and two patients received long term therapy three times weekly
(for up to 3 years). Four patients had no exposure to AMB. of the 5 patients exposed
to AMB, 4 developed resistance during therapy, and 1 patient first showed resistance
5 years after their 3-week course. All patients had prior azole exposure, and the
duration of azole exposure prior to AMB resistance was from 8 months to 5 years. All
AMB resistant isolates showed some level of azole resistance and 27 (82%) were pan-azole
resistant. Example patient timeline.
Conclusion: All patients reported with A. fumigatus with elevated MIC for AMB had
previously been treated with azole antifungals and developed resistance against one
or more azoles. Many AMB resistant isolates were pan-azole resistant suggesting a
link between AMB and azole resistance. Antifungal drug resistance can have significant
implications on patient care due to limited treatment options. A better understanding
of the development, genetic mechanism, and clinical relevance of AMB resistance is
needed. Therefore, all isolates identified in this study will next be sequenced and
typed.
Symposium 10 Symposium 10 Sensing the host
Papon
N.
1
Kabbara
S.
1
Hérivaux
A.
1
Bidon
B.
1
Kilani
J.
1
Govic
Y. Le
1
Gastebois
A.
1
Bernonville
T. Dugé De
2
Clastre
M.
2
Courdavault
V.
2
Gal
S. Le
1
Nevez
G.
1
Bouchara
J.-P.
1
1Host-Pathogen Interaction Study Group, SFR ICAT 4208, Univ Angers, Univ Brest, University
Hospital Center, Angers, France
2Ea2106 “biomolécules Et Biotechnologies Végétales”, Université de Tours, Tours, France
S10.2. Hybrid histidine kinases: major sensing proteins in pathogenic fungi
Protein phosphorylation is one of the most extensively used modifications in signal
transduction pathways in both prokaryotic and eukaryotic cells. Prominent families
of enzymes that perform these protein phosphorylations encompass serine/threonine
kinases, tyrosine kinases, and histidine kinases (HKs). While HKs dominate prokaryotic
signaling pathways, they are less prevalent in eukaryotes, and most importantly, absent
in animals. Historically, a small number of eukaryotic HKs have been studied in plants,
yeasts, filamentous fungi, and slime molds. Recent studies have expanded characterization
of HKs in other eukaryotic lineages and archaea, allowing a broader assessment of
the types of signaling systems mediated by HKs, their phylogenetic distribution and
evolution. HKs are central to regulatory systems that impact agriculture, the environment,
and both beneficial and pathogenic interactions of microbes with humans and other
animals. Their great diversity, versatility, and broad distribution make them attractive
targets for therapeutics, biotechnological interventions, and also as building blocks
for engineered biosensor systems. From a structural perspective, fungal HKs are commonly
composed of three main regions. The first region referred to as the “sensing domain”
corresponds to a highly variable N-terminal sequence that determines the stimulus
perceived. The central “transmitter region” is made of two conserved domains: a dimerization
histidine phosphotransfer (DHp) domain and a catalytic ATP-binding (CA) domain. The
DHp domain includes an H-box, usually containing the phosphorylatable histidine residue,
and an X-box. The CA subdomain includes four distinct sequence motifs: the N-, G1-,
F-, and G2-boxes. In contrast to prokaryotic HKs, most eukaryotic HKs harbor an additional
C-terminal receiver domain (REC) that includes a phosphorylatable aspartate residue.
Thus, eukaryotic HKs are generally called “hybrid HKs” (HHKs). From a mechanistic
point of view, HHKs constitute in fungal cells the initial sensing proteins of a four-step
phosphorelay signaling pathway involving phosphorylation events of two downstream
effectors, i.e. histidine phosphotransfer shuttle proteins (Hpts) and response regulators
(RRs) (Figure 1). The perception of a stimulus induces the trans-autophosphorylation
of a conserved His residue in the DHp domain by the CA catalytic part of the HHK.
The phosphate of this histidine is then transferred to the conserved Asp residue in
the REC domain prior to transfer to the Hpt domain, and finally to the conserved aspartate
residue of the protein belonging to the RR family. The phosphorylation state of this
RR orchestrates subsequent molecular events underlying the response to the input signal.
Here, we review the distinct genetic approaches carried out over the last fifteen
years and that allowed the elucidation of the function of several HKs in prominent
fungal pathogens. However, because their roles in regulating fundamental cellular
processes are still largely obscure in fungi, we will discuss the major challenges
that remain to improve our knowledge about the function of HKs in fungi and to design
specific inhibitors for future therapeutic development.
Chen
X.
1
Widiyanto
T.W.
1
Chibana
H.
2
Kajiwara
S.
1
1School of Life Science and Technology, Tokyo Institute of Technology, Yokohama, Japan
2Medical Mycology Research Center, Chiba University, Chiba, Japan
S10.5. The analysis on the genes related to the biofilm formation of Candida glabrata
Objectives: Candida glabrata is capable of developing biofilm on the surface of host
cells and medical materials. The biofilm formation is considered as one of pathogenic
factors on infection to the host, which shows increase in the resistance to antifungals
and host immune defense compared to the planktonic cells. Until now, the mechanism
of biofilm formation of C. glabrata has not been studied well compared with C. albicans.
Therefore, the aim of our study was to isolate and characterize some genes related
with the biofilm formation of C. glabrata. Methods: The in vitro model of biofilm
formation was constructed by using multi-well plates and the metabolic activity were
detected by XTT assay. The susceptibility of biofilm formation was also detected towards
different antifungals. Gene expression was analyzed by qPCR. Vacuolar morphology was
observed by staining with FM4-64 fluorescent dye. The growth fitness was detected
in buffered media with different pH and intracellular pH was measured with flow cytometry.
Results: Upon the result of a genetic comprehensive screening by using C. glabrata
mutant library, two gene mutants decreased metabolic activity of biofilms drastically
and the transcriptional expressions of these two genes in biofilm formation showed
more than two times higher than those of planktonic cell growth. One gene showed high
homology with Saccharomyces cerevisiae SYN8 gene, then was named as CgSYN8. S. cerevisiae
Syn8p was identified as a SNARE protein, acting in membrane fusion of vesicles. C.
glabrata syn8Δ mutant was defective in both the metabolic activity and the biomass
of the biofilm. Deletion of SYN8 also led to decrease in the adhesion ability during
the biofilm formation, which may link to the repression of two adhesin genes, EPA10
and EPA22. Additionally, C. glabrata syn8Δ mutant revealed the abnormal vacuolar morphology.
Therefore, it was suggested Syn8p is not only required for the normal vacuolar function
but also influence the biofilm formation of C. glabrata. Another gene related to the
biofilm formation of C. glabrata was named as CgQDR2. QDR (quinidine drug resistance)
family genes in S. cerevisiae belong to MFS (Major Facilitator Superfamily) transporters,
which mediate the ability of membrane transport to actively efflux the drug out of
the cell. C. glabrata qdr2Δ mutant exhibited significant reduction in the biofilm
formation and fluconazole drug susceptibility during biofilm formation. In addition,
our results suggested QDR2 deletion caused an impaired ability of C. glabrata to maintain
pH homeostasis, then might lead to the reduction of cell growth at neutral-basic pH
conditions. These results may explain how the QDR2 gene affect the biofilm susceptibility
and drug efflux towards antifungal drugs in C. glabrata. Conclusion: CgSYN8 and CgQDR2
were suggested to be involved in biofilm formation of C. glabrata. These findings
provide more understanding on the biofilm formation of this fungus and more information
for the development of clinical treatment in future.
Symposium 12 Environment & fungal outbreaks
Fisher
M.
Department of Infectious Disease Epidemiology, Imperial College London, London, United
Kingdom
Discovering that chytrid fungi cause the disease chytridiomycosis in amphibians represented
a paradigm shift in our understanding of how emerging infectious diseases contribute
to global patterns of biodiversity loss. In this lecture I describe how the use of
multidisciplinary biological approaches has been essential to pinpoint the origins
of pathogenic chytrids, to time their emergence and pathways of spread and to identify
the core mechanisms that underpin their pathogenicity. I will discuss the potential
for using bioinformatics toolkits and experimental methods to allow a fuller understanding
of these chytrids biology in order to leverage emerging technologies aimed at their
control. The impacts of infectious diseases in wild populations are not restricted
to wild animals and plants; they also threaten public health through the attrition
of ecosystem services as well acting as reservoirs and amplifiers of disease inocula.
I will argue that the lessons learnt from tracking amphibian chytrid fungi are also
of relevance to understanding and tackling emerging patterns of fungal disease in
humans. Paraphrasing Oscar Wilde, ‘to allow one fungal pandemic is misfortune, to
allow two is carelessness, to allow three…’.
S12.1. ‘Once is a misfortune, twice is carelessness…’: Lessons to be learnt from pandemic
fungal infections of amphibians
Hagen
F.
1
2
3
1Medical Mycology, Westerdijk Fungal Biodiversity Institute, Utrecht, Netherlands
2Medical Microbiology, University Medical Center Utrecht, Utrecht, Netherlands
3Department of Dermatology, Jining No. 1 People’s Hospital, Jining, China
The Cryptococcus gattii species complex placed itself in the spotlights of the medical
mycology community with the unprecedented and ongoing outbreak that emerged in the
temperate climate of Vancouver Island (British Columbia, Canada) during the late 1990s.
A few years thereafter the outbreak was found to have spread to the Canadian mainland
and the Pacific Northwest of the U.S.A. This pathogenic yeast, at that time named
Cryptococcus neoformans variety gattii, was mostly known as being a tropical or subtropical
pathogen that caused disease among apparently immunocompetent subjects. It was observed
that the outbreak in Canada and the U.S.A. was due to an until then rare genotype
named AFLP6/VGII, mostly known from Australia and the South American continents. Subsequent
molecular typing showed that the outbreak was caused by a major (AFLP6A/VGIIa) and
a minor (AFLP6B/VGIIb) genotype that had different virulence properties. In 2002 C.
neoformans variety gattii was raised to species level as Cryptococcus gattii. Using
several molecular techniques five lineages were recognized, nowadays known as molecular
types (genotypes) VGI (AFLP4), VGII (AFLP6), VGIII (AFLP5) and VGIV (AFLP7), the fifth
lineage is extremely rare and known as genotype AFLP10/VGIIIc. Although C. gattii
was just proposed as being a separate species, the debate was already ongoing whether
or not to further differentiate it into more species related to the molecular defined
lineages. A further refinement for a taxonomic revision was supported by the availability
of more molecular, phenotypic, antifungal sensitivity, geographic distribution and
predilection for certain hosts. In 2015 a proposal was made to raise the five lineages
to species level: C. gattii sensu stricto (AFLP4/VGI), C. bacillisporus (AFLP5/VGIII),
C. deuterogattii (AFLP6/VGII), C. tetragattii (AFLP7/VGIV) and C. decagattii (AFLP10/VGIIIc).
Due to the outbreak with C. deuterogattii in North America most of the focus was on
this species, but what do we know about the other members of the C. gattii species
complex in terms of environmental distribution and clinical relevance?
S12.2. The Cryptococcus gattii species complex
Desnos-Ollivier
M.
National Reference Center For Invasive Mycoses & Antifungals, Molecular Mycology Unit,
Cnrs Umr2000, Institut Pasteur, Paris, France
Rapid genotyping tools such as Multi Locus Sequence Typing (MLST) and Variable Number
Tandem Repeat (VNTR) are frequently used for common yeast species involved in invasive
human infection (Candida albicans, Candida glabrata, Candida tropicalis, Candida parapsilosis,
Pichia kudriavzevii, Cryptococcus neoformans/gattii complex). These methods are very
useful to determine potential link between clinical and/or environmental isolates
but implementation/validation requires a large number of unrelated isolates. Grouped-cases
of infection or colonization due to uncommon or emerging pathogenic yeast species
are usually considered to be due to a single strain. Since there is no genotyping
method for these uncommon species and considering that origin of the infection can
be endogenous and/or exogenous, this hypothesis should be verified. Whole genome sequencing
(WGS) has become relatively simple, fast and inexpensive but analysis remains time-consuming
and requires bioinformatic expertise. Since 2012 and the first French outbreak due
to Saprochaete clavata, we started using WGS to study relationship between isolates
involved in grouped cases and belonging to uncommon yeast species. Different approaches
involving different parts of the genome can be considered. For S. clavata, alignment
of whole genome allowed to identify different clades, one of which was responsible
for the national epidemic with specific Single Nucleotide Polymorphism positions (SNP).
Then, some SNPs were selected to design a real time PCR with allele-specific oligonucleotide
(ASO) which allowed us to rapidly detect isolate belonging to the epidemic clade.
Another approach was the use of WGS data of Yarrowia lipoytica to identify intergenic
regions highly polymorphic and usable as molecular tool. Finally, the alignment of
SNPs positions localized all over the genome was used to study genomic diversity of
Trichosporon asahii and Magnusiomyces capitatus population. Although the presence
of a rare species in several patients in the same hospital over a short period of
time suggest a common source of infection, this is not always the case especially
for species that are supposed to be part of the normal biota of skin and/or gastrointestinal
tract. Our data confirm the importance of data and isolate centralization in a Reference
Laboratory for molecular epidemiology. The routine WGS as a typing method for uncommon
yeasts requires prior validation with varied sampling together with bioinformatics
experts and mycologists to avoid misinterpretation.
S12.4. Whole genome sequencing: a valuable tool to study outbreaks due to uncommon
yeast species
Fekkar
A.
1
2
Bougle
A.
3
Normand
A.C.
1
Palous
M.
1
Piarroux
R.
1
Imbert
S.
1
1Laboratoire De Parasitologie-mycologie, APHP La Pitié Salpétrière, PARIS, France
2Sorbonne Université, Inserm, CNRS, Centre d’Immunologie et des Maladies Infectieuses,
Cimi-Paris, F-75013, PARIS, France
3Réanimation Chirurgie Cardiaque, La Pitie Salpetriere Hospital, Paris, France
S12.5. Outbreak of fluconazole-resistant Candida parapsilosis in a hospital ward:
arguments for clonal transmission and environmental persistence
Objectives: The emergence of multidrug-resistant fungal agent is a threat for human
health. In recent years, breakthroughs of infections due to fluconazole-resistant
Candida parapsilosis isolates have been increasingly reported in different countries.
We have recently faced with invasive infections due to resistant isolates and have
decided to further investigate this phenomenon.
Methods: We conducted a monocentric study at La Pitié-Salpêtrière Hospital, a tertiary
care center in Paris, France. All Candida parapsilosis isolates collected between
January 2012 and April 2019 for which sensitivity to antifungal agents had been determined
were analyzed. Antifungal susceptibility testing was performed using Etest (Biomerieux)
method and confirmed according the EUCAST broth microdilution reference method. Minimal
Inhibitory Concentrations were determined for azoles drugs (i.e. fluconazole, itraconazole,
voriconazole, posaconazole and isavuconazole). For fluconazole resistant isolates
and a random selection of susceptible isolates, the erg11 gene was sequenced and isolates
were genotyped by a microsatellite method based on 6 markers.
Results: 193 isolates (193 patients) sampled between 2012 and 2019 were analyzed.
We observed an increase of fluconazole resistance rate among Candida parapsilosis
isolates (MIC > = 8 µg/mL) between the periods 2012-2016 (3/97, 3.1%) and January
2017-April 2019 (10/96, 10.4%) (p<0.05 by Fisher’s Exact test): three fluconazole-resistant
isolates were detected in 2012-2013, none in 2014-2016 and 10 between January 2017
and April 2019. All the resistant isolates presented the previously described A395T
mutation in the erg11 gene that confers the amino acid change Y132F. This mutation
was absent in azole-sensitive isolates. Genotyping showed 4 different microsatellite
profiles for resistant isolates (R1, R2, R3 and R4) while susceptible isolates were
not related each other’s and showed a greater diversity (Figure 1).
Two resistant clusters (R1 and R3) were related to grouped cases in a single intensive
care unit (ICU). Isolates belonging to the R3 profile (n = 7) were detected since
November 2017, were responsible of 7 deep infections and presented the highest MICs
to fluconazole (> 64 mg/L) and a cross resistance with voriconazole (MIC > 0.5 mg/L).
Epidemiological and chronological analyses furnish clues for a patient-to-patient
transmission but also suggest an ability of resistant isolates to persist in the ward
environment. Indeed, two isolates of the same genotype R1 were detected 4 years apart
in the same ICU.
Conclusion: Our work shows an increase of the incidence of fluconazole-resistant Candida
parapsilosis isolates in intensive care units in relation to the clonal spread of
few strains. Our results provide evidence of i. patient-to-patient transmission and
ii. persistence of resistant clone over time in the same unit. Our study urges for
a better investigation of fungal resistance and improved eradication measures to limit
the spread of this resistant emerging pathogen.
Symposium 13 Fungal respiratory infections in cystic fibrosis (the ECMM/ISHAM working
Group Fri-CF)
Gal
S. Le
1
2
Nevez
G.
1
2
Favennec
L.
3
4
Bouchara
J.-P.
5
6
1Laboratory of Parasitology-Mycology, University Hospital Center, Brest, France, Brest,
France
2Host-pathogen Interaction Study Group Ea3142, University of Brest - University of
Angers, BREST, France
3Laboratoire De Parasitologie-mycologie, CHU de Rouen, Rouen, France
4Protozooses Transmises Par L’alimentation, University of Rouen - University of Reims,
Rouen, France
5Host-Pathogen Interaction Study Group, SFR ICAT 4208, Univ Angers, Univ Brest, Institute
of Biology in Health, IRIS, University Hospital Center, Angers, France, Angers Cedex,
France
6Laboratory of Parasitology-Mycology, University Hospital Center, Angers, France,
Angers, France
Cystic fibrosis (CF) is the most common genetic inherited disease in the European
Caucasian population. The disease is caused by mutations in the gene CFTR (Cystic
Fibrosis Transmembrane conductance Regulator) which encodes a membrane transporter
involved in electrolytic exchanges across the apical membrane of epithelial cells.
Several organs are therefore affected by these mutations, but the clinical picture
is dominated by respiratory infections. Indeed, because of the resulting thickening
of the bronchial mucus, the respiratory tract is often colonized by microorganisms,
leading to respiratory infections which are the major cause of morbidity and mortality
in patients with CF. Bacteria, mainly Staphylococcus aureus and Pseudomonas aeruginosa,
are the major cause of these infections. However, several yeasts and filamentous fungi
may also colonize the airways of patients with CF, sometimes leading to pulmonary
infections and to severe disseminated infections after lung transplantation. Moreover,
even in the absence of clinical signs, this fungal colonization could contribute to
the inflammatory reaction and thus to the progressive deterioration of the lung function.
If the epidemiology and pathophysiology of fungal infections still remain largely
unknown, the frequency of these infections increases together with life expectancy
of CF patients. Aspergillus fumigatus ranks first among the filamentous fungi colonizing
the airways of patients with CF, followed by Scedosporium species, Exophiala dermatitidis,
Lomentospora prolificans (formerly Scedosporium prolificans), and Aspergillus terreus.
The prevalence of these fungi varies from one study to another because of: i) differences
in patient recruitment (age, CFTR genotype); ii) lack of standardization of the procedures
used for mycological examination of respiratory secretions; iii) geographical and
climatic variations. In addition, other fungal species have been increasingly reported
during the past decade, such as the yeasts Candida blankii, Trichomonascus ciferrii
and Blastobotrys adeninivorans / Blastobotrys raffinosifermentans. These yeasts share
the capability to use diamines, such as putrescine, which may explain that they multiply
in the bronchial mucus during pulmonary exacerbations since the concentration of putrescine
increases in this context. Likewise, some filamentous fungi including Arthrographis
kalrae and species of the Rasamsonia argillacea complex (formerly Geosmithia argillacea,
but first reported in CF as Penicillium emersonii) seem to be emerging in CF. Although
it is usually well tolerated, the chronic colonization of the airways by these fungi
should not be disregarded since it constitutes a risk factor for disseminated infections
in the case of lung transplantation. The emergence of these fungi could be explained
at least in part by improvements in diagnostic methods, or by selection pressure caused
by repeated antifungal cures. Our knowledge of the ecology of these filamentous fungi
still remains in infancy; nevertheless, regarding species of the Rasamsonia argillacea
complex, some observations suggest that they could be xylophagous fungi that have
spread in Europe since the end of the last century as a consequence of global warming
and of the many storms that have crossed Europe since the end of the nineties.
S13.1. Rasamsonia species and other emerging fungi in CF
Ramirez-Garcia
A.
1
Martin-Souto
L.
1
Aparicio-Fernandez
L.
1
Buldain
I.
1
Antoran
A.
1
Bouchara
J.-P.
2
Martin-Gomez
M.T.
3
Rementeria
A.
1
Hernando
F.L.
1
1Immunology, Microbiology and Parasitology, University of the Basque Country (UPV/EHU),
Leioa, Spain
2Laboratory of Parasitology-Mycology, University Hospital Center, Angers, France,
Angers, France
3Microbiology, Vall d’Hebron Universitary Hospital, Barcelona, Spain
The detection and diagnosis of Scedosporium/Lomentospora still relays on traditional
methods and, in spite of being the second most common etiological agent among filamentous
fungal infections, very few advances have been achieved in this field during the last
decades. This fact is especially worrying in Cystic Fibrosis (CF) patients, whose
characteristics favor the microbial presence. In these patients, the fungal detection
does not mean that an infection is occurring, but it may increase the risk of suffering
from it or other health problems. Therefore, it would be interesting to dispose an
easy, rapid and non-invasive detection method for monitoring these patients and study
the evolution of the fungus. With this purpose, some groups are focusing their efforts
on finding new approaches to design a serologic system. In fact, techniques such as
counterimmunoelectrophoresis or ELISA using recombinant proteins of some well-described
virulence factors are being used by some laboratories, which design their own assays.
Our research group addressed this problem using different immunoproteomics-based approaches.
First, four different Scedosporium boydii protein extracts were studied by ELISA to
select the most useful one for IgG detection in CF patients. The extracts used were
a whole-cell extract, fungal cell-surface extract, conidia cell surface extract and
secreted extract, all of them being able to discriminate acceptably the Scedosporium/Lomentospora-infected
patients from Aspergillus-infected and non-infected patients. However, the best results
were obtained with the whole-cell extract, which showed specificity, sensitivity,
predicted positive and negative values comparable with commercial kits for other fungal
diagnosis purposes. In spite of the excellent results obtained, to increase the specificity
and avoid the problems derived from the use of a complex extract, the immunoreactivity
of a collection of sera from CF patients infected with Scedosporium/Lomentopora against
S. boydii was also studied in comparing with sera from CF patients infected with Aspergillus
or without fungus. The analysis, made by 2-DE and WB, allowed the identification of
antigens specifically detected by CF patients with Scedosporium/Lomentopora, two of
them being identified because of their promising results. In addition, to shed light
on the problematic of whether the fungal presence in CF patients is a colonization
or an infection, we used sera from mice infected intravenously with different species
of Scedosporium/Lomentospora to detect infection-related antigens of S. boydii. Interestingly,
all the sera showed a very similar immunorecognition patterns, but different from
the recognized by sera from Aspergillus-infected mice. Therefore, we purified six
antigens and tested their interest for diagnosis analyzing their immunoreactivity
by WB against sera from CF patients, two of them showing interesting results. Altogether,
these results obtained by immunoproteomics-based techniques open up door in the looking
for a serologic test for the detection of Scedosporium/Lomentospora in CF patients,
as well as the monitoring of the fungal presence. Specifically, although the results
have to be further studied and standardized, the ELISA test using whole-cell extract
appears to be a very useful, and the antigens identified seems to be interesting candidates
to be include in the diagnostic procedure.
S13.2. Immunodiagnosis of Scedosporium/Lomentospora infection - lessons from immunoproteomic
studies
Kirchhoff
L.
1
Rath
P.-M.
1
Steinmann
J.
1
2
1Institute of Medical Microbiology, University Hospital Essen, Essen, Germany
2Institute of Clinical Hygiene, Medical Microbiology and Clinical Infectiology, Klinikum
Nuernberg, Nuremberg, Germany
S13.4. Morphology, growth and biofilm formation of the black yeast-like fungus Exophiala
dermatitidis is influenced by Pseudomonas aeruginosa under in vitro cystic fibrosis
conditions.
Objectives:
Exophiala dermatitidis, belonging to the melanized fungi, is frequently colonizing
the respiratory tract of cystic fibrosis (CF) patients in rates up to 19 %. It was
recently reported that E. dermatitidis is capable to form biofilms in a strain-dependent
manner. However, little is known about E. dermatitidis behavior in co-culture with
other CF-relevant pathogens, e.g. one of the most prevalent bacterial species, Pseudomonas
aeruginosa. Growth, biofilm formation capabilities and morphology of E. dermatitidis
in an artificial sputum medium (ASM), mimicking the CF sputum conditions, were assessed
in mono- and in co-culture with P. aeruginosa.
Methods:
P. aeruginosa (PA07 & PA14, including PA14 ΔlasR and ΔrhlR) and E. dermatitidis (clinical
isolate) were analyzed in growth experiments over a period of 48 hours at 36°. Growth
was determined by colony forming unit (CFU) counts. Biofilm, formed on polystyrene
surfaces under standard protocols, was determined after 24 and 48 hours of incubation
at 36°C without agitation by stain with crystal violet, by CFU counts after biofilm
detachment using 0.1% dithiothreitol, for species-specific cell counts and by confocal
laser scan microscopy determining the thickness of extracellular matrix (ECM). Morphology
of the dimorphic E. dermatitidis was monitored in light microscopy. Lengths of fungal
hyphae were estimated and compared.
Results:
P. aeruginosa showed growth inhibiting effects on E. dermatitidis. Cell count of the
fungus was decreasing in co-culture with P. aeruginosa. In contrast, E. dermatitidis
biofilm formation was induced after 24 h and reduced after 48 h in co-culture with
P. aeruginosa. A lack of P. aeruginosa quorum sensing (QS) systems lasR and rhlR resulted
in induced fungal biofilm formation. At the same time, presence of lasR and rhlR lacking
mutants caused an increased amount of E. dermatitidis hyphal structures whereas co-cultivation
with the P. aeruginosa wildtype resulted in decreased hyphae amounts, compared to
mono-culture.
Conclusion: Interactions between P. aeruginosa and E. dermatitidis result in altered
growth, biofilm formation capabilities and morphology of the fungus in an in vitro
CF model. A role of P. aeruginosa QS systems in these interactions is suggested.
Martin-Souto
L.
1
Areitio
M.
1
Aparicio-Fernandez
L.
1
Buldain
I.
1
Antoran
A.
1
Bouchara
J.-P.
2
Martin-Gomez
M.T.
3
Rementeria
A.
4
Hernando
F.L.
4
Ramirez-Garcia
A.
4
1Immunology, Microbiology and Parasitology, University of the Basque Country (UPV/EHU),
Leioa, Spain
2CHU d’Angers, Angers, France
3Microbiology, Hospital Universitari Vall d’Hebron, Barcelona, Spain
4Immunology, Microbiology and Parasitology, University of the Basque Country (UPV/EHU),
Leioa, Spain
S13.6. Study of antigenic markers for serological detection of Scedosporium spp. in
Cystic Fibrosis patients
Objectives: Cystic fibrosis (CF) is the major genetic disorder among the Caucasian
population. CF patients produce a thick and sticky bronchial mucus in their respiratory
tract, which facilitates the growth of airborne pathogens. In addition to bacteria,
several fungi colonize the respiratory tracts of these patients. Species of Scedosporium
and Lomentospora genera are emergent fungal pathogens ranking the second, only behind
Aspergillus spp., among filamentous fungi causing a chronic colonization of the airways
of CF patients. This may lead to chronic inflammation or even to life-threatening
invasive disease in cases of severe immunosuppression. Detection of Scedosporium/Lomentospora
relies upon low sensitivity and specificity non-standardized procedures. To contribute
to the finding of new diagnostic methods, this work aims to characterize some molecular
targets and to study their usefulness for serological detection of infections caused
by Scedosporium spp. in CF patients.
Methods: A total of 191 CF patients’ sera were used in this study. First, we classified
these sera into 3 groups: patients with positive sputum cultures for Scedosporium
(S+), for Aspergillus (A+), and patients with negative fungal cultures (Ctrl). A serological
study of the sera was carried out to detect specific IgG response against Scedosporium
boydii total protein extract by ELISA. In the search of diagnostic markers that allow
the discrimination between colonization and infection, we performed an in vivo infection
in a murine model to obtain sera that allow the detection of antigens associated with
a disseminated infection by two dimensional western blot. These antigens were electroeluted
from protein gels and blotted individually against CF patients’ sera to observe the
immunological response.
Results: The ELISA assay provided good specificity, and positive and negative predictive
values to discriminate among S+, A+ and Ctrl patients. However, the sensitivity was
influenced by differences in the humoral response of the S+ group. This suggests different
levels of fungal presence, from colonization to infection, among S+ patients. To solve
this problem, we looked for S. boydii antigens associated with a disseminated infection
in a murine model selecting the six proteins with the highest immunoreactivity. Two
of them were specifically recognized by most of S+ sera, especially by those that
were more reactive in the ELISA assay.
Conclusion: Two proteins of S. boydii resulted to be potential diagnostic markers
for the detection of the infection caused by Scedosporium spp. in CF patients. The
use of them in combination with an ELISA using S. boydii total protein extract might
help in the detection of Scedosporium spp. in CF patients and the monitoring of the
fungal presence.
Symposium 14 Teaching Medical Mycology: what, how, to whom and when
Segal
E.
Sackler School of Medicine, Clinical Microbiology and Immunology, Tel Aviv University,
Tel Aviv, Israel
Mycology which is the discipline devoted to the study of fungi, is one of the four
parts comprising Microbiology, the basis for understanding infectious diseases. Medical
Mycology is the part of Mycology which focuses on those fungi known to cause or potentially
can cause infections in human hosts. As such, study of Medical Mycology is an integral
part of the education of physicians and is included in the curriculum of Medical Schools.
One of the questions associated with the teaching of Medical Mycology is at which
level should Medical Mycology be taught: at the basic sciences level, with other parts
of Microbiology, or at a later stage in the frame of Infectious Diseases, as part
of Internal Medicine. The answer to this dilemma may vary among different Medical
Schools. Another question associated with teaching Medical Mycology in Medical Schools
is as to the content of the subject: should it reflect the specific geographic–climatic
conditions of the given Medical School or should a more general, broader approach
be used. An additional dilemma regarding teaching of Medical Mycology in Medical Schools
is whether the specific student demography should affect what is taught and to what
extent. The latter is the subject of the following presentation. In the presentation
different teaching possibilities will be demonstrated and discussed, as to pro- or
contra- of the impact of student demography on what is taught. Examples of different
approaches to the dilemma will be shown and discussed. Participation of the audience
in the discussion will be encouraged.
S14.2. Mycology teaching in Medical School—Is student demography important to what
should be taught
Ashbee
R.
University of Leeds, Leeds, United Kingdom
S14.3. Specific courses outside European Continent – The Indian Example
HR Ashbee, RD Sahni & JS Michael Teaching medical mycology in the Indian setting presents
some unique challenges and opportunities. The diversity of mycoses seen is extensive
and presentations can be unusual with disease sometimes occurring in patients not
traditionally ‘at risk’. Whilst dermatophytosis and superficial candidosis is often
neither diagnosed nor treated, fungal keratitis and sinusitis, subcutaneous and systemic
mycoses represent a significant burden on the healthcare system. In combination with
this, diagnostic facilities vary from virtually nil to fully equipped laboratories
with well-trained staff. The Christian Medical College Hospital in Tamil Nadu, south
India has had a mycology laboratory since the 1950’s, has highly skilled staff and
a massive number of clinical samples – hence it is ideally suited to deliver comprehensive
mycology training. The first course in 2007 was aimed at technical staff working in
laboratories and junior faculty, primarily focusing on basic medical mycology and
identification of fungal pathogens. The course was attended by 30 participants from
all over India, some traveling for 30 hours by train to take part in the course. The
need for training was apparent and further courses were planned The current course
has been re-designed and is run in two parts. The first 3-day part is aimed at registrars/residents
in microbiology and provides an integrated approach to teaching with clinicians presenting
cases and the laboratory aspects taught in practical, hands-on sessions after the
cases. The full range of diseases are covered, including the superficial mycoses,
subcutaneous mycoses, candidosis, cryptococcosis, mucormycosis, aspergillosis and
endemic mycoses. Hands-on practical sessions also cover setting up of antifungal susceptibility
tests and their interpretation. The second part of the course held over 2 ½ days is
for consultant microbiologists and this includes a full day of practical molecular
mycology, including DNA extraction, setting up PCR and trouble-shooting molecular
assays, use of commercially available assays, as well as molecular detection of antifungal
resistance. Antifungal susceptibility testing is taught in another full day practical
session with participants taken through the whole CLSI method, from making up the
medium, preparing antifungal dilutions, making up plates and preparing the inoculum,
to reading and interpreting results. Other methods such as E-test and disc diffusion
are also taught. The practicals take place in small groups so every participant can
get hands-on experience of each technique. Discussion sessions make up the remainder
of the time with ample opportunity for participants to ask questions and clarify any
problems. Participants are also encouraged to maintain contact after the workshop
and help is provided with testing or fungal identification by CMC staff if required.
The courses represent a significant investment of time and resources by the staff
of CMC and overseas faculty but feedback has been extremely good and demonstrates
that provision of high quality, comprehensive mycology training is both very much
needed but also very well received.
Arikan-Akdagli
S.
Medical Microbiology, Mycology Laboratory, Hacettepe University Medical School, ANKARA,
Turkey
S14.4. Teaching Clinical Laboratory Mycology: How and who is the audience?
Expertise in Clinical Laboratory Mycology requires hands-on experience and in-depth
basic knowledge. Challenges regarding teaching Clinical Laboratory Mycology are numerous.
First, the number of invasive fungal infections is increasing with more infections
due to rare and less-recognized species and the education for management of this growing
problem is yet inadequate. Second, Mycology education was rather less emphasized until
recent years. Teaching for Clinical Laboratory Mycology practices is usually limited
during training of a Clinical Microbiologist and the numbers of well-equipped Clinical
Mycology Laboratories and experts are also relatively limited worldwide. Third, the
characteristics of Y (and Z) generation(s) are unique. The members of generation Y
are primarily among the current audience and they are largely influenced by technology.
Therefore, broader training and diverse teaching methods are required to meet all
these challenges. Clinicial Laboratory Mycology training is a part of speciality/subspeciality,
PhD or Master of Science training programs in Medical Microbiology/Mycology. Also,
Infectious Diseases/Infectious Diseases and Clinical Microbiology Joint Training,
and Pathology Training Programs include training in Mycology at required levels. For
Medical Faculty students, on the other hand, basic Clinical Laboratory Mycology teaching
is incorporated in the program at some institutions in order to integrate the previously
taught theoretical knowledge with that on mycological diagnosis in the last year(s)
of education. Laboratory technicians are also among the audience for teaching Clinical
Laboratory Mycology at required and specifically technical level. Combining conventional
teaching methods with digital technologies is needed for contemporary high quality
Clinical Laboratory Mycology education. The common approach for teaching requires
expert(s), a well-equipped Mycology Laboratory, and reliable basic information sources,
including physical books or e-books. In lack of these facilities or for advancement
for higher levels, in-person courses and workshops are very beneficial. However, they
are costly and require qualified instructors and resources. E-learning courses, webinars,
and educational websites are also very helpful in expanding the trained audience.
Teaching Clinical Laboratory Mycology is a difficult task and requires dedicated Mycologists.
Leclère
A. Sturny
1
Garcia-Hermoso
D.
2
Boyer-Chammard
T.
1
Kolte
I.
3
Dromer
F.
4
Alanio
A.
5
Lortholary
O.
6
Loyse
A.
3
1Molecular Mycology, IP Paris, Paris, France
2National Reference Center For Invasive Mycoses & Antifungals, Molecular Mycology
Unit, Cnrs Umr2000, Institut Pasteur, PARIS, France
3Institute of Infection and Immunity, St. George’s University of London, London, United
Kingdom
4National Reference Center For Invasive Mycoses & Antifungals, Molecular Mycology
Unit, Cnrs Umr2000, Institut Pasteur, Paris, France
5Paris-diderot, Sorbonne Paris Cité University, Institut Pasteur, Molecular Mycology
Unit, Cnrs Cmr2000, Parasitology-Mycology Laboratory, Lariboisière Saint-Louis Fernand
Widal Hospitals, Assistance Publique-Hôpitaux de Paris, Paris, France
6Infectious Diseases, APHP, Hopital Necker-Enfants malades, Paris, France
S14.5. Capacity building for invasive fungal infections in Africa: concrete examples
through mycology courses and the DREAMM implementation project
Across the world, fungal pathogens are omnipresent in the environment, affect over
a billion and kill more than 1.5 million people. Despite these figures, invasive fungal
infections (IFIs) do not gather as much press coverage as tuberculosis or malaria.
AIDS represents one of the major risk factors for IFIs together with other acquired
immunodeficiencies. Invasive fungal infections should be a concern everywhere by their
incidence, severity and high burden. Worldwide IFIs are not recognized due to lack
of surveillance systems together with a lack of mandatory reporting, limited teaching
in medical schools and therefore limited awareness and expertise of clinicians and
biologists, lack of reliable diagnostic procedures for many IFIs, and limited number
of commercialized antifungal drugs. In resource limited countries, the lack of basic
laboratory equipment and poor access to essential medicines are additional major barriers.
Outcomes of cryptococcal meningitis, a leading cause of HIV-related death, are still
poor despite updated WHO guidelines. The DREAMM project aims to reduce the mortality
of cryptoccocal meningitis in resource limited settingsby providing rapid diagnostic
tests, essential medicines and reinforcing basic and mycology trainings for all staff
members involved (technicians, nurses, physicians, biologists). Through this program,
a better awareness of all IFIs is expected and results are already visible.
Symposium 15 Aspergillus in the ICU
Wauters
J.
Medical Intensive Care Unit, University Hospitals Leuven, Leuven, Belgium
S15.1. Influenza-associated aspergillosis in ICU: when the flu get mouldy
Worldwide, 3-5 million people develop severe influenza every year, leading to 50.000-100.000
deaths annually in the European Union and the USA. of the patients hospitalized, 5-10%
needs ICU admission due to severe illness. Patients with severe illness initially
present with typical influenza symptoms, but are rapidly evolving into respiratory
deterioration due to bacterial superinfection but also influenza in itself can cause
severe acute respiratory distress syndrome (ARDS), the latter being associated with
a mortality of 14% to 41%. Although bacterial co-infection is already known for decades,
influenza-associated aspergillosis (IAA) was recently found to be a frequent and severe
complication of influenza pneumonia in critically ill patients. Classically, invasive
pulmonary aspergillosis (IPA) typically occurs in a severely immunocompromised host.
Influenza-associated aspergillosis (IAA) was occasionally described decades ago and
several small case series were reported recently. 65% of the reported cases did not
have classic host factors for IPA as defined by the EORTC/MSG. Recently, we published
a large multicentre case-control study within the Dutch-Belgian Mycosis Study Group
(7 Belgian and Dutch ICUs, 2009-2016) including 432 severe influenza patients (positive
influenza polymerase chain reaction (PCR)), 315 of them being EORTC negative. As a
control group, 315 influenza negative and EORTC negative patients, admitted to the
ICU with severe community-acquired pneumonia (CAP), were selected. In the 432 severe
influenza patients, IPA was diagnosed in 19% (83/432), a median of 3 (IQR 0-7) days
after ICU admission. The IPA incidence in the 117 immunocompromised (EORTC positive)
influenza patients was as high as 32% (38/117), while 14% (45/315) of the non-immunocompromised
(EORTC negative) influenza patients developed IPA. In contrast, only 5% (16/315) of
the non-immunocompromised influenza-negative controls developed IPA (p<0·0001). The
90-day mortality in influenza patients with and without IPA was 51% and 28%, respectively
(p<0·0001). Moreover, in this retrospective cohort study, influenza was found to be
independently associated with IPA (aOR 5·2, 95% CI 2·6-10·3, p<0·0001), besides a
higher APACHE II score, male sex and use of corticosteroids. Though awareness of IAA
is rising, it remains unclear why patients with severe influenza infection develop
IPA. Influenza-induced necrosis of the bronchial tree might provide a gateway for
Aspergillus infection. Further, severe influenza might alter innate and adaptive host
facilitating fungal infection and genetic variation might also influence the susceptibility
to IPA. Finally, corticosteroid (CS) therapy is a known independent risk factor for
the development of IPA at the ICU. In summary, IAA is an early and frequent complication
of influenza pneumonia and increases the probability of death with a factor 2 to 3
in critically ill patients with severe influenza. An aggressive diagnostic approach
should be pursued, but early diagnosis is difficult due to unspecific clinical and
radiological presentation. Future studies should evaluate whether a faster diagnosis
and/or antifungal prophylaxis could improve outcome of influenza-associated aspergillosis.
Reference: Schauwvlieghe AFAD, Rijnders BJA, Philips N et al. Dutch-Belgian Mycosis
study group. Invasive aspergillosis in patients admitted to the intensive care unit
with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6(10):782-792.
Prattes
J.
Section of Infectious Diseases and Tropical Medicine, Medical University of Graz,
Graz, Austria
Invasive aspergillosis (IA) has increasingly reported in critical ill patients without
classical risk factors like hematological malignancy, neutropenia or solid organ transplantation.
Liver cirrhosis represents the main underlying disease in some of these patients,
highlighting the relevance of cirrhosis as a risk factor for IA development. Whereas
the overall incidence of IA in patients with liver cirrhosis is very low (<1%), the
risk for IA increases when liver function deteriorates and patients are in need for
intensive care. Reasons for susceptibility of cirrhotic patients for developing IA
are diverse. Cirrhosis-associated immune dysfunction summarizes both, systemic inflammation
due to cirrhosis and cirrhosis associated immunodeficiency. The latter one may be
due to systemic glucocorticoid treatment but also due to alterations of the innate
as well as adaptive immune functions. Impaired expression of Fcγ –receptor on neutrophils,
impaired neutrophil mobilization, phagocytic activity, chemotaxis, bacterial phagocytosis
and killing are present in cirrhotic patients. However, the degree of immune dysfunction
correlates with the stage of the liver disease putting patients with end stage liver
disease at highest risk for IA development. Awareness for IA in cirrhotic patients
has to be warranted as diagnosis may be challenging. Blood biomarkers, like galactomannan
and beta-d-glucan, lack sensitivity and clinical presentation may be atypical. In
case of IA suspicion early chest CT scan and bronchoscopy may be needed.
S15.2. Invasive aspergillosis in patients with underlying liver cirrhosis
Rautemaa-Richardson
R.
1
2
3
4
1Division of Infection, Immunity and Respiratory Medicine, University of Manchester,
Manchester, United Kingdom
2Mycology Reference Centre, Excellence Center For Medical Mycology (ecmm), Wythenshawe
Hospital, Manchester University NHS Foundation Trust, Manchester, United Kingdom
3National Aspergillosis Centre, Manchester, United Kingdom
4Department of Infectious Diseases, Manchester University NHS Foundation Trust, Manchester,
United Kingdom
Renal failure is a common complication in critically ill patients. In addition, angioinvasive
aspergillosis is associated with renal lesions and renal failure. The challenge is
that the main intravenous drugs used to manage invasive aspergillosis (IA), namely
amphotericin B and voriconazole (with cyclodextrin) are nephrotoxic. Also, in critically
ill patients with multi organ failure, the pharmacodynamics and pharmacokinetics are
highly variable, which results in a significant risk for toxicity as well as suboptimal
dosing and treatment failure. The impact of renal failure on the management of IA
depends on whether the patient is on renal replacement therapy (RRT) or not. Once
on RRT, the main consideration is the risk for suboptimal drug levels until the system
is saturated with the drug. However, it is important to keep in mind that the initiation
of RRT does not remove the risk for further or permanent drug-induced kidney injury.
Once the patient starts to respond to the treatment, their haemodynamics stabilise
and their kidney function improves, there is a risk for both toxicity and subtherapeutic
levels. Therefore, in the case of azoles, critically ill patients on intensive care
unit (ICU) require regular therapeutic drug monitoring (TDM) and repeated dose adjustments.
This also applies to azoles not associated with kidney toxicity (isavuconazole). Whilst
TDM is an important part of monitoring the safety and efficacy of antifungal treatment,
microbiological diagnostics are another important tool in patient management. Sputum
high-volume culture, PCR and galactomannan measurements can be used to monitor treatment
response whereas blood galactomannan is less useful in non-neutropenic setting. However,
it is not rare that these tests remain positive for prolonged periods despite good
clinical response.
S15.3. Aspergillus in the ICU – Management of IA in Renal Failure
Kale
P.
1
Khillan
V.
1
Sarin
S.K.
2
1Clinical Microbiology, Institute of Liver and Biliary Sciences, New Delhi, India
2Hepatology, Institute of Liver and Biliary Sciences, New Delhi, India
S15.4. Fungal pneumonia in critically ill cirrhotics: Spectrum, Outcomes, Comparison
of diagnostic methods and Biomarkers
Objectives: Liver cirrhosis causes immune dysregulation and increased susceptibility
to fungal infections. Futher there are associated co-morbidities like diabetes mellitus,
renall impairment and malignancy which predispose to fungal infections. Hence early
diagnosis of fungal infections and initiation of appropriate empiric or pre-emptive
antifungal is essential to reduce morbidity and mortality associated with it. We studied
the epidemiology, spectrum, risk factors of fungal infections and antifungal susceptibility
pattern for guiding empiric therapy. We also compared the rapid diagnostic methods
and biomarkers for early diagnosis fungal pneumonia in critically ill cirrhotics.
Methods: Single-center, prospective cohort study of 100 critically ill cirrhotics
with fungal pneumonia between January to September 2018. All patients met the diagnostic
criteria for liver cirrhosis by clinical, biochemical, and ultrasonography findings.
Respiratory samples were processed for fungal culture and real time PCR. Antifungal
susceptibility testing was done by Broth microdilution method in accordance with CLSI
guidelines. Biomarkers; bronchoalveolar lavage (BAL) and serum Galactomannan, and
serum procalcitonin measured on day 1, 3 7. Mortality within one month of diagnosis
or discharge was analyzed.
Results:
Aspergillus flavus was the most common species (70/100,70%). Risk factors for fungal
pneumonia included neutropenia (p 0.03), steroids prior to ICU admission (p 0.02),
prolonged (>21 days) hospitalization (p<0.05). Culture positivity was 80%. Culture
was not inferior to real time PCR for diagnosis of fungal pneumonia. Antifungal susceptibility
testing revealed, all A. flavus and A. fumigatus isolates sensitive to azoles, amphotericin
B and echinocandins. BAL galactomannan was early prognostic marker with median rise
>3.5 over the index value. Median PCT level was higher from day 1in the fungal pneumonia
non-survivor than survivor group (3.29 vs. 0.8ng/ml), with higher 30-day mortality
(72%). Baseline PCT at admission to ICU was higher in non- survivors; levels on D3
and D7 were persistently higher. Higher PCT level was associated with bacterial co-infection
(48%), antibiotic (74%) and antifungal therapy and renal failure and mortality.
Conclusion: Cirrhotics who are neutropenic, have prolonged hospitalization and exposed
to steroids have a high risk of fungal pneumonia. Aspergillus flavus predominate as
in consensus with Asian epidemiology. Culture methods are reliable and combination
of molecular test with BAL galactomannan is useful for rapid diagnosis. However the
cutoff should be defined with epidemiological correlation. High serum procalcitonin
level is an independent prognostic biomarker of mortality risk in fungal pneumonia
which reaches nearly 70%. In our study the baseline PCT at admission to ICU was higher
in non- survivor group, levels on D3 and D7 were persistantly higher. High index of
suspicion and early detection is required in advanced cirrhosis
Holtappels
M.
1
Jacobs
C.
1
Chiller
T.
2
Fortenberry
J.
3
Jackson
B.
4
Lagrou
K.
5
Toda
M.
4
Veerdonk
F. Van De
6
Verweij
P.E.
7
Wauters
J.
8
1Laboratory For Clinical Infectious and Inflammatory Disorders, KU Leuven, Leuven,
Belgium
2Mycotic Diseases Branch, Centre for Disease Control, Altanta, United States of America
3Pediatric Critical Care, Children’s Healthcare of Atlanta, Atlanta, United States
of America
4Mycotic Diseases Branch, Centre for Diseases Control, Altanta, United States of America
5Department of Microbiology, Immunology and Transplantation, KU Leuven, Leuven, Belgium
6Infectieziekten, Afdeling Interne Geneeskunde, Nijmegen, Netherlands
7Medical Microbiology, RadboudUMC, Nijmegen, Netherlands
8Medical Icu, UZLeuven, Leuven, Belgium
S15.5. IPAFLU survey: Invasive Aspergillosis among Patients with Severe Influenza
in Intensive Care Units
Objectives: Invasive aspergillosis usually occurs in people with weakened immune systems.
However, several reports describe fatal Aspergillus pulmonary infections in previously
healthy patients who were hospitalized with a severe influenza virus infection. Recently,
a retrospective cohort study, conducted at seven intensive care units (ICUs) in Belgium
and the Netherlands during 2009–2016, found that invasive pulmonary aspergillosis
occurred in 19% of patients with severe influenza requiring admission to the ICU.
We conducted an international survey to determine broader knowledge and awareness
of influenza-associated aspergillosis (IAA), as well as the diagnostic use of galactomannan
(GM) in bronchoalveolar lavage (BAL) and serum in critically ill patients with severe
influenza.
Methods: Members of the Extracorporeal Life Support Organization (ELSO), the Society
of Critical Care Medicine (SCCM), and the European society of intensive medicine (ESICM)
network (n = 20,093) were invited to participate in an online 11-question survey.
Participants were asked about their roles in the ICU, ICU size, number of patients
with influenza admitted to the ICU per influenza season, use of neuraminidase inhibitors
(NAIs), frequency of obtaining lower respiratory specimens and GM testing on serum
and BAL samples, and their knowledge on the occurrence of IAA.
Results: A total of 468 (2.3%) members responded, with 225 (48%) residing in the United
States, 151 (32%) in Europe, and 92 (20%) in other countries, including Australia
and countries in South America, Asia and Africa. Most (89%) participants were critical
care physicians. Large ICU departments were more common in the United States compared
with Europe. The majority of participants reported <30 influenza cases per season,
and NAIs were the preferred antiviral treatment (92%). European participants reported
obtaining lower respiratory specimens and determining GM on BAL and serum more commonly
than U.S. participants (p < 0.001) (Figure 1). Globally, 66% (n = 303) of respondents
denied hearing of or seeing IAA cases in the past five years (Figure 2). However,
57% of European participants reported seeing ≥1 patient with IAA compared with only
17% of U.S. participants (p < 0.001) and 36% of participants in other countries (p
= 0.007).
Conclusion: More participants in Europe reported having seen IAA cases in compared
with the United States and other countries. Although the observed differences in IAA
cases could be explained by true variation in IAA prevalence, the condition might
be underdiagnosed outside Europe. The results suggest that such underdiagnosis might
partly explain the differences in IAA awareness in Europe compared with the United
States and other countries.
Choi
Y.H.
1
Kim
E.J.
1
Kwak
Y.G.
2
Yoo
H.M.
3
Choi
J.Y.
4
Shin
M.J.
5
Kim
S.R.
6
Yoo
S.-Y.
7
Cho
N.-H.
8
1Department of Infectious Diseases, Ajou University School of Medicine, Suwon-si,
Gyeonggi-do, Republic of Korea
2Department of Internal Medicine, Inje University Ilsan Paik Hospital, Goyang, Republic
of Korea
3Infection Control Office, Inje University Sanggye Paik Hospital, Seoul, Republic
of Korea
4Infection Control Unit, Chung-Ang University Healthcare System, Seoul, Republic of
Korea
5Infection Control Office, Seoul National University Bundang Hospital, Seongnam, Republic
of Korea
6Infection Control Office, Korea University Guro Hospital, Seoul, Republic of Korea
7Adjunct Assistant Professor, College of Nursing, The Catholic University of Korea,
Seoul, Republic of Korea
8Department of Infection Control, Gangnam severance hospital, Yonsei university, Seoul,
Republic of Korea
S15.6. Trend of Candidemia with Bloodstream infection in Intensive care units from
2006 to 2017: Results from the Korean National Healthcare-associated Infections Surveillance
System
Objectives: Candidemia is an important healthcare-associated infections (HAIs) in
intensive care units (ICUs). The incidence trend and distribution of candidemia over
a 12-year period through the Korean National Healthcare-associated Infections Surveillance
System (KONIS) data were analyzed.
Methods: The KONIS system was established in 2006, and has performed prospective surveillance
for HAIs including bloodstream infection (BSI) and causative pathogen in ICU. We evaluated
yearly trends of the frequencies of the causative pathogens and candidemia. All statistical
analyses were 2-sided and performed the Cochran-Armitage test for trend and the Cochran–Mantel–Haenszel
mean score test, by use of SAS software.
Results: From 2006 until 2017, 2,248 candidemia cases occurred in 9,184,264 patients-days.
For BSI, the proportion of candidemia gradually increased (for BSI from 15.2% in 2006
to 16.6% in 2017, P < 0.05) (Figure 1). The pooled mean incidence rate of candidemia
was 0.24 per 1,000 patient days. Most frequent pathogen for BSI was Staphylococcus
aureus from 2006 to 2012, however, candida species emerged as the most frequent pathogen
since 2013. Candida albicans (39.9%) was the most frequent pathogen for candidemia,
followed by C. tropicalis (20.2%) and C. parapsilosis (18.2%) (Figure 2). There was
no significant change in the distribution of candida species by year (P = 0.29). Central-line
associated BSI were the majority (92.5%). In subgroup analysis, there was a significant
association with the increase in the proportion of candidemia by year in the presence
of organ transplant wards (from 18.9% in 2006 to 21.1% in 2017), hospitals with less
than 500 beds (from 2.7% in 2006 to 13.6% in 2017), or surgical ICUs (from 16.2% in
2006 to 21.7% in 2017) (P < 0.05).
Conclusion: The proportion of candida as nosocomial pathogen for BSI has increased
in Korea. Especially, the proportion in hospitals with less than 500 beds and surgical
ICUs is increasing, so appropriate infection control program is needed. This work
was supported by the Research Program funded (M2018A060000011) by the Korea Centers
for Disease Control and Prevention.
Symposium 16 Dermatology
Arikan-Akdagli
S.
Medical Microbiology, Mycology Laboratory, Hacettepe University Medical School, ANKARA,
Turkey
Invasion of skin by Aspergillus is an uncommonly encountered clinical picture. Primary
cutaneous aspergillosis (PCA) indicates lesions due to direct inoculation of the fungus
at the otherwise injured site. The lesions usually develop following trauma, surgery
or burn wounds. One significant feature of PCA is that it may be observed in immunosuppressed
as well as immunocompetent individuals. Premature neonates also constitute a unique
group of predisposed hosts due to skin fragility. PCA may disseminate and be lethal
particularly in immunocompromised patients. Secondary cutaneous aspergillosis (SCA),
on the other hand, develops either due to hematogenous spread or direct invasion of
the skin from adjacent infected structures, such as paranasal sinuses. Skin and visceral
involvement or multiple skin lesions in two or more noncontiguous areas suggest disseminated
Aspergillus infection. It is estimated that skin involvement in invasive aspergillosis
occurs at rates of 1-5%. The skin lesions in CA appear to have variable presentations,
including macule, papule, plaque, subcutaneous nodule, ulceration with necrosis, and
hemorrhagic bulla. The exact prevalence of CA is unknown and there are limited number
of dedicated series and case reports. Cases of CA are probably underdiagnosed and
underreported due also to difficulties in diagnosing fungal infections in general.
In addition, the affected patient population is heterogeneous and complex with multiple
comorbidities and this further complicates the issue. For optimal management of patients
with CA, skin biopsy should immediately be taken from suspected lesions particularly
in predisposed individuals for histopathological and direct microscopic examinations
and mycological culture.
S16.1. Cutaneous Aspergillosis: Is it so rare?
Nenoff
P.
1
Monod
M.
2
Verma
S.B.
3
Burmester
A.
4
Ebert
A.
1
Singal
A.
5
Gupta
S.
6
Wiegand
C.
4
Hipler
U.-C.
7
Wittig
F.
1
Krüger
C.
1
Koch
D.
1
Vasani
R.
8
Saraswat
A.
9
Madhu
R.
10
Panda
S.
11
Das
A.
11
Kura
M.
12
Jain
A.
13
Graeser
Y.
14
Uhrlaβ
S.
1
1Partnership Dr. C. Krueger & Prof. P. Nenoff, Laboratory of medical microbiology,
Roetha OT Moelbis, Germany
2Dermatology Service, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland
3“Nirvan” and “In Skin” Clinics, Vadodara, India
4Klinik Für Hautkrankheiten, Universitätsklinikum Jena, Jena, Germany
5Department of Dermatology & Std, University College of Medical Sciences & GTB Hospital
(University of Delhi), Delhi, India
6Department of Dermatology and Venereolgy, Maharishi Markandeshwar Institute of Medical
Sciences and Research, Mullana, India
7Klinik Für Hautkrankheiten, Universitätsklinikum, Jena, Germany
8Bhojani Clinic, Mumbai, India
9Dermatology, Indushree Skin Clinic, Lucknow, India
10Department of Dermatology (mycology), Madras Medical College, Chennai, India
11Department of Dermatology, KPC medical college, Kolkata, India
12Department of Dermatology, Grant Medical College, Mumbai & Sir JJ Group of Hospitals,
Mumbai, India
1312415, Doctor’s Nest, New Rajeev Gandhi Nagar, Kota, India
14Institut Für Mikrobiologie Und Hygiene, Universitätsmedizin – Charité, Berlin, Germany
S16.2. Is resistance a problem for dermatophytosis?
Objectives: Resistance of dermatophytes against antifungal agents was not perceived
as a problem for decades. Therapy failure in onychomycosis or tinea capitis was never
related to a reduced in vitro susceptibility of the causative species of dermatophytes.
The situation has changed dramatically. The starting point, was, most likely, India.
An incredible increase in chronic recalcitrant dermatophytoses over the past few years
has been noted in India. The main causative pathogen is the zoophilic dermatophyte
Trichophyton (T.) mentagrophytes ITS genotype VIII.
Patients and methods: Three epidemiologic exercises, for the purpose of speciation
and also to look into the possibility of antifungal resistance were undertaken with
cooperation from nine Indian centers with German and Swiss mycologists. A total of
291 isolates (278 T. mentagrophytes VIII, and 13 T. rubrum) were included in the terbinafine
antifungal susceptibility testing and genetic point mutation analysis of the squalene
epoxidase (SQLE) gene. Itraconazole and voriconazole minimal inhibitory concentrations
(MICs) were determined.
Results: High resistance rates of 66.7% (T. mentagrophytes VIII), and 27.3% (T. rubrum)
to terbinafine were found in the first multicentric study from India. 76.0% T. mentagrophytes
VIII and 57.1% T. rubrum strains of two further studies (New Delhi and Mullana) showed
in vitro resistance to terbinafine. The T. mentagrophytes VIII strains collected from
multiple geographic regions of India showed high frequency of single point mutations
in the SQLE gene leading to terbinafine resistance. All resistant T. mentagrophytes
strains (200) harboured missense mutations with subsequent amino acid substitutions,
most frequently Phe397Leu (90.0%), either as a single substitution or in combination
with Ala448Thr. On the other hand, terbinafine sensitive strains (78) showed almost
exclusively a SQLE wild type (23.1%) or a single Ala448Thr substitution (73.1%). Substitutions
less commonly encountered included Leu393Phe, Leu393Ser, Ser395Pro, Gln408Leu, His440Tyr
and Ser443Pro. The number of terbinafine resistant dermatophytes in Northern India
has increased significantly from 2017 to 2019 (69.0% in 2017; 72.0% in 2018; 78.0%
in 2019). Additionally, we noted a rise in SQLE double mutants, which shows a selection
advantage for this genotype combination.
Conclusion: The dramatic increase in terbinafine resistant T. mentagrophytes ITS VIII
from all over India within such a short period of time underscores the issue of development
of resistance in patients with chronic dermatophytoses. A strong association was found
between in vitro terbinafine resistance of T. mentagrophytes and the occurrence of
single point mutations of the SQLE gene and distinct amino acid substitutions, respectively.
Alterations at position Phe397Leu, Leu393Ser, Leu393Phe, Gln408Leu, His440Tyr, of
the SQLE were associated with terbinafine resistance. Increasing number of patients
in Europe (Germany, Finland, Estonia) and from United States have been seen to be
presenting with chronic recalcitrant dermatophytosis due to terbinafine-resistant
T. mentagrophytes VIII from India. Transmission of the Indian T. mentagrophytes VIII
to other countries due to travel, migration and in general, globalization, appears
to be a serious issue even from a public health perspective. Further studies are the
need of the day to understand this phenomenon better and find answers to combat it.
Schwartz
I.S.
University of Alberta, Edmonton, Canada
Among the main endemic mycoses of North America, blastomycosis is least understood.
Until recently, blastomycosis was thought to be a single clinical disease caused by
a single pathogen, Blastomyces dermatitidis. However, recent re-analyses of isolates
from North American and global collections have revealed substantial genetic and phenotypic
diversity in Blastomyces. Classical blastomycosis, now known to be caused by B. dermatitidis
and the cryptic species B. gilchristii, is limited primarily to eastern and midwestern
parts of North America. In addition, B. helicus, a species morphologically and physiologically
distinct from B. dermatitidis species complex, has been described as the cause of
atypical, disseminated blastomycosis in immunocompromised hosts from western and mountainous
parts of the United States and Canada. Moreover, blastomycosis from Africa and the
Middle East - long recognized to have clinical differences from the disease in North
America - has been determined to be primarily caused by B. percursus and an additional
novel species. Clinicians and microbiologists should be aware of these additional
species, how they differ from B. dermatitidis, and the clinical and epidemiological
spectrum of blastomycosis.
S16.4. Blastomycosis
Ramírez
A.M. Celis
1
Giraldo
C.M. Parra
2
Pineda
E.N. Pinzón
1
1Biological Sciences, Universidad de los Andes, Bogotá, Colombia
2Microbiology, Pontificia Universidad Javeriana, Bogotá, Colombia
S16.5. Galleria mellonella as a novelty model to study host – pathogen interaction
in Malassezia furfur CBS 1878
Objectives:
Malassezia furfur is a lipid-dependent yeast that is part of the animal and human
skin mycobiota. This species can cause dermatological infections in the immunocompetent
host until fungemia in immunosuppressed patients and neonates with parental lipid
nutrition. However, aspects related to virulence traits and antifungal resistance
is not entirely understood. Thus, the establishment of a model to study host-pathogen
interaction has been a matter of attention in the last decade. The silkworm Galleria
mellonella is a useful animal model characterized by its tolerance to a wide range
of temperatures, including human temperature 37 °C; also, the immune response against
pathogens is similar to the innate immunity in humans. Hence, this exciting model
has been used for the large-scale screening of fungal pathogens such as Candida spp.,
Cryptococcus neoformans, and the activity of antifungal drugs in dermatophytes. This
study aim to evaluate the feasibility of using G. mellonella larvae as an invertebrate
model for infection with Malassezia furfur CBS 1878
Methods:
M. furfur CBS 1878 (Westerdijk institute, Utrecht, The Netherlands) was used for all
experiments. An inoculum was adjusted to four different concentrations (1.5 × 109
UFC/mL to 1.5 × 106 UFC/mL) and 10 μl were injected in the last pro leg of G. mellonella.
Survival analysis was performed for statistical processing using Prism software (GraphPad
Software, Inc.). Infected larvae and controls were maintained at 33°C and checked
daily. Hemolymph analysis was conducted to evaluate the capacity of phagocytosis of
hemocytes. Experiments to corroborate the fungal load were also conducted. Each treatment
was conducted in triplicate (technical replicates), and the whole experiment was repeated
three times (biological replicates). Statistical analysis were performed using R package.
Results: Survival test reveals that at least all silkworms injected with M. furfur
CBS 1878 at 1.5 × 109 UFC/mL died within five days, whereas all silkworms injected
with saline were alive. Fungal load data were in agreement with the inoculums tested.
Besides, activation of the immune system of G. mellonella and the interaction between
the yeast with different cells of the hemolymph was observed.
Conclusion: This study is the first approach to the implementation of an invertebrate
model Galleria mallonella to the future study of the host-pathogen interaction and
the antifungal activity in M. furfur CBS1878. We demonstrated that injection of this
yeast into silkworm hemolymph killed silkworms. We found the most effective inoculum
was 1.5 × 109 UFC/mL with a short time of survival on average of five days. This model
promise to be an efficient infection model to conduct easy and reliable approaches
that perform to underpin our knowledge in Malassezia genus.
Symposium 17 HIV associated cryptococcal meningitis
Kolte
I.
1
Leclère
A. Sturny
2
Boyer-Chammard
T.
2
Kivuyo
S. Lesikari
3
Mfinanga
S.
3
Kanyama
C.
4
Kouanfack
C.
5
Lortholary
O.
6
Dromer
F.
7
Bicanic
T.
1
Molloy
S.
1
Harrison
T.
1
Loyse
A.
1
1Infection & Immunity, St Georges University of London, London, United Kingdom
2Molecular Mycology, IP Paris, Paris, France
3National Institute for Medical Research, Dar es Salaam, Tanzania
4UNC Project-Malawi, Lilongwe, Malawi
5Hôpital Central de Yaoundé, Yaoundé, Cameroon
6Infectious Diseases, APHP, Hopital Necker-Enfants malades, Paris, France
7National Reference Center For Invasive Mycoses & Antifungals, Molecular Mycology
Unit, Cnrs Umr2000, Institut Pasteur, Paris, France
The results of the ACTA trial underpinned the development of new 2018 WHO guidance
for the treatment of cryptococcal meningitis with short course amphotericin B deoxycholate
(AmBd) + flucytosine (5FC) becoming the new gold standard for resource limited settings.
The alternative recommended regimen is a two-week oral course of 5FC and fluconazole.
However, access to key diagnostic tests such as the cryptococcal antigen lateral flow
assay (CrAg LFA) and essential antifungal medicines including 5FC for the treatment
of HIV-related cryptococcal meningitis is severely lacking in African low-and middle-income
countries (LMICs) where disease burden and mortality is highest. This talk will highlight
longstanding advocacy and implementation efforts to ensure access to these lifesaving
tools and train frontline healthcare workers (HCWs) working on their optimal use so
as to effectively reduce mortality in resource limited settings. We will focus in
particular on illustrating the respective work of the cryptococcal meningitis action
group (cryptoMAG), the DREAMM project, and outline the recently announced Unitaid/CHAI
project on advanced HIV disease. The cryptoMAG group is an international group of
stakeholders including St George’s University of London (SGUL), Centers for Disease
Prevention & Control (CDC), WHO, Médecins sans Frontières (MSF) and Institut Pasteur
amongst notable others that has been advocating for access to diagnostic tests and
essential medicines for cryptococcal meningitis since 2013. DREAMM (Driving REduced
AIDS-associated Meningo-encephalitis Mortality) is an implementation project currently
ongoing in Tanzania, Cameroon and Malawi. It uses mixed methodology including co-designed
training programs and local health system strengthening centred around African leadership
to effectively and sustainably reduce mortality linked to HIV-related meningitis,
a leading cause of advanced HIV disease. Lastly, in January 2019 Unitaid announced
its program on AHD in partnership with the Clinton Health Access Initiative (CHAI).
This program will enable access to the CrAg LFA and essential antifungal medicines
including 5FC in 7 African LMICs, cryptococcal meningitis being a leading cause of
death from advanced HIV disease.
S17.3. Towards having antifungal drugs in low-resource areas
Bicanic
T.
Infection & Immunity, St Georges University of London, London, United Kingdom
S17.4. Fluconazole resistance in cryptococcal meningitis
In much of sub-saharan Africa, where Cryptococcus is the most common cause of community
acquired meningitis, the gold standard treatment of Amphotericin B with flucytosine
is not available, and fluconazole monotherapy is the norm for all phases of CM treatment:
induction, consolidation and maintenance. Even at higher doses of 800-1200 mg/d, fluconazole
is slow to clear Cryptococcus from CSF. Although primary resistance is rare in global
surveys using MIC testing, the intrinsic mechanism of fluconazole resistance, reversible
disomy of chromosome 1, is unstable: in the absence of drug pressure, it occurs in
only a very small subpopulation of colonies and will be missed by conventional MIC
testing. In a study in Tanzania in patients with HIV-associated cryptococcal meningitis,
we performed serial lumbar punctures in 20 patients receiving fluconazole monotherapy
versus fluconazole combined with flucytosine, plotting the rate of clearance of the
total population and subpopulation able to grow on fluconazole, and saving colonies
for subsequent whole genome sequencing. Fluconazole heteroresistance was detectable
in all clinical Cryptococcal isolates.from CSF of all patients prior to initiation
of therapy. The proportion of resistant colonies in CSF increased during the first
two weeks of treatment in all patients receiving Fluconazole at 800-1200mg/d, but
was suppressed in those receiving combination with flucytosine. Genomic analyses revealed
high rates of aneuploidy in heteroresistant colonies, as well as in clinical isolates
from patiets experiencing relapse. The predmonant mechanism in human infectionwas
disomy of chromosome 1 (containing the drug target gene ERG11 and the efflux pump
AFR1) and heteroresistance positively correlated with in vitro drug efflux pump activity.
We also undertook fluconazole PK analyses in CSF for a subpopulation of patients:
Monte Carlo simulations from the clinical PK/PD model predicted that only a minority
of patients (13%) receiving doses of fluconazole at 1200 mg/d sterilise their CSF
after 2 weeks, with 83% having a persistant subpopulation resistant to fluconazole.
Our findings demonstrate that fluconazole when used alone is a sub-optimal agent for
treating cryptococcal meningitis due to rapid selection of a resistant sub-population
which comes becomes predominant within 2 weeks. Efforts are underway to make the combination
oral therapy of cluconazole with flucytosine more widely available and implementable
in Africa.
Harrison
T.
Centre For Global Health, Institute of Infection and Immunity, St George’s University
of London, London, United Kingdom
S17.5. Current and future clinical trials on HIV-associated cryptococcal meningitis.
The ACTA trial demonstrated that in centres in Sub-Saharan Africa, an induction regimen
of 1 week amphotericin B (AmB) and flucytosine (5FC), followed by fluconazole (FLU)
1200 mg/d in the second week, was associated with reduced 10-week mortality compared
with the then internationally recommended regimen of 2 weeks of AmB+5FC. 2 weeks of
the oral combination of FLU plus 5FC was non-inferior to this standard; and 5FC was
superior to FLU, as the partner drug given with AmB. The results have been further
supported through 12-month outcome and economic analyses, and have driven both a change
in WHO guidelines for the treatment of HIV-associated cryptococcal meningitis, and
increasing access to 5FC through a Unitaid programme. The ongoing AMBITION-CM trial
is testing single high dose (10mg/kg) liposomal amphotericin B (L-AmB), given with
a 2-week oral FLU+5FC backbone, against the new standard of 1 week AmB+5FC. A randomized
phase II study had shown non-inferior early fungicidal activity (EFA) with single
high dose L-AmB vs daily L-AmB at 3 mg/kg/d for 14 days. The hope is that the L-AmB
arm will be at least as effective and better tolerated and easier to deliver than
one week of AmB deoxycholate. The trial includes a pre-planned health economic evaluation.
Over 300 participants have been enrolled. Any further improvements in treatment may
depend on introduction of new drugs into induction regimens, or novel host-directed
or mechanical strategies. Viamet-1598, a fungal Cyp51 inhibitor, and a compound series
from Amplyx Pharmaceuticals that target Gwt1, an enzyme required for fungal cell wall
localization of GPI-anchored mannoproteins, are 2 promising candidates, and it is
hoped both can advance to phase II EFA studies. Immunotherapeutic approaches should
be further investigated but will need to be carefully targeted based on rapid assays
of patient specific immunity. Investigators at Duke University are exploring the possibility
of using neurapheresis to filter fungal cells from the CSF and rapidly reduce the
organism load. Meanwhile efforts continue to prevent the development of clinical cryptococcal
infection in patients with advanced HIV disease, and improve the results of the cryptococcal
antigen (CrAg) screen and pre-emptive treatment strategy for patients with low CD4
cell counts. Despite pre-emptive fluconazole, mortality for those screening CrAg positive
is significantly higher than for those who are CrAg negative; many of those with high
CrAg titre have evidence of sub-clinical meningitis; and fluconazole failures have
been increasingly well documented. A study looking at single high dose L-AmB to treat
CrAg positive patients in screening will take place in Uganda, and a trial to test
the oral FLU+5FC combination against FLU alone for CrAg positive patients identified
at screening is planned in South Africa and Tanzania.
Symposium 18 Antifungal stewardship in the era of resistance
Verweij
P.E.
Center of Expertise in Mycology Radboudumc/CWZ, Nijmegen, the Netherlands
S18.1. Stewardship and azole resistant aspergillosis: a challenge for farmer or physician?
Aims of antibiotic stewardship include treating infectious diseases with the appropriate
drug, preventing overuse and minimizing resistance selection. These goals are frustrated
by the emergence of azole resistance in Aspergillus fumigatus. Recent studies showed
that voriconazole-treated patients with invasive aspergillosis have a 20% lower survival
when the infection was caused by a voriconazole-resistant isolate compared with voriconazole-susceptible
infection. Appropriate initial therapy is thus critical but diagnosis of resistance
is slow when based on fungal culture and MIC-testing. To ensure appropriate initial
antifungal therapy combination antifungal therapy is recommended if resistance frequencies
exceed 10%. The Dutch guideline for management of invasive aspergillosis indeed recommends
voriconazole/isavuconazole in combination with an echinocandin of liposomal amphotericin
B is the azole-susceptibility is unknown as resistance surveillance indicated a resistance
rate of 15%. Therefore, many patients are exposed to increased toxicity and drug interactions,
while the majority still have an infection due to an azole-susceptible isolate. To
improve antifungal stewardship programs accurate resistance surveillance is required
as well as rapid and sensitive diagnostic resistance tests. As the main driver of
resistance to medical azoles in A. fumigatus is azole fungicide use in the environment,
interventions are needed to reduce the burden. Recent studies have identified specific
conditions that facilitate resistance selection and the next step is to design and
evaluate interventions that preclude resistance selection in the environment.
The azoles represent an important class for both medical treatments and food production.
Maintaining this class for both application is therefore critical and requires investment
in rapid diagnostic tests and stewardship programs.
Kanj
S.
Department of Internal Medicine, Division of Infectious Diseases, American University
of Beirut Medical Center, Beirut, Lebanon
S18.2. Rapid diagnosis of fungal infections: Impact on stewardship
Invasive fungal infections (IFI) remain a challenge to the treating physician because
of difficulty in diagnosis and high rates of morbidity and mortality associated with
these infections, which occur predominantly in the immunosuppressed and critically
ill patients. The incidence of invasive fungal infections, especially candidiasis,
aspergillosis, and mucormycosis, continues to rise. With the excessive use of azole
antifungal agents, we have witnessed a global shift of fungal population to more resistant
species and genera. In addition, the increased consumption of antifungal agents in
agriculture have selected for resistant Aspergillus species. There is a wide variation
in the epidemiology of fungal infections worldwide. It is no longer acceptable to
manage IFI without identifying the species of the infecting organism and its susceptibility
profile to the various antifungal agents. Neighboring countries might have a different
epidemiology depending on antifungal practices in humans and the environment. Whereas
guidelines can be of great help in the management of patients, making the correct
diagnosis is of paramount importance. Studies have shown that delay in the initiation
of appropriate antifungal therapy is associated with a significant increase in mortality.
Therefore, there is an urgent need to make a prompt diagnosis of IFI. In the past,
histopathology, fungal cultures, and Candida scores in the right clinical setting
guided the diagnosis and therapy of IFI. More recently, the availability of newer
diagnostic tools such as Aspergillus galactomannan, β-D glucan, and polymerase chain
reaction, has been of great help in speeding the diagnosis of IFI. These tools have
also been used for screening and guiding pre-emptive therapy in high-risk patients.
Studies have shown that combining these new tools increases the sensitivity and specificity
with an impact on fungal infection-free survival in some patients and allows for early
discontinuation. More recently, the development of new molecular tools such as Matrix-Assisted
Laser Desorption/Ionization – Time of Flight, FilmArray, Light Cycler, T2Candida assay,
and others, allows the rapid detection of fungal pathogens and determines resistance
markers with a turnaround time of only a few hours. Investigational tools such as
Proximity Ligation Assay, Breath Fungal Secondary Metabolite Signature, and Siderophore-based
Molecular Infection Imaging are also promising in facilitating the diagnosis of IFI
in the future. In addition, the development of point-of-care testing is helpful, especially
in diagnosing cryptococcal meningitis in some developing countries where access to
microbiology laboratory is limited, or in diagnosing invasive Aspergillosis using
Lateral Flow Devices. All these new diagnostic tools have a tremendous impact on antifungal
stewardship as they allow physicians to discontinue antifungal agents earlier when
not needed or to de-escalate them to narrower-spectrum agents. These efforts will
ultimately translate into decreasing antifungal resistance and reducing costs to healthcare,
which are essential elements of stewardship.
Cottom
L.
1
Jones
B.
2
1Department of Medical Microbiology, Glasgow Royal Infirmary, NHS Greater Glasgow
& Clyde, Glasgow, United Kingdom
2Department of Medical Microbiology, Glasgow Royal Infirmary, Glasgow, United Kingdom
S18.5. Optimising antifungal stewardship: An evaluation of candidaemia guideline compliance
and clinical outcome
Objectives: Candidaemia remains an important cause of nosocomial infection, associated
with significant morbidity and mortality. Over the last decade there has been a growing
awareness that improvements to stewardship and robust surveillance is needed to ensure
the judicious use of antifungal therapy. A guideline should never be construed or
serve as a standard of care, rather recommendations should always be interpreted alongside
individual patient findings and be guided by local epidemiology and antifungal susceptibility
knowledge. The aim of this study was to evaluate compliance with the current 2016
Infectious Diseases Society of America (IDSA) guidelines and assess the impact on
clinical outcome. Adherence to management recommendations was assessed using the EQUAL
Candida Score of the European Confederation of Medical Mycology (ECMM). A secondary
aim was to assess clinical outcome (30 day, 90 day and all-cause mortality) with an
echinocandin compared to fluconazole as the initial treatment (primary therapy) for
candidaemia.
Methods: A retrospective analysis was performed over 18-months (January 2017 to June
2018). Data was analysed from five large university teaching hospitals within Greater
Glasgow and Clyde; the largest NHS organisation in Scotland serving a population of
1.2 million. Patients were identified using our laboratory information management
system and electronic clinical record systems. Only cases where a diagnosis of candidaemia
had been confirmed and the patient had been actively treated were included for analysis.
The source of infection and antifungal management was reviewed for each patient. The
EQUAL candida score was calculated for each case. The 30-day, 90-day and all-cause
mortality rate was determined for the study population.
Results: A total of 131 patients were identified as having a candidaemia. The most
common Candida species isolated was C.albicans (40%; 53/131) followed by C.glabrata
(27%; 35/131). An echinocandin was initiated as primary therapy in 52% (68/131) of
cases. In 62% of cases the patient had an indwelling line that was attributed as the
direct source/nidus of infection. 21% of cases were related to a urinary tract source
and/or urological instrumentation. The mean EQUAL Candida Score was found to be 13
(range 9-17), with the maximum score for guideline adherence being 16 for non-CVC
patients and 19 for CVC carrier (score adjusted as initial blood culture volume was
not known for study population). No statistically significant difference in clinical
outcome (30 day mortality) was found when comparing an EQUAL Candida Score of ≤13
with > 13 (chi-square calculation; p-value 0.76). For the study population, a statistically
significant difference in clinical outcome (30 day mortality) was found when comparing
fluconazole to an echinocandin as initial treatment/primary therapy with a survival
benefit being found in favour of fluconazole (chi-square calculation; p-value 0.039).
Conclusion: Our study provides a valuable evaluation of compliance. Interesting, increased
compliance was not found to improve clinical outcome. Furthermore, the results from
our study suggest that fluconazole as initial therapy was non-inferior to an echinocandin
for the treatment of candidaemia, with a survival benefit being observed. This study
supports the importance of an effective program of surveillance to promote antifungal
stewardship and the judicious use of antifungal agents.
Symposium 19 Pneumocystis
Alanio
A.
Paris-diderot, Sorbonne Paris Cité University, Institut Pasteur, Molecular Mycology
Unit, Cnrs Cmr2000, Parasitology-Mycology Laboratory, Lariboisière Saint-Louis Fernand
Widal Hospitals, Assistance Publique-Hôpitaux de Paris, Paris, France
S19.1. Recent diagnostic strategies in Pneumocystis pneumonia
Pneumocystis jirovecii pneumonia (PCP) is one of the most commonly diagnosed opportunistic
infection in HIV-positive patients. In addition, the rising number of immunocompromised
HIV-negative patients at risk of P. jirovecii infection (immunosuppressive therapies,
allogeneic bone marrow or solid organ transplantation) is an emerging concern. PcP
is difficult to diagnose in HIV-negative patients owing to the non-specific pulmonary
symptoms and signs associated. Microscopic visualization of cysts or trophic forms
in respiratory specimens based on immunofluorescence stainings is the most sensitive
microscopic method and still considered as the gold-standard test to diagnose this
infection. Bronchoalveolar lavage fluids are the most sensitive specimen type, since
P. jirovecii is an organism associated to lung alveoli. PCR-based methods play an
increasing role in the lab, initially developed to circumvent decreased fungal load
in HIV-negative patients and detection in upper respiratory specimens. Real-time quantitative
PCR (qPCR) is definitely the only format adapted to detect P. jirovecii since the
risk of contamination is minimal and quantification is possible. A negative qPCR in
BAL, but not in non-invasive respiratory specimens, allows to rule out PCP diagnosis.
Quantitative results have been used for years to try to discriminate PCP (high fungal
load) from carriage/colonization (low fungal load). However, these methodologies have
limited use since intermediate fungal load are inconclusive. Recent advances in the
comparison of the performances of the different qPCR assays showed that standardization
is certainly one of the clues to move forward with this question. In parallel, the
combination with (1-3)-b-D-Glucan (BG) detection in serum helps but do not completely
resolve the problem. BG detection has a high sensitivity and a high negative predictive
value for diagnosis of PcP in immunocompromised HIV-positive and -negative patients,
but a positive result may also indicate the presence of other invasive fungal infections.
The clinical utility of these diagnostic tests and the diagnostic strategies in HIV
or non-HIV patients should be further assessed in prospective, randomized interventional
studies.In the meantime, the status of the host should be better defined with regard
to the evolution of P. jirovecii carriage. New screening and prophylaxis strategies
to prevent transmission in hospital settings should be proposed and discussed
Kovacs
J.
Associate Clinical Professor of Medicine, The George Washington University School
of Medicine and Health Sciences, Washington, DC, United States of America
S19.2. Management of Pneumocystis Pneumonia in HIV-infected Patients
Pneumocystis pneumonia (PCP) remains one of the most common life-threatening opportunistic
infections in HIV-infected patients, despite the tremendous benefits seen with combination
antiretroviral therapies. PCP continues to be seen in the current era primarily because
at-risk patients are unaware of their HIV infection or are not in continuous medical
care. Infection appears to represent recent acquisition in many cases rather than
reactivation of latent infection. Primary clinical manifestations include fever, shortness
of breath, and a non-productive cough. CXR will typically show diffuse infiltrates
though it may be normal in 10-20% of cases; chest CT scans will almost invariably
be abnormal, however. Diagnosis is based on detection of the organism in respiratory
samples such as induced sputum or BAL. Because the organism cannot be cultured, organism
detection relies on colorimetric or immunofluorescent staining, or PCR assays. The
latter are highly sensitive but less specific than the former, in part because PCR
can identify colonization or subclinical infection not requiring therapy. Assays to
measure (1-3)-β-D-glucan levels have poor specificity and should not be relied on
as the exclusive diagnostic modality. The preferred first-line drug for treatment
is trimethoprim-sulfamethoxazole, which targets 2 steps in Pneumocystis folate metabolism.
Alternatives for patients with mild to moderate disease (A-a O2 gradient <45 mm Hg)
include clindamycin-primaquine, trimethoprim plus dapsone, and atovaquone. Alternatives
for patients with more severe disease requiring parenteral therapy include clindamycin-primaquine
(primaquine is available only for oral administration) and pentamidine. Caspofungin
should not be used as a single agent since it affects only the cyst form and not the
more numerous trophic form of the organism; further, there are no controlled studies
evaluating its efficacy. Corticosteroids should be administered in patients with PaO2
<70 mm Hg or (A-a) O2 gradient >35 mm Hg, ideally within 3 days of starting therapy,
and be continued using a tapering regimen while anti-Pneumocystis therapy is administered.
Although there are no randomized trials, meta-analyses suggest that clindamycin-primaquine
is more efficacious than pentamidine. For patients failing therapy, there are no randomized
trials to address optimal management, and whether it is better to add drugs or to
change regimens. PCP can be effectively prevented in at-risk patients (e.g. CD4 <200
cells/mm3) by using trimethoprim-sulfamethoxazole, or alternatively dapsone, dapsone-pyrimethamine,
atovaquone, or aerosol pentamidine. In patients recovering from an episode of PCP,
secondary prophylaxis should be initiated after completion of therapy. Prophylaxis
can be discontinued after the immune system has reconstituted to CD4 >200 cells/mm3
for at least 3 months. In patients with poor immune reconstitution (CD4 >100 but <200
cells/mm3) but with HIV suppression to below detection limits of commercial assays
(typically <20 copies/ml) for 3-6 months, prophylaxis can also be discontinued. Prophylaxis
should be continued in patients with CD4 <100 cells/mm3 even with HIV suppression.
Mutations have been identified in the dihydropteroate (DHPS) gene of Pneumocystis,
the target of sulfamethoxazole and dapsone, primarily in patients receiving those
drugs for prophylaxis. Although these mutations appear to confer at least low level
resistance, their clinical significance is uncertain.
Calderon
E.
Instituto de Biomedicina de Sevilla and CIBER de Epidemiología y Salud Pública, Seville,
Spain
S19.4. Pneumocystosis in neonates
Pneumocystosis has been well recognized as a severe pulmonary disease and an important
cause of morbidity and mortality in premature infants or debilitated neonates since
the mid-twentieth century when Pneumocystis was acknowledged as the causing agent
of interstitial plasma cell pneumonia. This form of pneumocystosis was observed before,
during and after World War II in Europe, where it reached epidemic proportion with
more than 500 cases confirmed by autopsy. Unexpectedly, this epidemic pneumocystosis
disappeared in the same manner it had been emerged in Europe. Although data on pediatric
Pneumocystis pneumonia (PcP) from developing countries are scarce mostly based on
small series of cases, PcP in infants seem to remain as an important medical problem
in those areas. Nowadays, the interest in Pneumocystis infection goes beyond PcP because
a new spectrum of Pneumocystis-related disease seems to emerge in immunocompetent
infants. Serological studies and use of molecular techniques to identified Pneumocystis
jirovecii have shown that this microorganism probably is one of the more frequent
infectious agents faced by humans in everyday life and that the first exposition to
this pathogen happen in most children early in life, probably in the neonatal period.
However, primary Pneumocystis infection currently goes unrecognized because it has
been presumed to be an asymptomatic or mild nonspecific disease. However, recent reports
indicate that it can present clinically as a self-limiting upper or lower acute respiratory
tract infection. Pneumocystis infection was identified by specific PCR in 24% of infants
with bronchiolitis from France and in 29.4% of Cuban infants and toddlers with whooping
cough. In another study of 422 children hospitalized with acute respiratory tract
infection, Pneumocystis was detected in 16% of infants. However, a marked difference
occurred in the age distribution, as the prevalence was 48% in infants ages 50 to
112 days, 13% in infants ages 113 to 265 days, and 2% in in infants younger than 49
days. In a study carried out in Santiago de Chile, Pneumocystis DNA was detected in
specimens from 51.7% of infants who died unexpectedly in the community, but only 15%
had pneumonia. This study also showed that Pneumocystis infection is more frequent
than viruses before the age of 6 months, and that it has a consistent peak between
2 and 5 months of age. The significance of these findings is unclear but shows the
high prevalence of Pneumocystis infection in infants. Recently, in Spain, a high prevalence
of Pneumocystis infection has been described in preterm neonates and this infection
was associated to higher risk to develop neonatal respiratory distress syndrome. Increasing
evidence suggests that the most common respiratory infection-affecting infants is
the mild and sneaky, primary infection by Pneumocystis. This infection goes currently
unrecognized and has been neglected as a subclinical irrelevant infection by contrast
with the severe PcP affecting the immunocompromised patients. However, compelling
new evidence suggests that this infection may be pathogenic to certain infant age
groups and that microbiome host interactions in early life may condition the development
of altered immune responses in older infants or adults.
Schmidt
D.
Scharmann
U.
Buer
J.
Rath
P.-M.
Institute of Medical Microbiology, University Hospital Essen, Essen, Germany
S19.5. Evaluation of a Novel Commercial Loop-mediated Isothermal Amplification (LAMP)
Assay for Rapid Detection of Pneumocystis jirovecii
Objectives:
Pneumocystis jirovecii pneumonia (PCP) is an increasing problem in immunocompromised
patients. Detection of P. jirovecii in respiratory samples is usually based on microscopy
or PCR. Both methods require experienced technical personnel and are either insensitive
or time-consuming. In this study, a prototype of the eazyplex Pneumocystis jirovecii
assay (Amplex Diagnostics, Germany) was established. The assay is based on LAMP technique
(loop-mediated isothermal amplification). It requires minimal hands on time for sample
manipulation (below 5 minutes) and performance of the assay itself (25 minutes) and
could be a reliable technique to detect P. jirovecii.
Methods: In total, 145 respiratory samples from 134 critically ill patients (65% male,
median age = 64 years) were investigated, using the LAMP assay in comparison with
the RealStar Pneumocystis jirovecii PCR 1.0 (altona Diagnostics, Germany). For the
LAMP assay 25µl of sample were mixed with 125µl RALF buffer (resuspension and lysis
fluid, Amplex) and incubated for 3 minutes at 99°C. 25µl of this suspension were added
to the wells of the test strip afterwards. The LAMP assay was performed in a Genie
II Mk2 device (Amplex). For qPCR, 300µl of sample were extracted using the Maxwell16
nucleic acid extraction platform (Promega, Germany). 10µl of DNA were used for qPCR
according to the manufacturer´s recommendations. QPCR was performed on a RotorGeneQ
cycler (Qiagen, Germany) in a final volume of 30µl. To determine the detection limit
of the LAMP assay, compared to the qPCR, tenfold serial dilution of extracted P. jirovecii
DNA and of a high positive P. jirovecii respiratory sample were investigated in both
assays. Additionally, for each serial dilution time-to-positivity of the LAMP assay
was plotted against cycle threshold values (ct values) of the qPCR. For quantification
of the fungal load the quantification standards of the RealStar qPCR were used (1x104
-1x101 copies/µl).
Results: The LAMP assay detected 17 of 19 qPCR positive samples and 117 of 117 qPCR
negative samples. The assay was invalid in nine samples that were negative by qPCR.
Time-to-positivity of the LAMP assay ranged from 11-22 minutes in patient samples.
Detection limit of the assay was approximately 15-20 copies of genomic DNA/µl in patient
samples and in extracted DNA which corresponded with ct values of 28-29 in the qPCR.
Conclusion: The LAMP assay is an accurate and time saving tool for the detection of
P. jirovecii in respiratory samples. Two samples that were positive by qPCR were not
detected by the assay which is probably due to the significantly lower sample volume
and the crude extraction method the assay uses. Nevertheless, the convenience of the
performance of the assay and interpretation of the results allows laboratories with
less experience and less technical equipment to perform molecular assays on a high
standard.
Symposium 20 Candida auris
Meis
J.
Department of Medical Microbiology and Infectious Diseases, Excellence Center For
Medical Mycology (ECMM), Canisius Wilhelmina Hospital, Nijmegen, Netherlands
S20.1. Schizophrenic gram negative yeast conquering the world
Candida auris has been named as the new “fungal superbug” which poses a significant
threat to public health during outbreaks. C. auris was first named and described only
10 years ago. Follow-up genomic analysis has revealed that C. auris has emerged almost
simultaneously in this short period on 3 different continents. At present 4 major
clades have been identified and last month a fifth clade was discovered. In the beginning
C. auris was often mistaken for other Candida or yeast species when using phenotypic
assimilation/fermentation identification tests. Wide introduction of MALDI-TOF technology
has significantly improved accurate identification in the past few years and is now
the gold standard in addition to molecular sequencing. C. auris has also shown resistance
to several different antifungal drugs and classes of drugs. While its resistance profile
varies geographically, this new yeast pathogen has acquired resistant to fluconazole,
a key drug in the azole class of antifungal drugs. C. auris spreads in healthcare
facilities in a similar way as bacteria while the occurrence and niche in the environment
is elusive. Infections have been reported in a range of countries across continents,
but current estimates are probably inaccurate. Impervious to both antiseptics and
the three major classes of anti-fungal medications, C. auris has been quietly transmitted
within hospitals and nursing homes in at least 37 countries such as the UK, Spain,
India, Pakistan, Middle East, South Africa, Latin America and parts of the USA. Patients
can be colonised with C. auris without becoming sick, but the pathogen seems to prefer
to colonise patients who are already sick or immunocompromised (eg, cancer or transplant
patients), and the very young or very old among hospitalised inpatients. Among at-risk
patients who have C. auris candidemia or invasive candidiasis, depending on the world
region, high mortality rates (~ 30–60%) have been observed. But because infection
typically occurs when a person is already very sick, it may be difficult to disentangle
the attributable mortality. It is important to realize that C. auris is predominantly
a healthcare-associated infection. Prior broad-spectrum antibiotics and antifungal
use can influence the risk of infection. Finally an important question is still unanswered
C. auris: Unde venis et quo tendis? Reference Meis JF, Chowdhary A. Candida auris:
a global fungal public health threat. Lancet Infect Dis. 2018;18(12):1298-1299
Ruiz-Gaitan
A.
1
2
1Severe Infection Research Group, Medical Research Institute La Fe, Valencia, Spain
2Department of Clinical Microbiology, La Fe University and Polytechnic Hospital, Valencia,
Spain
S20.3. Outbreak control: "Controlling a multidrug-resistant Candida auris outbreak"
Background:
Candida auris is an emerging, multidrug-resistant yeast causing hospital outbreaks.
The outbreaks by C. auris described in Spain as well as in other countries with large
outbreaks, are characterized by an exponential increase in the number of cases in
a short period, suggesting a high transmission rate. Objectives We report the first
24 months of the ongoing C. auris outbreak in a tertiary hospital in Spain. The epidemiological,
clinical and microbiological characteristics of candidemia episodes and environmental
samples by C. auris were also analyzed. Results: 228 patients were involved in the
case–control study (114 colonized/candidemia and 114 controls). All candidemia episodes
were observed in adult patients (21–81 years old) and 87.8% of them were admitted
to SICU. The most common underlying condition observed in both colonized and candidemia
patients was polytrauma (n = 13, 32%) followed by cardiovascular disease (n = 10,
25%) and cancer (n = 7, 17%). Indwelling CVC (odds ratio {OR}, 13.48), parenteral
nutrition (OR, 3.49), and mechanical ventilation (OR, 2.43) were the more frequently
invasive procedures observed in these two groups and remained significant predictors
of C. auris colonization/candidemia. All C. auris isolates were resistant to fluconazole
(MICs >64 mg/L) and had significantly reduced susceptibility to voriconazole (GM,
1.8 mg/ L). All isolates were susceptible to itraconazole, posaconazole, isavuconazole,
and echinocandins. Environmental sampling showed presence of the C. auris on sphygmomanometer
cuffs (25%) patient tables (10.2%), keyboards (10.2%), and infusion pumps (8.2%).
Conclusions: - Predictor conditions to C. auris colonization/candidemia are similar
to other Candida species. C. auris colonizes multiple patient’s environment surfaces.
All isolates are resistant to fluconazole and had significant reduced susceptibility
to voriconazole. - Due to its high transmissibility and survival in the hospital environment,
C. auris can cause long duration outbreaks that are difficult to detect in early stages,
and it makes it difficult to control and eradicate. - The implementation of early
and strict surveillance and control measures is essential to preventing the spread
of the outbreak representing a significant risk to critical patients. - Immediate
notification of C. auris to clinical and infection control teams, as well as to health
authorities and institutions, is essential to implementing infection control precautions
at all levels in a timely way, to prevent transmission inside and outside the hospital
and to prevent the development of infections in patients who are already colonized.
- This report is intended to demonstrate not only the complexity of handling and containing
a C. auris outbreak, but also how, with the use of a series of effective infection
prevention measures, the spread of this pathogen can be successfully controlled.
Chowdhary
Anuradha
Department of Medical Mycology, Vallabhbhai Patel Chest Institute, University of Delhi,
Delhi, India
S20.4. Candida auris: in developing world
Candida auris a multidrug-resistant(MDR) yeast that exhibits resistance to fluconazole
and markedly variable susceptibility to other azoles, amphotericin B, and echinocandins
has globally emerged as a nosocomial pathogen that can cause invasive infections.
Candida auris was first described in 2009 by Satoh et al. as a novel Candida species,
in the Candida haemulonii complex(Metchnikowiaceae), from a patient in Japan after
its isolation from the external ear canal. Subsequent to the first nosocomial outbreak
in South Korea in 2011, several hospitals associated outbreaks have been reported
in developing world especially in India, Pakistan and Africa. Alarmingly, in less
than a decade this yeast, which is difficult to treat, has become widespread across
several countries causing a broad range of healthcare associated invasive infections
that display clonal inter- and intra-hospital transmission. C. auris in routine microbiology
laboratories remains an unnoticed pathogen as 90% of the isolates characterized by
commercial biochemical identification systems such as API 20C, Vitek 2 (bioMérieux),
Phoenix (BD), and MicroScan (Beckman Coulter), misidentify as a range of other Candida
species. Most commonly, these isolates have been misidentified as C. haemulonii, but
also C. famata, C. sake, Rhodotorula glutinis, Rhodotorula mucilaginosa, and Saccharomyces
species. Rarely, C. auris has been identified as C. catenulate, C. lusitaniae, C.
guilliermondii, or C. parapsilosis. However, MALDI-TOF MS is considered a more rapid
and robust diagnostic technique for C. auris identification. Mass spectra can be easily
added to the MALDI-TOF MS database, leading to accurate identification of C. auris
to the species level. Sequencing of genetic loci, including D1/D2, RPB1, RPB2, and
internal transcribed spacer (ITS) domains of the rRNA, has proven useful in the identification
of C. auris, but it is not routinely used.
However, in the routine microbiology laboratories this yeast remains unidentified
especially in developing world due to lack of fully equipped mycological diagnostic
facilities. Further, the true burden of C. auris remains unexplored as effective surveillance
of Candida spp is not available in several countries in the developing world. Antifungal
susceptibility data for C. auris is of primary concern as C. auris exhibit consistently
high fluconazole MICs and variable susceptibility to the other azoles, echinocandins
and amphotericin B. No antifungal clinical breakpoints reported for C. auris. Studies
examining the susceptibility of this organism to antifungals have used a variety of
methods, including Clinical and Laboratory Standards Institute (CLSI) broth microdilution,
Etest, and the Vitek 2 yeast susceptibility system. Regarding azole resistance mutations
in Erg11 associated with the development of fluconazole resistance in C. albicans
have also been detected in C. auris isolates. The fact that this yeast exhibit MDR
clonal strains which are nosocomially transmitted is unusual in other Candida species.
Therefore, the possible threat of its rapid spread in affected countries and its emergence
in unaffected countries will not only challenge clinicians for its effective therapeutic
management but will also bring high economic burden to countries especially in resource
limited settings where modern identification facilities and access to antifungals
other than FLU are limited.
Kordalewska
M.
1
Lee
A.
1
Garcia-Rubio
R.
1
Park
S.
1
Berrio
I.
2
Chowdhary
A.
3
Zhao
Y.
1
Perlin
D.S.
1
1Center For Discovery and Innovation, Hackensack Meridian Health, Nutley, United States
of America
2Hospital General de Medellín “Luz Castro de Gutiérrez” ESE, Medellin, Colombia
3Department of Medical Mycology, Vallabhbhai Patel Chest Institute, University of
Delhi, Delhi, India
S20.5. Understanding Echinocandin Activity Towards Candida auris
Objectives:
Candida auris is a recognized cause of invasive infections and health care associated
outbreaks around the world. Development of echinocandin resistance has been documented
in C. auris isolates recovered from patients treated with these drugs. Thus, a thorough
understanding of C. auris echinocandin susceptibility profiles and resistance mechanisms
is crucial for improved management. In this study, we performed a comprehensive analysis
of C. auris echinocandin susceptibility and molecular resistance determinants.
Methods: A total of 106 C. auris isolates (Colombia, India, AR Bank) were investigated
in this study. Antifungal susceptibility testing (AFST) with echinocandins (anidulafungin,
caspofungin, micafungin) was performed in accordance with CLSI document M27-A3. FKS1
gene, encoding the target enzyme for echinocandin class antifungal drugs, was amplified
and sequenced. Mutation prevention concentration (MPC) determination assay was performed
for echinocandin-susceptible (micafungin MIC range 0.03-0.5 mg/l), FKS1 wild-type
C. auris, C. albicans and C. glabrata control strains. Isolates were cultured in RPMI-1640
containing 2-fold increasing concentrations (0.03 to 128 mg/l) of micafungin, then
culture aliquots were plated onto YPD plates and number of surviving cells were calculated.
Recovered colonies were screened for the presence of FKS1 mutations. Moreover, in
vivo drug response of C. auris isolates to caspofungin was assessed in a murine model
of invasive candidiasis.
Results: Four Indian isolates from a total of 106 isolates (3.8%) exhibited highly
elevated MICs ≥4 mg/l at 24 h and were considered presumptively resistant to all tested
echinocandins. These isolates were found to harbor an S639F amino acid substitution
in Fks1. The remaining 102 echinocandin-sensitive isolates presented wild-type genotype.
Micafungin was the most potent echinocandin (MIC50 = 0.125 mg/l). We encountered a
significant challenge obtaining an accurate MIC readout for caspofungin since all
tested isolates exhibited an Eagle effect (paradoxical growth effect), with the intensities
of the Eagle effect varying among the isolates. In MPC determination assay with micafungin,
C. auris isolates did not present a classical bi-modal killing pattern characteristic
for fungicidal drugs activity against other Candida species. Response of C. auris
to micafungin differed between isolates, but micafungin did not reach the fungicidal
endpoint for any of the isolates, even when high drug concentrations (128 mg/L) were
tested. Since no FKS1 mutation was revealed by sequencing, we hypothesize the presence
of extreme tolerance mechanism in C. auris. Echinocandins were effective in vivo in
the treatment of invasive murine candidiasis caused by FKS1 wild-type C. auris isolates
(significant (P < 0.05) ~1 log10 CFU/g kidney burden reduction upon caspofungin treatment
relative to vehicle controls), despite the presence of the Eagle effect in vitro.
Mice challenged with fks1 S639F mutants failed to respond to the drug and their kidney
burdens were not much different between caspofungin treated and untreated groups.
Conclusion: Without established breakpoints for C. auris, AFST results interpretation
and conclusions regarding resistance, especially when testing caspofungin, should
be made with caution. The high echinocandin tolerance of C. auris observed in vitro
should be further explored. Presently, the only validated determinant impacting C.
auris pharmacodynamic response to echinocandins is the FKS1 genotype.
Symposium 21 Immunotherapy for opportunistic fungal infections
Kontoyiannis
D.
Ut Md Anderson Cancer Center, 1515 Holcombe, Houston, United States of America
S21.1. CAR T cell and NK cell therapy of invasive mycoses
Invasive fungal infections remain a major threat to the survival of immunocompromised
patients and no curative therapies are currently available to treat drug resistant
opportunistic fungi. In this brief talk, I will provide a conceptual overview of recent
developments to use specific chimeric antigen receptor (CAR) expressing T cells and
NK cells as adoptive immunotherapy. CAR T cells have been successfully used to treat
B-cell malignancies. To render CAR T cell therapy specific for fungi, we designed
a CAR which combines the carbohydrate-binding domain of Dectin-1 with T cell signaling
domains, designated as Dectin-1-CAR (D-CAR). The pattern recognition receptor Dectin-1
was selected based on its property to bind selectively to β-1,3-glucan present on
the cell wall of life threatening fungal species. I will be discussing our efforts
to manufacture clinical grade D-CAR+ T cells and to increase T cell persistence. However,
allogeneic CAR T cells carry a significant risk of graft-versus-host disease, and
logistical problems of rapid and robust production remain. As the role of natural
killer (NK) cells in fungal immunity has been increasingly deciphered, I will be discussing
the potential benefits of cord-blood derived NK cells as an attractive, allogeneic,
off-the-self source for future, logistically feasible immunotherapeutic approaches
to treat invasive mycoses.
Pagano
L.
Fondazione Policlinico Universitario A. Gemelli - IRCCS - Universita Cattolica del
Sacro Cuore, Rome, Italy
S21.2. WBC transfusions Granulocyte Transfusions As Adjunctive Treatment of Invasive
Fungal Diseases In Neutropenic Patients
The degree and duration of neutropenia are crucial prognostic factors in hematological
patients (pts) with invasive fungal infections (IFIs). Since the introduction of granulocyte
colony stimulating factor (G-CSF), there has been a renewal of interest in granulocyte
transfusions (GTX). Granulocyte transfusions (GTX) are seldom used as a life-saving
therapy for neutropenic patients with severe infections. Despite several compelling
evidences of GTX efficacy in retrospective and prospective case series, no study has
been successful in demonstrating a definite advantage for recipients in controlled
clinical trials. Some specific issues relevant to the efficacy of this therapeutic
approach, such as the primary infection, the delivered doses and schedules, and the
immunological effects of GTX, are still subject to discussion today. Importantly,
the awareness of biological effects accompanying the transfusion of neutrophils might
support their use at standardized doses and may definitely convey significant advantages
to the recipient patients. At present, despite statistical evidences are lacking,
GTX are still perceived as a lifesaving tool to support neutropenic patients with
life threatening IFIs until their bone marrow recovery. Sharing procedures for donor
identification and cell mobilization, pursuing common criteria to identify which patients
will benefit of GTX during febrile neutropenia and define indications and therapeutic
cell doses are absolutely urgent to pinpoint the true advantage of using GTX. On the
other hand, adopting equal end points and outcomes to evaluate both clinical response
to treatment and biological functions of neutrophils and chemokines during IFI, need
to be clarified.
Seif
M.
1
Nerreter
T.
1
Machwirth
M.
1
Ebel
F.
2
Einsele
H.
1
Hudecek
M.
1
Löffler
J.
1
1Medizinische Klinik Und Poliklinik Ii, Universitätsklinikum Würzburg, Würzburg, Germany
2Institut Für Infektionsmedizin Und Zoonosen, Ludwig-Maximilians-Universität München,
München, Germany
S21.4. Novel chimeric antigen receptor T cells for invasive aspergillosis immunotherapy
Objectives: Immunocompromised patients are susceptible to invasive fungal infections
mainly caused by Aspergillus fumigatus (Af). Adoptive transfer of Aspergillus-specific
T cells has been shown to reduce the burden of invasive aspergillosis. Such T cells
are hard to isolate and expand. An alternative option is the use of T cells modified
to express a chimeric antigen receptor (CAR). CARs are recombinant receptor constructs
composed of an extracellular targeting element linked to an intracellular signaling
module. A recent study using Dectin 1 as targeting element proved the applicability
for antifungal CAR T cells in vitro and in vivo. One main limitation of Dectin 1 is
the differential expression of its target β-glucan on Af morphotypes cell wall. β-glucan
is mainly exposed on the surface of swollen conidia and early germ tubes but less
on hyphae. Thus, better specificity would be highly valuable. Here we propose a highly
specific and effective way of redirecting T cells towards Af.
Methods: For the construction of AF-specific CARs, we fused an scFv, derived from
an antibody directed against the Af hyphal cell wall, to extracellular IgG4-Fc spacer
domains of different lengths followed by CD28 and CD3-ζ signaling domains. We non-virally
expressed the CARs on the surface of primary human CD4+ and CD8+ T cells using the
Sleeping Beauty retrotransposon system and co-cultured CAR T cells with Af germ tubes
to evaluate specific T cell activation and direct hyphal damage.
Results: We could show that our CAR T cells are specific for Aspergillus fumigatus,
as no cross-reactivity with other Aspergilli was observed. Activated CD8+ CAR T cells
secreted mainly Th1-related cytokines (IFN-γ, TNFα, IL-8 and GM-CSF) and chemokines
(mainly CCL3 and CCL4), and only one Th2 cytokine (IL-13). Similarly, CD4+ CAR T cells
secreted mostly Th1 cytokines and chemokines, but also some Th2 cytokines (mainly
IL-13 and IL-4). CARs equipped with a long extracellular spacer containing the full
hinge-CH2-CH3-motif conferred superior T cell activation as compared to CARs having
a short "hinge-only" spacer. Noteworthy, mainly CAR T cells containing a long spacer
underwent proliferation upon activation. Upon binding to the target, the cytolytic
machinery of CD8+ CAR T cells was activated, leading to the release of perforin and
granzyme B and up to 45% hyphal damage. Furthermore, when cocultured with macrophages,
both CD4+ or CD8+CAR T cells enhanced antifungal activity of macrophages.
Conclusion: Taken together, our results show that we successfully redirected CD4+
and CD8+ CAR T cells against Af hyphae and that fine-tuning of CAR constructs might
allow the control of fungal infections in high-risk patients.
Charpentier
E.
1
2
Marques
C.
1
Menard
S.
1
Blanchard
N.
1
Chauvin
P.
2
Guemas
E.
1
Cassaing
S.
2
Fillaux
J.
2
Valentin
A.
2
Berry
A.
1
2
Iriart
X.
1
2
1Center for Pathophysiology of Toulouse Purpan - INSERM UMR 1043, Toulouse, France
2Parasitology and Mycology, CHU de Toulouse, Toulouse, France
S21.5. Comparison of circulating lymphocyte populations CD4+ and CD8+ T cells, B and
NK lymphocytes according to the favorable or worsening evolution of patients with
pneumocystosis
Objectives: Pneumocystosis is a severe opportunistic disease in which the host inflammatory
response to the infection is decisive for the disease evolution. Although a reduced
count of CD4+ T lymphocytes has been associated to a major risk of pneumocystosis,
especially in HIV-positive subjects, little is known about other lymphocyte populations
(CD8+ T cells, B and NK lymphocytes) or about CD4+ and CD8+ T cells subpopulations.
This study aimed to compare circulating lymphocyte populations in patients suffering
from pneumocystosis according to the patient evolution.
Methods: Levels of circulating lymphocyte populations were evaluated in the blood
of 24 patients diagnosed with a pneumocystosis at Toulouse Teaching Hospital between
2016 and 2018. Among them, five were solid organ transplant (SOT) recipients, seven
had a haematological malignancy (HM), five had a solid cancer (SC), three had an inflammatory
disease (ID) and four were HIV-positive. CD4+ and CD8+ T cells, B lymphocytes and
NK cells were evaluated with flow cytometry as well as Th1, Th2, Th17, Treg and naive,
effector, memory subpopulations. The lymphocyte populations were compared between
patients with favorable evolution (n = 16) and deceased patients (n = 8; 2 SOT, 2
HM, 3 SC, 1 HIV).
Results: Interestingly, the most significant results concerned CD8+ T cell subpopulations.
Although there was no difference in global CD8+ T lymphocyte count, the proportions
of memory CD8+ T lymphocytes (both central memory and effector memory) were lower
in deceased subjects. At the opposite, the proportions of naive and effector CD8 lymphocytes
were increased in this group. Effector memory CD8+ lymphocytes of deceased patients
harboured less CD127 (IL7 receptor that induces lymphocyte survival and proliferation)
whereas their CD8+ effector cells contained more CD127+ CD25+ (IL2 receptor and activation
marker) cells in comparison to the CD8+ effector cells of surviving patients. These
differences in CD8+ T cells were associated with reduced Th1 CD4 subpopulation and
Th1/Th2 ratio in deceased patients. NK cells were also reduced in this group. There
was no significant difference, either in CD4+ T cells count or in other CD4 subpopulations
(Th2, Th17, Treg, naive, effector, memory) or in B cells count. Patients age, fungal
load and CMV presence or absence were not significantly different in the two groups.
Conclusion: The implication of CD8+ T cells in pneumocystosis is currently a controversial
topic with a discussed role of these cells in the fungus clearance and a suspected
association with alveolar lesions. According to this study, high CD8+ memory subpopulations
would be associated with a better evolution of the disease, along with Th1 CD4+ T
cells. However, high naive and effector CD8 populations would be associated to a worsening
evolution of the patient. The disparity of CD8+ lymphocytes subpopulations might be
related to a difference of immunity stimulation by Pneumocystis jirovecii antigens
or it could be connected with a difference of CD8+ subpopulations homeostasis possibly
affected by some therapeutics or living conditions. Despite the dogma that establishes
CD4+ T lymphocytes as the center of pneumocystosis pathogenesis, CD8+ T cells may
also have an important role in the disease pathogenesis.
Xisto
M.I.D.S.
1
Muñoz
J.E.
2
Dias
L.S.
3
Santos
G.M.P.
2
Calixto
R.O.R.
1
Bernardino
M.C.
1
Taborda
C.P.
4
Barreto-Bergter
E.
1
1Instituto De Microbiologia Paulo De Góes, Universidade Federal do Rio de Janeiro,
Rio de Janeiro, Brazil
2Instituto Biomédico, Departamento De Microbiologia E Parasitologia, Universidade
Federal Fluminense, Niterói, Brazil
3Department of Pediatric, School of Medicine and Public Health, University of Wisconsin-Madison,
Madison, United States of America
4Institute of Biomedical Sciences, department of Microbiology, University of São Paulo,
São Paulo, Brazil
S21.6. Glucosylceramides from Lomentospora prolificans Induce Cytokines Production
and Increase the Microbicidal Activity of Macrophages
Objectives:
Lomentospora prolificans is an emerging opportunistic fungus with a high resistance
to antifungal agents and it can cause localized infections in immunocompetent patients
and disseminated infections with a high mortality rate in immunosuppressed patients.
Glucosylceramides (GlcCer) are synthetized in the majority of known fungal pathogens.
They are bioactive molecules presenting different functions such as involvement in
fungal growth and morphological transitions in several fungi. In this study, we report
the characterization of GlcCer species in L. prolificans and the role of these molecules
in the activation of the innate immune response.
Methods: GlcCer species were isolated from mycelium and conidia forms of L. prolificans
and their chemical structures were elucidated by mass spectrometry (ESI-MS). The reactivity
of L. prolificans GlcCer species to anti-GlcCer Mab was analyzed by ELISA. The GlcCer
distribution on the surface of L. prolificans was analyzed by immunofluorescence using
anti-GlcCer Mab. The cytokine analysis was performed by the GlcCer injection in BALB/c
mice and the cytokines were quantified by ELISA according to the manufacturer’s instructions.
Recruitment of Cells to the Peritoneum Cavity was analyzed FACS.
Results: GlcCer purified from both forms presented a major species at m/z 750 that
corresponds to N-2-hydroxyhexadecanoyl-1-β-D-glucopyranosyl-9-methyl-4,8-sphingadienine.
Monoclonal antibodies against GlcCer could recognize L. prolificans GlcCer species
from mycelium and conidia, suggesting a conserved epitope in fungal GlcCer. In addition,
in vivo assays showed that purified GlcCer species from both forms was able to induce
a high secretion of pro-inflammatory cytokines by splenocytes. GlcCer species also
promote the recruitment of polymorphonuclear, eosinophils, small peritoneal macrophage
(SPM) and mononuclear cells to the peritoneal cavity. GlcCer species were also able
to induce the oxidative burst by peritoneal macrophages with NO and superoxide radicals
production, and to increase the killing of L. prolificans conidia by peritoneal macrophages.
Conclusion: Our results indicate that GlcCer species from L. prolificans are potent
immune response activators. These molecules induce a strong production of NO in peritoneal
macrophages with a high killing activity. In vivo, GlcCer species induce an immune
response composed by a Th1 and Th17 cytokine profile, with recruitment of inflammatory
cells to the peritoneum cavity. In this way, we believed that these molecules are
very important to the immune response against L. prolificans, and could be used to
produce antibodies, vaccines or as an adjuvant.
Symposium 22 NGS and mycobiota
Cope
E.
Kask
O.
Kyman
S.
Zhang
I.
Lee
K.
The Pathogen and Microbiome Institute, Northern Arizona University, Flagstaff, United
States of America
S22.1. Bacterial-Fungal Interactions at the Airway Mucosa and Implications for Chronic
Rhinosinusitis
Emerging studies of the human microbiome (the trillions of bacteria, fungi, viruses,
and archaea that inhabit the human body) have demonstrated the myriad of ways that
host-associated microbial communities contribute to host health. In the airways, the
composition and diversity of the mucosal microbial communities are related to health
status. However, the relationship between airway microbiota and inflammation is not
well understood. Chronic rhinosinusitis (CRS) is a significant healthcare issue; current
estimates suggest that CRS is responsible for 5% of the total healthcare costs in
the US and affects up to 16% of the population. In a prior study of the bacterial
microbiota of CRS patients, we’ve shown that CRS patients have decreased sinonsasal
bacterial diversity compared to healthy individuals. CRS patients were characterized
by enrichment of one of four bacterial pathobionts, including Staphylococcaceae or
Pseudomonadaceae. The objectives of this study were to determine whether bacterial
and fungal microbiota interact in the airways to influence host immune response. We
used ITS2 sequencing in a cohort of 100 CRS patients and healthy controls and found
that, although bacterial community composition is variable across CRS patients, Malassezia
is the predominant fungal genus in the upper airways of healthy individuals and CRS
patients. We then hypothesized that species-specific bacterial-fungal interactions
differentially influence host mucosal immune response. Thus, we investigated in vitro
and in vivo interactions between Malassezia sympodialis, P. aeruginosa, and S. aureus.
In vitro interactions were evaluated using the modified Kirby-Bauer Assay and Crystal
Violet assay for biofilm quantification. A murine model of acute sinusitis was used
to investigate relationships with the host immune response. S. aureus and P. aeruginosa
were intranasally instilled in the presence or absence of M. sympodialis. 16S rRNA
gene sequencing and FungiQuant were used to evaluate changes in microbiota composition
and burden, and host immune response was measured using quantitative real-time PCR
(qRT-PCR). In vitro, late stage planktonic and biofilm P. aeruginosa inhibited M.
sympodialis growth. Co-infection of mice with M. sympodialis and P. aeruginosa or
S. aureus differently influenced the immune response. In co-infected mice, expression
of fungal sensing receptors (Dectin-1), allergic responses (IL-5 and IL-13) and inflammation
(IL-10, and IL-17) in the murine sinonasal cavity depended on the bacterial species
that was co-instilled with M. sympodialis (p<0.05, Mann Whitney). Mice infected with
M. sympodialis alone did not have increased Th1, Th2, or Th17 gene expression, suggesting
that this taxon can act as a commensal. However, mice that were co-infected with M.
sympodialis and S. aureus had increased IL-5 gene expression (p = 0.01, Mann Whitney)
compared to M. sympodialis or S. aureus alone. These results show that species-specific
interactions in airway associated microbiota relate to distinct host immune response.
These interactions may be implicated in some subsets of CRS disease. Our understanding
of the role of bacterial-fungal interactions in CRS will contribute to development
of novel therapies toward manipulation of the airway microbiota.
Gangneux
J.P.
1
Sassi
M.
1
Cann
P. Le
2
Lemire
P.
2
1Laboratoire De Parasitologie-mycologie, CHU de Rennes, Rennes, France
2Inserm Irset- Umr 1085, EHESP, Rennes, France
S22.3. Characterization of indoor dust microbiota in homes of asthma and non asthma
patients
Objective: The exposure of occupants of housing to indoor air pollutants has increased
in recent decades. Among microbiological contaminants, bacterial and fungal aerosols
remain poorly studied and the debate on the impact of these aerosols on respiratory
health is still opened. This study aimed to assess the diversity of indoor microbial
communities in relationship with the health of occupants.
Methods: Measurements were taken from dwellings of 2 cohorts in Brittany (France),
one with children without any pathology and the other with children and adults with
asthma. Thirty dust samples were analyzed by next generation sequencing with a 16S
and 18S targeted metagenomics approach. Analysis of sequencing data was performed
using qiime 2, and univariate and multivariate statistical analysis using R software
and phyloseq package. Dust samples were collected by vacuuming the floor in the child’s
bedroom using a Dustream Collector (Indoor biotechnologies, United Kingdom) sampler-fitted
vacuum cleaner (40 μm mesh nylon filter, domestic vacuum cleaner).
Results: A total of 2,637 prokaryotic (589 at genus level) and 2,153 eucaryotic taxa
were identified (856 fungal taxa (39%) and 573 metazoa (26%)). Among Fungi, only 136
taxa were identified at genus level. The four main bacterial phyla were identified:
Proteobacteria (53%), Firmicutes (27%), Actinobacteria (11%), Bacteroidetes (8%).
The three main fungal phyla identified were: Ascomycota (84%), Basidiomycota (12%)
and Mucoromycota (3%). No bacterial nor fungal taxa were significantly associated
with asthma versus control group.
A trend of over representation in Asthma group versus control was observed for Corynebacterium
(p-value = 0.013, adj. p-value = 0.09) and Streptococcus (p-value = 0.025, adj. pvalue
= 0.112) genus. No correlations between the populations of fungi and the asthma conditions
as well as the habits of the occupants/the characteristics of the dwellings or other
environmental characteristic were observed.
Conclusion: Our findings provide evidence that dust samples harbor a high diversity
of human-associated bacteria and fungi. Molecular methods such as NGS are reliable
tools for identifying and tracking the bacterial and fungal diversity in dust samples,
a less easy strategy for the detection of eucaryots at least using18S metagenomics
approach. This study showed that the detection of some bacteria may be associated
to indoor air of asthmatic patients. Regarding fungi, a higher number of samples and
sequencing with more depth could allow to reach significant signatures.
Gangneux
J.P.
1
Guegan
H.
1
Vandenborght
L.-E.
2
Buffet-Bataillon
S.
1
Enaud
R.
3
Delhaes
L.
4
The Esghami-Efisg (Escmid) and ecmm NGS Study Group
1
1Laboratoire De Parasitologie-mycologie, CHU de Rennes, Rennes, France
2Genoscreen, Lille, France
3University of Bordeaux, Centre de Recherche Cardio-Thoracique de Bordeaux, U1045,
CHU de Bordeaux, CRCM Pédiatrique, CIC 1401, Bordeaux, France
4Parasitology Mycology, Univeristy of Bordeaux, Centre de Recherche Cardio-Thoracique
de Bordeaux, U1045, F-33000, Bordeaux, France, Bordeaux, France
S22.5. An ECMM-ESCMID survey on goals and practices for mycobiota characterization
using Next Generation Sequencing in European laboratories
Objectives: Although substantial efforts have been made to investigate about the composition
of the microbiota, fungi that constitute the mycobiota play a pivotal role in maintaining
microbial communities and physiological processes in the body. Next generation sequencing
(NGS) techniques have clearly renewed methodology to characterize host-associated
microbiota in a more comprehensively way, and have consequently revealed a much more
diverse microbiota than was previously thought to exist.
Methods: We constituted a panel of experts from the European Society of Clinical Microbiology
and Infectious Diseases (ESCMID-ESGHAMI and -EFISG study groups) and the European
Confederation of Medical Mycology (ECMM) to address an international-survey focusing
on laboratory’s current procedures regarding their goals and practices of mycobiota
characterization using NGS.
Results: The laboratories reported their main reason of using NGS to study the mycobiota
primary to understand the pathophysiology of a dysbiosis (n = 20), at a second level,
to contribute to a diagnosis (n = 16) to implement a therapeutic strategy (n = 12),
or to evaluate the exposome (environmental studies)(n = 10). Respiratory tract represented
the most studied site, followed by the digestive tract, Sample originated in half
of the laboratories from sputum (n = 14), or from stool (n = 12), broncho-alveolar
lavage (n = 11), environmental (n = 6), oral wash (n = 4) and skin (n = 1). Target
used for mycobiota analysis was equal among 18S rRNA (n = 11), ITS1 (n = 11) and ITS2
(n = 11), while some centers used the shotgun approach (n = 5). When asked about data
analysis, database used for alignment were mostly UnitE (n = 8 labs), and both Genebank
and CBS databases (n = 7 labs). Other details will be presented.
Conclusion: We can therefore report a photography of mycobiota analysis in 28 laboratories
from 19 countries, affiliated to working groups from ESCMID and ECMM and draw some
conclusions of the diversity of approaches. NGS to study mycobiota is a great challenge
for understanding pathophysiological questions in experimental studies as well as
for driving diagnostic strategies. Techniques will rapidely develop, moving from targeted
NGS to shotgun approaches and whole genome sequencing will also be very useful in
the next years in the field of mycology, but also for an holistic reasoning.
Symposium 23 The funny life of C. albicans in the oral tract
Kannan
A.
1
Laval
G.
2
Bougnoux
M.-E.
3
DEnfert
C.
4
Sanglard
D.
1
1Microbiology Institute, University Hospital Lausanne, University of Lausanne, Lausanne,
Switzerland
2Center For Bioinformatics, Institut Pasteur, Paris, France
3Laboratoire De Parasitologie Mycologie, APHP Hôpital Necker-Enfants-Malades, Paris,
France
4Fungal Biology and Pathogenicity, Institut Pasteur, INRA, Paris, France
S23.3. Modulation of the Fungal-Host Interaction by the Intra-Species Diversity of
C. albicans.
Candida albicans clinical isolates intrinsically display high diversity in their population
genetic structure. Regulation of several host factors and development of C. albicans
diseases can be influenced by diverse genetic backgrounds of the fungus. Using a collection
of 96 genome-sequenced isolates, we aim here to understand by so-called Expression
Quantitative Trait Loci (eQTLs), the influence of C. albicans genome diversity on
fungal transcriptomic profiles in contact with human keratinocytes (TR146 cells).
The impact of the fungal genomes on the host transcriptome can thus be probed. Pilot
experiments using 5 distinct commensal clinical isolates (including the reference
isolate SC5314) in contact with TR146 cells at different time points (0.3-, 1-,2-
and 6h) revealed distinct transcriptional differences between the isolates. After
6h co-culture, only 194 C. albicans genes were found to be commonly differentially
regulated (2-fold threshold) as compared to SC5314 in all four isolates. These data
suggest a heterogeneous response between these different isolates, even with a small
number of isolates. This heterogeneous response underpins the large genome diversity
existing between the different C. albicans clades. Based on the overall summary of
top 200 variable C. albicans genes among all the five isolates, we observed that the
isolate CEC3665 most varied from SC5314 upon infection. This isolate differed from
the others principally by genes involved in hyphal development and adherence. Consistently,
CEC3665 exhibited low adherence to TR146 cells as compared to the others. The use
of these 5 initial isolates allowed the optimization of several other experimental
parameters (optimal exposure time to TR146 cells, variations within biological triplicates)
that was used for probing the transcriptional profiles of the entire 96 genome-sequenced
collection. The current state of the 96 genome-sequenced collection analysis will
be presented.
Bacher
P.
Institute of Clinical Molecular Biology & Institute of Immunology, Kiel, Germany
S23.4. Intestial Th17 drives airway inflammation in ABPA
Th17 cells provide protection at barrier tissues but may also contribute to immune
pathology. The relevance and induction mechanisms of pathologic Th17 responses are
poorly understood. In humans, Th17 responses are particularly important against Candida
albicans, a mucocutaneous fungal pathobiont. In contrast, the role of Th17 cells for
other pathogenic fungal species is unclear. We used antigen-reactive T cell enrichment
(ARTE) for the ex vivo analysis of human T helper cell responses against 30 common
members of the human mycobiome. We identified the mucocutaneous pathobiont Candida
albicans as the major direct inducer of human anti-fungal Th17 cells. Th17 cells directed
against other fungal species are induced by T cell cross-reactivity to C. albicans.
Intestinal inflammation expands total C. albicans- and cross-reactive Th17 cells.
Strikingly, Th17 cells cross-reactive to the airborne fungus Aspergillus fumigatus
are selectively activated and expanded in patients with airway inflammation, such
as asthma, COPD and cystic fibrosis and especially during acute allergic bronchopulmonary
aspergillosis (ABPA), suggesting their specific contribution to lung pathology. This
indicates a direct link between protective intestinal Th17 responses against C. albicans
and lung inflammation caused by airborne fungi. We identified heterologous immunity
to a single, ubiquitous member of the mycobiota as a central mechanism for systemic
induction of human anti-fungal Th17 responses and as a risk factor for pulmonary inflammatory
diseases.
Ceballos-Garzon
A.
1
2
Wintaco
L.M.
3
Padilla
C. Hernández
4
Hoz
A. De La
4
Beltrán
S.L. Valderrama
4
Moreno
C. Alvarez
4
Pape
P. Le
1
Ramirez
J.
3
Parra-Giraldo
C.M.
2
Velez
N.
2
1Department of Parasitology and Medical Mycology of The University of Nantes., Universite
de Nantes, Nantes, France
2Unidad de Proteómica y Micosis Humanas, Grupo de Enfermedades Infecciosas Departamento
de Microbiología, Facultad de Ciencias, Pontificia Universidad Javeriana, Bogotá D.C,
Colombia, Bogota, Colombia
3Grupo De Investigaciones Microbiológicas-ur (gimur), Universidad del Rosario, bogota,
Colombia
4Grupo De Investigación En Enfermedades Infecciosas, Unidad De Infectología, Hospital
Universitario San Ignacio (husi) Pontificia Universidad Javeriana, Hospital Universitario
San Ignacio, bogota, Colombia
S23.5. Persistence of clonal azole-resistant isolates of Candida albicans from a patient
with Chronic Mucocutaneous Candidiasis in Colombia
Objectives: To evaluate and to characterize phenotypically and molecularly clinical
Candida albicans isolates, obtained from a patient with Chronic Mucocutaneous Candidiasis.
Methods: sixteen C. albicans were isolated from a 25-year-old male with several recurrent
fungal infections and admitted to the San Ignacio Hospital in Bogota, Colombia. The
isolates were recovered during four-year from a different anatomical sites. The isolates
were typified by MultiLocus Sequence Typing, and evaluate for susceptibility to triazoles,
Caspofungin and Amphotericin B by microdilution method (CLSI). Their pathogenic capacity
was evaluated in the Galleria mellonella model.
Results: Genotyping of all clinical isolates showed the persistence of the same Diploid
Sequence Type (DST). Isolates changed their susceptibility profile over time acquiring
resistance to azole drugs. In Contrast there were no significant statistical differences
in pathogenicity during the period.
Figure 1. Characteristics of Candida spp isolates A. Antifungal treatment over time
B. Antifungal susceptibility results.
Figure 2. Survival curve of average according to anatomical origin. Kaplan-Meier plots
of G. mellonella survival after infection with C. albicans 3x105 UFC/ml. Inside the
brackets the number of samples.
Symposium 24 Fungal neglected tropical diseases
Sande
W. Van De
Department of Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam,
Netherlands
Mycetoma is a subcutaneous inflammatory and infectious disease of mainly the foot.
It is endemic in tropical and subtropical regions where it causes a high morbidity.
It was the first fungal infection recognized as a Neglected Tropical Disease by the
World Health Organization by resolution WHA69.21 in 2016. Within this resolution,
endemic regions were encouraged to assess the burden of mycetoma and the scientific
community was encouraged to develop tools for early diagnosis and to improve treatment
of mycetoma. It is now more than 3 years since this resolution and within these three
years new information became available on all these subjects. In terms of assessing
the burden of infection, case reports and single center studies were published from
different regions in the world. Studies from Brazil, China, India, Mexico, the Philippines,
Senegal, Sudan and Togo. Also reports from non-endemic regions such as Italy, Spain,
Switzerland and the United States of America demonstrated that immigrants and refugees
can also take mycetoma to their new homelands where they often cause problems in diagnosis
due to lack of awareness. Within these reports it became evident that there are still
new mycetoma causative agents discovered every year. To identify the causative agents,
morphological identification and/or histology often leaded to misidentification demonstrating
the need for more reliable diagnostic tools. To speed up diagnosis and make it more
reliable, techniques were developed to isolate DNA directly from mycetoma grains and
novel PCR primers were developed to be able to identify more causative agents. Isothermal
alternatives were also explored and for histology sections an in situ hybridization
probe was developed. For identification on cultures, a Maldi-Tof database was generated.
To optimize treatment, an open source drug discovery platform was founded (MycetOS)
which identified the fenarimols as a new class of potent anti-mycetoma drugs and other
classes such as the orotomides were tested in vitro for their activity against mycetoma
causative agents. Based on the in vitro potency of ravuconazole, the first ever clinical
trial on a new drug was started at the mycetoma research centre in Khartoum. It is
obvious that since the recognition as a Neglected Tropical Disease the mycetoma field
has moved forward and will continue as such in the upcoming future.
S24.1. Updates on mycetoma
Queiroz-Telles
F.
Public Health, Federal University of Parana, Curitiba, Brazil
S24.2. Chromoblastomycosis: A true neglected tropical disease
Several infectious diseases, including helminthic, protozoal, bacterial, and viral
infections but not fungal diseases other than mycetoma and chromoblastomycosis, are
currently defined as “neglected diseases” by the WHO. After sporotrichosis, chromoblastomycosis
is the second most prevalent implantation mycosis in the world. Chromoblastomycosis
(CBM) is defined as a chronic and indolent granulomatous fungal disease caused by
the transcutaneous inoculation of propagules from several species of melanized fungi
from the Herpotrichiellaceae family. The most prevalent species causing chromoblastomycosis
are Fonsecaea pedrosoi, F. monophora, and Cladophialophora carrionii. Advances in
molecular taxonomy have shown that the biodiversity of the CBM agents has increased
without substantial correlation with specific type of lesions or severity of the disease
and response to therapy. Because several CBM sibling etiologic agents are found in
soil and plant microbiota, this disease is strongly associated with cutaneous macro
or micro traumas during diverse outdoor activities, such as agro pastoralism, hunting,
eco-tourism, etc. Chromoblastomycosis mainly occurs in tropical and subtropical zones
in Latin America, Africa, Asia and Oceania. According to recent data, the the highest
incidence density of CBM (number of reported cases /10 million), occurs in the Dominican
Republic (50.1), Madagascar (26.4), Gabon (23.5), Congo and Costa Rica (11.8), respectively
(Santos DW, PhD Thesis) Melanized cell wall, muriform cell architecture, hydrophobicity
and cell adhesion are considered virulence factors leading to increased pathogenicity,
a key point for disease development. After an uncertain period of incubation, the
initial lesion starts at the site of inoculation. With time, these can evolve to polymorphic
cutaneous lesions. The clinical forms of the disease might be associated with cytokine
cellular-mediated response and in severe cases, the production of TNF α and interleukin
10 has been detected. To confirm diagnosis, the visualization of pathognomonic muriform
(sclerotic) cells is the most simple, inexpensive, rapid and 100% sensitive method.
In culture, the organisms are fastidious. Molecular methods are mandatory for species
identification, but they remain expensive and are not important for therapeutic guidelines.
Although serologic tests (immunodiffusion and ELISA) have been validated, they are
not commercially available. The therapeutic options, for CBM, include physical methods
and chemotherapy. Without a doubt, excisional surgery is the best method for the treatment
of CBM but only for initial small and well-delimitated cutaneous lesions. According
to a few open non-comparative clinical studies, itraconazole (ITZ) is currently the
best therapeutic option for CBM, at the daily dose of 200 to 400 mg from 8 to 36 months.
The success rate ranges from 15 to 80%. The duration and cure rates are related to
the severity of the disease, ITZ gut absorption and the etiologic agent. Terbinafine
(TBF) at the daily dose of 250 to 500 mg is the second most used drug. For refractory
cases, posaconazole may be used as well as the combination of ITZ with TBF or 5-flucytosine.
The only way to prevent CBM is to avoid trauma Individual under occupational risk
should use protective equipment such as adequate clothes, shoes and gloves. Similarly,
to other implantation mycoses, there are no available immunoprophilaxis for CBM.
Bezerra
L. Lopes
CIETEC/USP, São Paulo, Brazil
S24.3. Sporotrichosis: past and future
The description of new cryptic species with different pathogenic potentials has changed
the perspectives on sporotrichosis. The Pathogenic Clade of the Sporothrix genus has
four pathogenic species: S. schenckii, S. brasiliensis, S. globosa and S. lurei. The
new emerging pathogen, S. brasiliensis, shows higher virulence and is mainly related
to zoonotic transmission while S. schenckii and S. globosa are sapronotic agents.
After 20 years of the zoonotic outbreak of sporotrichosis in Brazil the challenges
to manage the disease caused by S. brasiliensis are enormous, including clinical-epidemiological
aspects, treatment and diagnosis. The cat-transmitted disease has already spread from
the South to the Northeast of Brazil with impressive number of cases in either humans
or felines being reported every year. The increment of cases reported by Centers of
Zoonotic Control account for over 500% between 2016 and 2018. For this reason, a national
effort among scientists, clinicians and veterinaries was established to propose a
guideline for the management of S. brasiliensis infection. This group of experts from
several endemic areas is working since 2018. The data and conclusions achieved by
the Feline and Human Brazilian Working Groups will be presented.
Le
T.
Duke University School of Medicine, DURHAM, United States of America
S24.4. Novel diagnostic and treatment strategies for the Southeast Asian fungus Talaromyces
marneffei
Novel diagnostic and treatment strategies for the Southeast Asian fungus talaromyces
marneffei – Thuy Le, MD DPhil Talaromyces marneffei (Tm) is one of seven endemic human
fungal pathogens that causes substantial global morbidity and mortality. Over just
two decades, Tm has emerged as a leading cause of opportunistic infection and death
in adults and children with advanced HIV disease in southern China and Southeast Asia.
The mortality on antifungal therapy in both HIV and non-HIV infected patients is as
high as 30%. The current diagnosis still relies on microscopy of skin lesions (which
is a late manifestation and is absent in 40% of patients) and on cultures of the pathogen
which takes up to 14 days. Late diagnosis is the most challenging clinical problem
and drive mortality from 24% to 50%. The current treatment options are suboptimal.
Intravenous amphotericin B deoxycholate is recommended for induction therapy but has
serious side effects and is still not widely available. Itraconazole tablets are cheap
and are widely available, but has low oral bioavailability, variable pharmacokinetics
and is inferior to amphotericin B in efficacy. This talk presents new data on the
comparative performance of real-time PCR assays versus antigen detection assays for
rapid diagnosis of talaromycosis. Both assays have excellent specificity of >95%;
however, the antigen tests are significantly more sensitive than the real-time PCR
tests (>90% compared to 70-80%). Tm antigen can be detected up to 16 weeks prior culture
positivity in symptomatic hospitalized AIDS patients, and Tm antigen independently
predicts 12-month mortality in patients with a CD4 cell count <100 starting antiretroviral
therapy. This talk will also present results of the IVAP trial, a multi-center clinical
trial comparing amphotericin B and itraconazole for induction therapy in Vietnam,
which demonstrated a 50% mortality reduction in the amphotericin B group. The trial
showed that 98% of patients in the amphotericin B group cleared fungemia within 8
days of therapy, paving the road for a planned phase II clinical trial testing short
courses of liposomal amphotericin B therapy to reduce toxicity. Efforts to test novel
antifungal compounds are ongoing and should offer new and less toxic treatment options
in the coming years.
Stoianoff
M.A. Resende
Navarro
D.B. Silva
Silva
D.L.
Santos
D. Assis
Dept. of Microbiology, Federal University of Minas Gerais, Belo Horizonte MG, Brazil
S24.5. Urban sporotrichosis: neglected micro-epidemia in the State of Minas Gerais,
Brazil
Objectives: Sporotrichosis is a subcutaneous mycosis caused by dimorphic fungus of
the Sporothrix complex that can affect both humans and other animals, being more common
and severe in cats, and occurring more frequently in regions of tropical climate,
as is the case in Brazil. Because it is not a compulsory notification disease in most
Brazilian states, the true incidence of the disease is unknown. The aim of this study
was to report the occurrence of cases of human sporotrichosis in the state of Minas
Gerais, Brazil. Methods: The secretions of the ulcerated lesions were collected with
the aid of sterile swab and the material was subjected to Gram staining, as well as
to the culture at 28°C and 37°C. It was confirmed by the micromorphological examination
that the samples belong to the genus Sporothrix. The molecular identification of the
species is underway and the patients were referred for treatment. Results: It was
reported the occurrence of 11 cases of human sporotrichosis between August 2018 and
May 2019, nine of them from the city of Ribeirão das Neves and two from Belo Horizonte,
state of Minas Gerais, Brazil. Among the patients, nine belonged to the female gender
and two to the male. The patients’ ages ranged from two to 61 years and all of them
reported contact with sick cats. The mean time between contact with the animals and
the appearance of the lesions was 15 days. Nine patients presented lymphocutaneous
form and two presented fixed cutaneous form. The patients presented lesions in the
arms, forearms, fingers or in the heel. The Gram staining revealed presence of yeasts
of elongated and/or rounded form, sometimes with single bud. The thermal dimorphism
and the micromorphology confirmed the identification of Sporothrix spp. Conclusion:
The results suggest that there may be a sporotrichosis microepidemia in the municipality
of Ribeirão das Neves, with marked importance of the zoonotic transmission of the
disease. According to official information, the conditions of infrastructure and sanitation
are precarious in this region. The notification of this disease and studies that relate
the socio-environmental and behavioral conditions that can determine the variation
in sporotrichosis transmission are of great importance, in order to adopt the appropriate
prevention and control measures. The contact of individuals with cats at home environment
may be increasing in the last years, a fact that contributes to the increase of the
possibility of infection of the population. The control of the source of infection
is fundamental in this situation, with priority being given to the investigation of
factors involved in the transmission dynamics of the disease, to assist in the surveillance
and control measures necessary to contain its growth. Key words: Sporotrichosis, Sporothrix
spp., Urban sporotrichosis Financial Support: CNPq, CAPES.
Posters
Araujo
E. Marques De
1
2
Pape
P. Le
2
Lima-Neto
R. Gonçalves De
1
Gonçalves
T.
1
Gusmao
N. Buarque De
1
1Biologia De Fungos, universidade federal de pernambuco, Recife, Brazil
2Ea1155 Iicimed, Nantes University Hospital, NANTES, France
P001. induction of antifungal resistance exerted by the exposure of paclobutrazol
to Aspergillus fumigatus
Objectives: The resistance of Aspergillus fumigatus to azolic drugs occurs mainly
through two routes: in vivo as a consequence of antifungal therapy, and in the enviroment
as a consequence of the use of fungicides in agriculture. Placobutrazol is a plant
growth retardant used in agriculture, which inhibits cellular growth and hormones
composition. Since it is excessively applied and presents chemical stability, paclobutrazol
accumulates in the soil and can affect the existing microbiota.The present study aimed
to induce to the resistance and consequently the induction of CYP51 gene mutation
on azoles-susceptible isolates of Aspergillus fumigatus after exposition against paclobrutazol.
Methods: he A. fumigatus (2 from environmental origin and 1 ATCC) were submitted to
antifungal susceptibility testing by the microdilution method (MIC-EUCAST) to prove
that all strains were sensitive at concentrations of 32 μg/mL of paclobrutazol and
0.5 μg/mL of voriconazole and itraconazole. For the resistance induction test, these
fungi were exposed to paclobrutazol for five weeks in RPMI culture medium at 37°C
in initial concentration of 4 μg/mL, and increasing to a concentration of 64 μg/mL,
it was also checked whether concentration progression influenced induction using the
same method, without elevation in concentration of the molecule in the medium. Over
the five weeks of the test, the MIC was verified through the micro dilution method
established by EUCAST and the yeastone sensitrite commercial method in order to evidence
the elevation of MIC after exposure.
Results: The MICs obtained over the five weeks of the test showed a decreasing of
sensitivity in all strains (100%) against antifungals used in the hospital routine
after exposure to the paclobrutazol.
Conclusion: Thus, is possible to affirm the exposure of the Aspergillus fumigatus
to drugs used on agriculture may induce resistance against azoles usually prescribed.
These finds demonstrating the need of a better and more rigid regulation in the use
of this molecule in agriculture. The next steps in this research are to confirm that
placobutazol exposure may induce a permanent mutation on resistance gene.
Chaudhary
P.
Microbiology, Sagarmatha chaudhary eye hospital, lahan, siraha, Nepal
P002. Oral Candidiasis in HIV Infected Patients and its Antifungal Susceptiblity Pattern
by Disc Diffusion Method.
Objectives: Oropharyngeal candidiasis (OPC) is an opportunistic fungal infection that
is commonly found in (Human Immunodeficiency Virus) HIV infected patients. Acquired
Immunodeficiency syndrome (AIDS), a disease of human immune system caused by HIV,
has emerged as a global crisis since its discovery in summer of 1981 in United States.
The low absolute CD4+ T-lymphocyte count has traditionally been cited as the greatest
risk factor for the development of OPC and current guidelines suggest increased risk
once CD4+T-lymphocyte counts fall below 200 cells/mm³.Hence, a cross-sectional study
was conducted in Sukraraj Tropical and Infectious Disease Hospital (STIDH), Teku,
Kathmandu over 3 months period with a primary objective, to isolate and identify Candida
species among HIV infected patients and susceptibility pattern of isolates by disc
diffusion method and secondary objective, to estimate the frequency of oral candidiasis
according to age, sex, and other factors (CD4+ count, tobacco consumption, URTI, recent
antibiotic consumption) in HIV infected patients.
Methods: A total of 408 throat swab samples were collected from patients with and
without active lesion visiting ART center of STIDH. Flow chart for sample processing
and identification of isolates is shown below.
Results: 114 isolates were obtained from total sample processed. Among them, 65 isolates
were Candida species and remaining 49 was bacterial flora and other yeasts. Based
on various phenotypic methods, Candida albicans, 53 (81.5%), was the most commonly
isolated organism followed by Candida tropicalis 3 (4.6%), Candida krusei 2 (3.1%),
Candida glabrata 1 (1.5%) and 6 (9.2%) were other Candida species. of the 65 yeast
isolates from HIV seropositive individuals, 26 patients had CD4+ ≤ 200 cells/mm³ and
39 patients had CD4+ ˃200 cells/mm³. 14 patients exhibited severe immune suppression
with CD4+ count less than 100 cells/mm³. There was a significant association between
oral Candida carriage and CD4+ cell count ≤ 200 cells/mm³ (p<0.001). Antifungal susceptibility
test of Candida species showed highest sensitivity with Amphotericin B and resistance
with Fluconazole respectively. Table below depicts about the susceptibility pattern
of the Candida spp.
Conclusion:
C. albicans was the predominant species for oral yeast colonization in our study.
Different isolates presented different susceptibility to various antifungal agents.
Among the antifungal discs tested, Fluconazole was found to show resistance in most
of the isolates and Amphotericin B was the most sensitive drug which is an excellent
antifungal to control Candida spp. since it showed the best result among all antifungal
tested. Oral yeast colonization was associated with low CD4+ count (<200 cells/mm³).
Thus, oral lesion serves as early marker for HIV infection. The increase incidence
of oral candidiasis in HIV patients may be due to low CD4+ count, URTI and recent
antibiotic consumption.
Griffin
A.
Microbiology, St. James’s Hospital, Dublin, Dublin, Ireland
P003. The validation of VIPcheck™ plates to screen Aspergillus fumigatus isolates
for phenotypic resistance to triazole antifungal agents in St. James’s Hospital, Dublin.
Objectives: Triazole resistance is an emerging problem in Aspergillus fumigatus (AF)
resulting in failure of azole therapy. Triazole resistant AF is acquired through one
of two routes - previous exposure to triazole therapy or an environmental source.
In vitro antifungal susceptibility testing (AFST) on all AF strains isolated in a
microbiology laboratory would be both labour intensive and impractical. A method to
screen for triazole resistance would be more favourable. The objective was to validate
VIPcheck™ plates (Mediaproducts BV, Groningen, Netherlands) in SJH to investigate
whether they would be a suitable screening method for triazole resistance in AF clinically
isolated from patients in a large tertiary hospital with a significant cohort of immunocompromised
patients.
Methods: A total of 18 retrospective isolates (clinical and environmental) of AF were
tested using the VIPcheck™ plates (n=2 wild type, n=16 resistant to ≥ 1 triazole drug
as previously determined by AFST and/or molecular methods). VIPcheck™ plates are a
simple agar based screening method, each 4 well plate containing a growth control
(GC) well and 3 wells containing itraconazole (4mg/L), voriconazole (2mg/L) and posaconazole
(0.5 mg/L). Briefly, 25ul of a 0.5-2McF suspension AF is inoculated into each well
and plates are read after 48hrs incubation at 37 °C. Any growth in a triazole containing
well is suggestive of resistance prompting full AFST be carried out.
Results: A total of 18 isolates (clinical and environmental) of AF were tested using
the VIPcheck™ plates as described above. The wild type isolates showed growth only
in the GC well while the resistant strains all showed growth in one or more of the
triazole containing wells.
Conclusion: VIPcheck™ plates are not intended to give an exact MIC; the preparation
of the inoculum has a broad McFarland range of 0.5-2 and only one concentration of
each azole drug is included in the plates. Rather, they are useful as a screening
method to determine the need for further AFST and/or molecular testing and more importantly
for earlier detection of triazole resistance in patients who will require treatment.
Currently in SJH, AFST is carried out using gradient strips (Liofilchem™) and results
are interpreted using EUCAST breakpoints We validated the VIPcheck™ plates with the
intention to include this screening method as part of our AFST for AF isolated from
clinical samples and our results suggest that they provide a reliable screening method
for triazole resistance.
Broutin
A.
1
Bigot
J.
1
Normand
A.C.
2
Senghor
Y.
3
Moreno
A.
3
Hendrickx
M.
4
Guitard
J.
1
Hennequin
C.
3
1Parasitology-mycology, APHP, hôpital St Antoine, Paris, France
2Parasitology-mycology, APHP, Hôpital Pitie-Salpêtrière, Paris, France
3Parasitology-mycology, APHP, Hôpital St Antoine, Paris, France
4Mycology, Scientific Institute of Public Health, Brussels, Belgium
P004. In vitro susceptibility to isavuconazole of Fusarium clinical isolates; comparison
between eucast and etest methods
Objectives: To evaluate the in vitro susceptibility to isavuconazole of a panel of
precisely identified clinical Fusarium isolates and to assess the value of Etest as
an alternative method in this indication.
Methods: We randomly selected 75 clinical isolates previously identified as Fusarium
sp. The strains were identified using MALDI-TOF technology (MSI System). They were
then tested comparatively against isavuconazole (ISA) either using the broth microdilution
method proposed by the Eucast consortium according to the recent document E.DEF 9.1
and the Etest technique as recommended by the manufacturer (Liofilchem).
Results: All strains could be identified at the cryptic species-level revealing the
existence of 14 different species in the panel (Table 1). F. solani (FSSC), F. oxypsorum
(FOSC) and F. fujikuroi (FFSC) were the species complexes the most frequent, encompassing
22, 17 and 31 strains, respectively. Overall, ISA exhibited a limited activity against
the Fusarium strains with MICs90 >16 µg/ml whatever was the species complex. Strains
of the FOSC appeared a little bit more susceptible with a geometric mean at 9.41 µg/ml
versus 14.02 µg/ml and 13.68 µg/ml for FSSC and FFSC, respectively. Considering the
diversity of species, it was not possible to test a possible difference in susceptibility
between the cryptic species within a given complex. Finally, we observed MIC between
2 to >16 µg/ml for strains of the single species of the FOSC, suggesting that precise
identification may not be enough to predict the susceptibility to ISA. The concordance
between Etest and Eucast results was calculated at 100% within an interval +/- 2 dilutions.
The Intraclass Correlation Coefficient was calculated at 0.91, indicative of a strong
concordance between the 2 methods.
Table 1: Isavuconazole MICs distribution against 75 clinical isolates of Fusarium.
Conclusion: ISA exhibits a limited in vitro activity against clinical isolates of
Fusarium, with few variations between the species complexes. In the case the drug
should be used, Etest appears a valuable alternative to Eucast for evaluating the
in vitro susceptibility of the infecting strain.
Shin
J.H.
Kwon
Y.J.
Byun
S.A.
Choi
M.J.
Won
E.J.
Kim
S.H.
Department of Laboratory Medicine, Chonnam National University Medical School, Gwangju,
Republic of Korea
P005. Species Distribution and Antifungal Susceptibility of Candida Ear Isolates from
a Hospital in Korea
Objectives:
Candida auris was first isolated from the ears of Japanese and Korean patients. However,
the prevalence of Candida species from ear cultures and their antifungal susceptibility
profiles in these nations are unclear.
Methods: We assessed Candida isolates recovered from cultures of ear specimens from
a university hospital in Korea over the 4-year period from January 2014 to December
2017. Species identification was performed by matrix-assisted laser desorption/ionization
time of flight mass spectrometry and/or sequence analysis. Antifungal minimal inhibitory
concentrations (MICs) were determined by the broth microdilution method (M27A) of
the Clinical and Laboratory Standards Institute.
Results: Candida species were isolated from the ears of 80 patients (75 adult and
5 pediatric patients). Most of these patients suffered from chronic otitis media (83.8%),
and had a history of antibiotic use (73.8%) and ear surgery (60.0%). Among 80 non-duplicate
isolates from ear cultures, Candida parapsilosis was the most frequently detected
Candida species (35.0%), followed by C. auris (28.8%), Candida metapsilosis (10.0%),
Candida orthopsilosis (8.8%), Candida albicans (7.5%), and other species (11.1%).
The MICs of the isolates were 0.125 to > 64 µg/mL, ≤ 0.03 to 4 µg/mL, 0.25 to 1 µg/mL,
0.125 to 1 µg/mL, and ≤ 0.03 to 2 µg/mL for fluconazole, voriconazole, amphotericin
B, caspofungin, and micafungin, respectively. of the 80 isolates, 43.8% (35/80) showed
decreased susceptibility to fluconazole (MIC ≥ 4 µg/mL). of 23 C. auris isolates,
19 (82.6%) had a fluconazole MIC of ≥ 32 μg/mL. None of the isolates showed resistance
to amphotericin B or echinocandins.
Conclusion:
C. parapsilosis complex and C. auris were the Candida species identified most frequently
in ear cultures and exhibited a high rate of fluconazole non-susceptibility, particularly
C. auris.
Romald
N.
1
Kindo
A.J.
2
Veeraragavan
M.
3
1Department of Microbiology, Sri Ramachandra Institute of Higher Education and Research,
Chennai, India
2Microbiology, Sri Ramachandra Medical College and Research Institute, SRIHER, Chennai,
India
3Dermatology, Sri Ramachandra Medical College and Research Institute, Chennai, India
P006. Epidemiological pattern of Malassezia, its phenotypic identification and anti-fungal
susceptibility profile to azoles by broth micro-dilution method.
Objectives: Analyse the epidemiological pattern of disease with regards to age, sex
and site of predilection To Speciate Malassezia phenotypically. To study the anti-fungal
susceptibility (AFST) pattern of Azoles to pathogenic Malassezia species by recording
their MIC values (MIC50 & MIC90) using Broth Micro dilution method in accordance with
CLSI M27-A3. To identify resistant drug/drugs to Malassezia species.
Methods: Prospective study was done from August 2016-October2018 with sample size
of 150
Sample collection-Hyper/hypo pigmented skin lesions by Skin scrapings and swabs Microscopic
examination with 10%KOH Those showing meat ball and spaghetti appearances were included
in the study. Culture Samples inoculated into Sabourauds Dextrose Agar (SDA), SDA
with olive oil overlay (SDA-O) and Modified Dixon’s agar (MDA) containing chloramphenicol
and incubated at 32o C upto 3 weeks. Phenotypic Identification Done by colony growth
characteristics, gram staining, urease test, catalase test, bile esculin with tween
60 hydrolysis and tween assimilation AFST AFST was performed by the broth micro-dilution
method, according to the Clinical and Laboratory Standard Institute (CLSI) guidelines
M27-A3 (2008)
Systemic anti-fungals used-Fluconazole, Itraconazole, Voriconazole
Topical Anti-fungals used-Clotrimazole, Miconazole, Luliconazole
Since Malassezia are lipid dependant yeast RPMI 1640 was supplemented with glucose,
peptone, ox bile, malt extract, glycerol, tween 80, tween 40 and Chloramphenicol Culture-Malassezia
isolates grown on MDA for 72 h at 32°C± 2°C were used for inoculum preparation. Anti-fungal
stock solutions of drugs were prepared and stored at - 700C Quality Control-Each microtitre
plate included growth control well with inoculum and supplemented RPMI -1640 medium
without the antifungal agent. Candida krusei ATCC 6258 was used as a quality control
strain for antifungal susceptibility testing. Incubation and Reading Results-The microtitre
plates were incubated at 32˚C.Results read after 24hrs for candida isolate and after
72-96 hours for Malassezia isolates. The minimum inhibitory concentration (MIC90)
(reduction of 90% growth compared to growth control well) and MIC50 (50% reduction
of growth compared to growth control well) were recorded.
Results:
Age distribution- The most common age affected was 20 to 30yrs (36%), followed by
30-40yrs (21%) and 10-20yrs (19%) extremes of age showed lower incidence. Sex distribution-The
incidence among male and female was almost equal, showing little female predominance
Site of infection - Among the total 150, the most common site was back (47 samples),
followed by neck and shoulder (31 samples), and scalp (27 samples). Skin lesions-Both
hypo and hyperpigmented skin scrapings were collected. But more of hypopigmented lesions
were noted in our study ANTI-FUNGAL SUSCEPTIBILITY PATTERN-fig
Conclusion: Among 150 samples only 95(62%) yielded growth on both SDA with olive oil
and MDA. Growth on MDA was soon than SDA with olive oil Overlay. All organisms were
urease and catalase positive. Among the 95 samples grown the predominant species was
Malassezia sympodialis-29(33%) followed by M.globosa-26(29.5%), M.furfur-21(23%),
M.obtusa-12(13%) by conventional method. The systemic anti-fungal with the least MIC
values were Itraconazole and Voriconazole. Fluconazole shows resistant Pattern with
high MIC values The most effective topical anti-fungal was Clotrimazole with low MIC
values Malassezia sympodialis responded to anti-fungal better than other species isolated
Shin
J.H.
1
Hwang
I.J.
2
Won
E.J.
1
Choi
M.J.
1
Jung
S.-I.
3
Song
E.S.
2
Choi
Y.Y.
2
1Department of Laboratory Medicine, Chonnam National University Medical School, Gwangju,
Republic of Korea
2Paediatrics, Chonnam National University Medical School, Gwangju, Republic of Korea
3Chonnam National University Medical School, Gwangju, Republic of Korea
P007. Prevalence and genotype distribution of fluconazole-resistant Candida parapsilosis
bloodstream isolates from a neonatal intensive care unit during a 14-year period
Objectives:
Candida parapsilosis bloodstream infection (BSI) is a significant problem in neonatal
intensive care units (NICUs). C. parapsilosis is usually susceptible to azole antifungals;
however, some recent reports have indicated the emergence of BSIs caused by fluconazole-resistant
(FR) C. parapsilosis isolates. We investigated the prevalence and genotype distribution
of FR C. parapsilosis BSI isolates recovered from a NICU during a 14-year period.
Methods: We assessed a total of 64 C. parapsilosis BSI isolates comprising 22 non-duplicate
isolates from 22 neonates and 42 isolates from 21 neonates (two isolates from each
neonate obtained on the first and the last days of C. parapsilosis-positive culture).
All isolates were obtained at the same NICU between 2002 and 2015. In vitro antifungal
susceptibility testing for fluconazole, amphotericin B, caspofungin, and micafungin
was performed using the Clinical and Laboratory Standards Institute (CLSI) M27-A3
microdilution method. All isolates were genotyped via microsatellite typing using
four specific microsatellite markers (CP1, CP4, CP6, and B). Clonal strains were defined
as the isolation of two or more strains with identical genotype as determined by microsatellite
typing.
Results: of the 64 C. parapsilosis BSI isolates, 11 (17%) obtained from eight patients
were FR (fluconazole minimal inhibitory concentration [MIC] ≥ 8 mg/L). All 64 isolates
were susceptible to amphotericin B, caspofungin, and micafungin. Based on microsatellite
typing, the 64 isolates yielded 19 different genotypes, including 12 genotypes unique
to a single isolate and seven genotypes shared by 49 isolates (77%). All 11 FR isolates
shared two genotypes (1 and II) that displayed closer genetic relatedness than other
isolates examined in the microsatellite analysis, according to as an unweighted pair
group method with arithmetic mean (UPGMA) tree. of these two genotypes, type I strains
(three patient isolates) were isolated within a 6-month period, whereas type II strains
(five patient isolates) were isolated over a 4-year period. Among 21 patients showing
more than one C. parapsilosis isolate on 2 or more separate days, two sequential strains
from each patient had identical types in 76% (16/21) of patients and different types
in 24% (5/21). No difference in antifungal susceptibility was detected between two
sequential isolates from the same patient. Among eight patients with FR C. parapsilosis
isolates, 88% had no known prior antifungal exposure. We detected no significant difference
in the overall 30-day mortality of C. parapsilosis BSI due to FR strains, or in that
of BSI due to fluconazole-susceptible (FS) strains (25 vs. 20%).
Conclusion: The results of the present study suggest that clonal transmission may
occur frequently among both FS and FR blood isolates of C. parapsilosis in a NICU
setting. Increased incidence of BSI due to the FR C. parapsilosis strain in this NICU
may be associated with clonal transmission rather than prior antifungal exposure.
Shin
J.H.
1
Won
E.J.
1
Jung
S.-I.
2
Hwang
I.J.
3
Choi
M.J.
1
Byun
S.A.
2
Kim
S.H.
1
Park
Y.-J.
4
Kim
M.-N.
5
1Department of Laboratory Medicine, Chonnam National University Medical School, Gwangju,
Republic of Korea
2Chonnam National University Medical School, Gwangju, Republic of Korea
3Paediatrics, Chonnam National University Medical School, Gwangju, Republic of Korea
4The Catholic University of Korea College of Medicine, Seoul, Republic of Korea
5University of Ulsan College of Medicine and Asan Medical Center, Seoul, Republic
of Korea
P008. Bloodstream Isolates of Azole-Resistant Candida glabrata in Korean hospitals:
Resistance Mechanisms and Clinical Features
Objectives:
Candida glabrata has recently become the most common non-albicans Candida species
causing bloodstream infection (BSI) in South Korea, and the occurrence of azole-resistant
C. glabrata isolates has been observed. We investigated the azole resistance mechanisms
and clinical features of patients with azole-resistant C. glabrata BSI isolates recovered
from multicenter surveillance in Korea.
Methods: In total, 248 C. glabrata BSI isolates comprising 43 azole-resistant (fluconazole
minimum inhibitory concentration [MIC], ≥ 64 μg/mL and voriconazole MIC, ≥ 1 μg/mL)
and 205 non-azole-resistant (non-AR) isolates (fluconazole MIC, ≤32 μg/mL and voriconazole
MIC, ≤ 0.5 μg/mL) were analyzed. The isolates were collected from 10 university hospitals
over 8 years (2009–2017). The PDR1 genes of the isolates were sequenced to identify
mutations. Expression levels of CDR1, CDR2, and ERG11 genes after fluconazole exposure
were quantified by real-time PCR. The clinical features related to risk factors for
BSI and the mortality rates were compared. Risk factors for BSI caused by AR isolates
and survival rates in patients were assessed using multivariate logistic regression.
Results: Pdr1 mutations were identified in 42 of 43 azole-resistant isolates, while
no Pdr1 mutation was found in 201 of 205 non-AR isolates. Azole-resistant isolates
of C. glabrata had higher mean expression levels of CDR1 (8.3-fold) gene than non-AR
isolates (P <0.05). However, there were no significant differences in the mean expression
levels of CDR2 and ERG11 between two groups. Previous azole exposure (odds ratio [OR],
6.369; 95% confidence interval [CI], 2.434-16.664; P < 0.001), central venous catheter
use (OR, 11.406; 95% CI, 2.462-52.837; P = 0.00211), and total parenteral nutrition
(OR, 4.042 95% CI, 1.381-11.828; P = 0.011) were the independent risk factors for
BSI caused by AR C. glabrata. C. glabrata BSIs caused by AR isolates resulted in a
higher 30-day mortality rate (55.8%, 24/43) than BSIs caused by non-AR strains (36.6%,
75/205) (P = 0.019). In multivariate analyses, severe sepsis (OR, 5.592; 95% CI, 2.689-11.630;
P < 0.001), a high age-adjusted Charlson comorbidity index (ACCI) score (OR, 1.131;
95% CI, 1.012-1.263; P = 0.03), lack of antifungal therapy (OR, 5.213; 95% CI, 2.487-10.930;
P < 0.001), and azole-resistance in C. glabrata BSI isolates (OR, 2.856; 95% CI, 1.161-7.025;
P = 0.022) were independent predictors of the 30-day mortality among the 248 patients.
Conclusion: This study provides that Pdr1 mutation with overexpression of CDR1 may
be the main resistance mechanism of C. glabrata in Korea, and that azole-resistance
in C. glabrata BSI isolates is independent predictor of 30-day mortality, and is associated
with antifungal exposure, central venous catheter, and total parenteral nutrition
use.
Barton
R.
Sethi
K.
Microbiology, Leeds Teaching Hospitals Trust, Leeds, United Kingdom
P009. A case of urinary tract candidosis: a risky business
Case Report: A 62 year old man with a history of psoriasis treated with methotrexate,
poorly controlled type 2 diabetes, and chronic obstructive pulmonary disease had a
laparoscopic cholecystectomy for cholecystitis and repair of a cholecystoduodenal
fistula. He then presented with severe dysuria, polyuria and complained of passing
cloudy urine ever since the surgery. Following a series of urine samples which were
positive for yeasts which were not identified and a course of fluconazole (details
not available), he was seen at a haematuria clinic with continued dysuria, frequency
and urgency, and a flexible cystoscopy showed a "snowstorm" appearance within bladder,
and an ultrasound revealed mobile echogenic debris. He was then put on a 14/7 course
of 200mg oral fluconazole, pending a cystoscopy and bladder biopsy which revealed
erosive cystitis. A subsequent urine sample was identified as Candida glabrata which
was resistant to fluconazole (MIC >256mg/L) but sensitive to flucytosine (MIC 0.06mg/L).
Despite a further 14 day course of 800mg fluconazole, signs and symptoms of Candida
cystitis were not resolved. The patient was then referred to a urologist at our hospital
and various treatment choices were discussed with oral flucytosine and IV Amphotericin
deoxycholate being the main two options, associated with the risk of resistance and
toxicity respectively. It was decided to treat with a 7 day course of 25mg/kg of oral
flucytosine (levels were not monitored due to the short course) but follow up urine
samples 9 days after completing the flucytosine course were still positive for C.
glabrata resistant to both fluconazole (MIC >256mg/L) and flucytosine (MIC >64mg/L).
Approximately 2 weeks after completing the course of flucytosine, the patient was
admitted via Accident and Emergency with suspected sepsis relating to a biliary source,
and a new ultrasound showed that the left kidney was moderately hydronephrotic, the
bladder was filled with echogenic debris. A nephrostomy was performed and blood cultures
taken which grew C. glabrata with identical sensitivities to that from the most recent
urine sample. The patient was prescribed a 2 week course of Anidulafungin (200mg loading
dose then 100mg 24-hourly IV) to combat the systemic candidosis, and a 7 day course
of intravenous Amphotericin deoxycholate at 0.6mg/kg/day. During the Amphotericin
B deoxycholarte treatment, renal function was not markedly affected, though increases
in alkaline phosphatase levels led to the treatment being stopped at 6 days. Subsequent
blood and urine cultures remained negative, CRP declined from 150 to 5.8 mg/L and
symptoms resolved. This case is one of the examples of how the significance of the
presence of yeasts in urine of patients is not clearly understood by the clinicians.
Our patient presented here highlights the increasing prevalence of C.glabrata in funguria
and non-albicans candidaemia in at risk settings, the challenges in its management,
the risks and drug related factors which need to be assessed when treating fluconazole
resistant urinary tract candidosis.
Aneke
C.
Rhimi
W.
Otranto
D.
Cafarchia
C.
Veterinary Medicine, University of Bari, Aldo Moro, Bari, Italy
P010. Effects of efflux pump modulators on the azole antifungal susceptibility of
Microsporum canis
Objectives: This study investigated the effect of efflux pump modulators, EPMs (i.e.
haloperidol-HAL and promethazine-PTZ) and their inhibiting activity on the minimum
inhibitory concentrations of itraconazole (ITZ) and fluconazole (FLZ), against selected
M. canis strains.
Methods:
M. canis strains with low (≤ 1mg/ml itraconazole and <64mg/ml fluconazole) and high
(>1mg/ml itraconazole and ≥64 μg/ml fluconazole) azole MIC values were tested, and
data analyzed using the model-fractional inhibitory concentration index (FICI) method.
Results: The MIC values of ITZ and FLZ of M. canis decreased in the presence of sub-inhibitory
concentrations of HAL and PTZ, the latter being more effective with a greater increased
susceptibility. Synergism was observed in all strains with high azole MICs (FICI<0.5)
and no synergism in the strains with low azole MICs.
Conclusion: These results suggest that the drug efflux pumps are involved in the defense
mechanisms to azole drugs in M. canis strains. The synergism might be related to an
increased expression of efflux pump genes, eventually resulting in azole resistance
phenomena. Additionally, the most effective double combinations were those of PTZ
with either FLZ or ITZ as this had a greater increase in azole susceptibility. Complementary
studies on M. canis resistance are advocated in order to investigate the molecular
mechanisms of this phenomenon.
Mgbeahuruike
A.
Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine,
University of Nigeria, Nsukka, Nsukka, Enugu, Nigeria
P011. Antifungal resistance pattern of Candida species isolated from women of child-bearing
age in Nsukka Nigeria suffering from different clinical conditions.
Objectives: To isolate and identify Candida species in child bearing women suffering
from malaria, typhoid and diabetes To investigate the resistance partarn of the identified
Candida species using conventional azoles and plant extracts To trace the evolutionary
relatedness of the isolated fungi using phylogenetic analysis
Methods: High vaginal swabs were analyzed for Candida presence in women of child bearing
age suffering from malaria, typhoid and diabetes using Chromogenic agar. Evolutionary
relatedness of the Candida species was inferred using phylogenetic reconstruction.
Growth rate assay was done using Sabourand Dextrose medium supplemented with NaCl
and glucose. The resistance pattern of the Candida species was tested using fluconazole
and extracts (methanol and ethyl) from Byrsocarpus coccineus.
Results: Out of the 79 fungal samples analyzed, 21 (26.6%) were C. tropicalis, 25
(31.6%) were C. krusei, 10 (12.7%) were C. parapsilosis and 8 (10.1%) were C. albicans.
C. tropicalis was highest in the malaria patients, C. albicans dominated in the typhoid
patients while C. krusei was more in the diabetic patients. The healthy women presented
a very low percentage (8.9%) of Candida species. Phylogenetically, the sequenced Candida
species was more closely related to C. tropicalis. High concentration of glucose (1g/ml)
and NaCl (0.5M) decreased the growth of the fungus. From the study, fluconazole showed
a higher activity than the two extracts (methanol and ethyl acetate).
Conclusion: In conclusion, although fluconazole showed a higher activity than the
two extracts (methanol and ethyl acetate), the plant extracts demonstrated very high
activity and can be used as potential antifungal agents. C. tropicalis and C. krusei
were the most abundant Candida species found in this study.
Schwarz
P.
1
2
Dannaoui
E.
3
4
1Department of Internal Medicine - Respiratory and Critical Care Medicine, University
Hospital Marburg, Marburg, Germany
2Center For Invasive Mycoses and Antifungals, Philipps University Marburg, Marburg,
Germany
3Hôpital Européen Georges Pompidou, Laboratoire de Parasitologie-Mycologie, Paris,
France
4Working Group Dynamyc, Faculté de Médecine, Hôpital Henri Mondor, Créteil, France
P012. Colistin and isavuconazole interact synergistically in vitro against Aspergillus
nidulans and Aspergillus niger
Objectives: Invasive aspergillosis is a devastating disease in immunocompromised patients.
It mostly affects patients with hematological malignancies, especially those with
severe and prolonged neutropenia. Colistin is a polypeptide antibiotic from the class
of polymyxins. Polymyxins bind to lipopolysaccharides and phospholipids of the outer
cell membrane of gram-negative bacteria. Binding leads to competitive displacement
of divalent cations from the phosphate groups of the membrane lipids, leading to bactericidal
action by disruption of the outer cell membrane and leakage of intracellular contents.
Colistin has also shown in vitro activity against some fungi. The aim of the present
study was to assess the in vitro interaction of the broad-spectrum azole isavuconazole
with colistin against the most important Aspergillus species responsible for human
disease.
Methods: A panel of 31 Aspergillus isolates belonging to 5 species responsible for
human invasive aspergillosis was used for the experiments (10 Aspergillus fumigatus,
5 Aspergillus flavus, 5 Aspergillus terreus, 6 Aspergillus nidulans, and 5 Aspergillus
niger). Combinations of isavuconazole with colistin were tested by a broth microdilution
checkerboard technique based on the EUCAST reference methodology. Plates were read
spectrophotometrically, and ninety percent of inhibition was used as an endpoint for
both, the drugs alone and in combination. Results were interpreted with the fractional
inhibitory concentration index (FICI). Drug interactions were defined as synergistic
(FICI≤0.5), indifferent (FICI>0.5≤4) or antagonistic (FICI>4).
Results: Isavuconazole MICs ranges from 0.5 to 16 µg/ml. When tested alone, colistin
exhibited no antifungal activity with MICs of 128 µg/ml for all isolates. Combination
of isavuconazole with colistin was synergistic for 100 and 60% of the A. nidulans,
and A. niger isolates, respectively (Table). All other interactions were indifferent.
Antagonism was never seen.
Conclusion: Combination of colistin with isavuconazole showed strong synergy against
all tested A. nidulans isolates and synergy for the majority of the tested A.niger
isolates. In vitro interaction of colistin and isavuconazole will be further evaluated
by checkerboard and gradient concentration strips against a larger panel of A. nidulans
and A.niger isolates.
Risum
M.
1
Datcu
R.
1
Johansen
H.K.
2
Schønheyder
H.C.
3
Rosenvinge
F.S.
4
Knudsen
I.J.D.
5
Røder
B.L.
6
Antsupova
V.S.
7
Gertsen
J.B.
8
Kristensen
L.
8
Møller
J.K.
9
Hare
R.
1
Astvad
K.M.T.
1
Jørgensen
K.M.
1
Dzajic
E.
10
Arendrup
M.C.
1
2
11
1Mycology Unit, Statens Serum Institute, Copenhagen, Denmark
2Department of Clinical Microbiology, Rigshospitalet, Copenhagen, Denmark
3Department of Clinical Microbiology, Aalborg University Hospital, Aalborg, Denmark
4Department of Clinical Microbiology, Odense University Hospital, Odense, Denmark
5Department of Clinical Microbiology, Hvidovre Hospital, Hvidovre, Denmark
6Department of Clinical Microbiology, Slagelse Sygehus, Slagelse, Denmark
7Department of Clinical Microbiology, Herlev Hospital, Herlev, Denmark
8Department of Clinical Microbiology, Aarhus University Hospital, Aarhus, Denmark
9Department of Clinical Microbiology, Vejle Sygehus, Vejle, Denmark
10Department of Clinical Microbiology, Esbjerg Sygehus, Esbjerg, Denmark
11University of Copenhagen, Dept. of Clinical Medicine, Copenhagen, Denmark
P013. Update 2016-2018 of the nationwide Danish fungaemia surveillance study: epidemiologic
changes in a 15-year perspective
Objectives: The objectives of this study was to provide nationwide contemporary data
on the epidemiology of candidaemia, often a serious complication of hospital stay
with a crude 30-day mortality rate of about 40% despite recent advantages in diagnosis
and management. The motivation was that outcome depend on early appropriate therapy
necessitating knowledge on susceptibility pattern of involved species. Moreover, epidemiological
changes reflect current practises in prophylactic, empirical and pre-emptive use of
antifungals and therefore are also informative regarding the need for adjustment of
antifungal stewardship programmes or treatment guidelines.
Methods: As part of an active national surveillance programme, fungal isolates from
blood culture were referred to Statens Serum Institut (Copenhagen) for confirmatory
species identification and EUCAST E.Def 7.3.1 susceptibility testing. A total of 1452
unique isolates (same patient and species isolates were regarded unique if more than
21 days apart or with different susceptibility) were included. 13 isolates (0.9%)
were not referred. When MICs for echinocandins were above the clinical breakpoints,
FKS sequencing of the isolates was performed. An isolate was deemed echinocandin resistant
if the MIC was above anidulafungin breakpoint and/or if the isolate had a known FKS
hotspot-mutation. Results were compared to previous data in a 15-year perspective.
Results: The mean incidence rate during 2016-2018 was 8.13/100.000 inhabitants. Candidaemia
was more frequent in males than females (60% vs 40%) as in accordance with previous
years. In comparison to previous data, the fungaemia rate was stable (8.64, 9.03 and
8.38 per 100.000 inhabitants in 2004-7, 2008-11 and 2012-15 respectively). In the
years 2016-2018 C. albicans accounted for 42.1% of all unique isolates with a decline
in 2017 to below 40%. C. glabrata accounted for 32.1%. The proportion of candidaemia
in 2018 involving C. albicans decreased by a third from 64% in 2004 (P<0.0001), and
C. glabrata almost doubled from 17% in 2004 (P<0.0001) (Figure). We found a fluconazole
susceptibility rate across all Candida species of 59% of isolates which has decreased
since 2004-7 from 69% (P = 0.02). Echinocandin resistance was detected in 18/1294
isolates (1.4%) including 11/459 (2.4%) in C. glabrata specifically. No echinocandin
resistance was detected in 2004-7. FKS-mutations were identified in the following
species C. glabrata: two L630Q/S663F, four F659S, one L662W, three S663F and one S663P;
C. krusei: four L701M, two S659F and one S659S/F; C. albicans one P1354P/S; C. dubliniensis
one S645P; and for Candida lusitaniae one H657Y/L1243F/I283V. H657Y/L1243F/I283V,
P1354P/S and L701M are outside the hot spot, and L701M is not associated as a molecular
cause of echinocandin resistance.
Conclusion: We report a stable incidence rate of candidaemia, but the proportion of
C. glabrata continues to rise at the expense of C. albicans which accounted for less
than 40% in 2017. The consequent decrease in fluconazole susceptibility along with
the presence of FKS-mutations particularly in C. glabrata raises concern for future
perspectives in the treatment of candidaemia.
Shahandashti
R. Vahedi
Division of Hygiene and Medical Microbiology, Medical University of Innsbruck, Innsbruck,
Austria
P014. In vitro potpourri: Aspergillus terreus and the activity of amphotericin B
Objectives: Invasive aspergillosis caused by intrinsically resistant non-fumigatus
Aspergillus species displays a poor outcome in immunocompromised patients. Aspergillus
terreus holds an exceptional position within the aspergilli due to its intrinsic resistance
against the polyene amphotericin B (AmB). Till now, the exact mode of action in polyene
resistance is not well understood. Micro-environmental factors at the infection site
influence the growth of fungal pathogens and most likely also the efficacy of antifungal
drugs. The purpose of this study was to evaluate the impact of hypoxia versus normoxia
on the in vitro activity (fungistatic and fungicidal) of AmB against A. terreus.
Methods: In total, 100 clinical strains of Aspergillus section terrei, collected from
21 various countries were analyzed in detail. Antifungal susceptibility testing was
carried out according to EUCAST guideline, with normoxic (20% oxygen/5% carbon dioxide/75%
nitrogen) and hypoxic (1% oxygen/5% carbon dioxide/94% nitrogen) conditions. Final
drug concentrations used for AMB were 0.03–16 μg/ml. Minimum inhibitory concentration
(MIC) was defined as the lowest drug concentration showing no fungal growth and minimum
fungicidal concentrations (MFCs) was taken as the first well with ≥90% killing of
fungi. MFC was determined by agar and broth-based (flash microbicide method) methods.
MICs (μg/ml) and colony forming units were determined after 48h at 37°C. Testing was
performed in duplicates.
Results: AmB MICs of A. terreus section terrei ranged from 0.125-2 µg/ml for hypoxia,
and from 0.5-8 µg/ml for normoxia. These data indicate that some strains were more
susceptible to AmB in hypoxic conditions compared to normoxia (Figure 1). The majority
of strains showed that AmB had no fungicidal effect against tested A. terreus under
any methods and atmospheric conditions (Figure 2). A. terreus representing lower MICs
(n = 41, ≤0.5 µg/ml) did not result in modest MFCs (≥90% killing in 1-2 µg/ml).
Figure 1. In vitro susceptibility to AMB of 100 A. terreus isolates in normoxia versus
hypoxia, measured by EUCAST.
Figure 2. The effect of oxygen on MFC distributions of AMB, agar-based method measured
by colony forming units.
Conclusion: Simulating the host environment in in vitro susceptibility testing will
contribute to a better understanding of how these conditions influence antifungal
activity. Hypoxic conditions had a marginal influence on the in vitro antifungal susceptibility
pattern of A. terreus species to AmB. A slight reduction of MICs was demonstrated,
however, A. terreus did not turn to be classified as AmB susceptible (AmB MICs < 0.125
µg/ml) under hypoxic conditions. Remarkably, AmB lacked any fungicidal activity in
the majority of examined strains; comparing with MICs, MFC seems to be related more
with in vivo outcome and might partially explain the high failure rate of AmB therapy
in vivo.
Stauf
R.
1
Todt
D.
2
Steinmann
E.
2
Rath
P.-M.
3
Gabriel
H.
4
Steinmann
J.
5
Brill
F.H.H.
4
1Institute For Clinical Hygiene, Medical Microbiology and Infectiology, Paracelsus
Medical University Nuremberg, Nuremberg, Germany
2Department For Molecular and Medical Virology, Ruhr-University Bochum, Bochum, Germany
3Institute of Medical Microbiology, University Hospital Essen, Essen, Germany
4Institute For Hygiene and Microbiology, Dr. Brill + Partner GmbH, Hamburg, Germany
5Institute of Clinical Hygiene, Medical Microbiology and Clinical Infectiology, Klinikum
Nuernberg, Nuremberg, Germany
P015. In vitro activity of active ingredients of disinfectants against drug-resistant
fungi
Objectives: Prevention of immunosuppressed patients against fungal pathogens is essential
in times of emergence of antifungal-resistant yeasts and moulds. Therefore, the use
of fungicidal disinfectants is an important measure preventing infections. In this
study, the fungicidal activity of common microbial agents of chemical disinfectants
against antifungal-resistant and antifungal-susceptible yeasts and moulds was tested
in comparison to the reference strains Candida albicans, Candida tropicalis, Aspergillus
brasiliensis and Aspergillus niger.
Methods: In total, nine clinical isolates from the fungal collection of the University
Hospital Essen and four reference strains were included. Species identification was
performed by morphological methods and sequencing of the internal transcribed spacer
region (ITS). The antifungal susceptibility testing was performed via microdilution
assay according to EUCAST standard 9.3 for moulds and 7.3.1 for yeasts. The sensitivity
of clinical yeasts and moulds isolates in comparison to the reference isolates was
tested in a quantitative suspension test against peracetic acid and ethanol as the
common active ingredients of chemical disinfectants.
Results: Ethanol was sufficiently active with a log10 reduction factor of ≥ 4.0 against
all yeasts in a concentration of 50 % v/v within 1 min and against all moulds as 80
% solution within 1 min. Peracetic acid was active as 0.25 % solution in 5 min against
all yeasts and as 0.5 % solution in 5 min against all moulds. Compared to the reference
strains the clinical isolates showed similar or higher sensitivity to the active ingredients.
In addition, antifungal-resistant strains exhibited equivalent sensitivity in vitro
against ethanol and peracetic acid compared to the susceptible ones.
Conclusion: Clinical fungal isolates including multidrug resistant ones did show a
similar or higher sensitivity to active ingredients of disinfectants than the reference
strains.
Buil
J.
1
Oliver
J.
2
Birch
M.
2
Law
D.
2
Rex
J.
2
Tehupeiory-Kooreman
M.
1
Lee
H. Van Der
1
Melchers
W.
1
Verweij
P.E.
1
1Medical Microbiology, Radboudumc, Nijmegen, Netherlands
2F2G Ltd, Manchester, United Kingdom
P016. In vitro susceptibility of olorofim against 1,682 clinical Aspergillus isolates
Objectives: Olorofim (OLO) is a novel antifungal agent with potent activity against
filamentous fungi such as Aspergillus spp., Scedosporium spp. and Lomentospora prolificans.
We included OLO in our routine MIC-panel for filamentous fungi and analysed the in
vitro susceptibility of OLO against clinical Aspergillus isolates received in our
laboratory between May 2017 and March 2019.
Methods: The in vitro activity of OLO was tested against 1,682 clinical Aspergillus
isolates (987 azole susceptible A. fumigatus isolates, 414 azole-resistant A. fumigatus,
72 A. flavus species complex (SC), 32 A. nidulans SC, 110 A niger SC, 21 A. terreus
SC, 7 A. versicolor SC, 39 other species). OLO susceptibility was determined using
broth microdilution based on EUCAST guidelines. Isolates were identified to the SC
level using microscopy and growth characteristics.
Results: The olorofim MICs of all clinical A. fumigatus, A. flavus species complex,
A. terreus species complex, A. niger species complex, A. versicolor species complex
were found to be uniformly <1 mg/L. The MIC50 and MIC90 of species with >10 different
isolates are displayed in the table. No significant differences were found in the
OLO MICs of azole-susceptible A. fumigatus isolates compared to azole-resistant A.
fumigatus isolates. Isolates of some cryptic species within the Aspergillus aspergillus
species complex (A. hollandicus, n = 1 A. glaucus, n = 4 and A. chevalieri n = 1)
were found to have elevated MIC’s: 1 isolate (A. glaucus) with a MIC of 1 mg/L and
the remainder with a MIC of >2 g/L).
Conclusion: OLO showed potent in vitro activity against a large collection of clinical
Aspergillus species, including 1401 A. fumigatus isolates, with many of these resistant
to one or more azoles. Interestingly, OLO was less potent against a small number of
isolates belonging to the Aspergillus aspergillus SC (formerly Eurotium).
Delavy
M.
Corthesy
M.
Greub
G.
Sanglard
D.
Coste
A.T.
Microbiology Institute, University Hospital Lausanne, University of Lausanne, Lausanne,
Switzerland
P017. Detection and Typing of Fluconazole Resistance on Candida albicans with Matrix-Assisted
Laser Desorption/Ionization Time-of-Flight Mass
Objectives:
Candida albicans causes life-threatening systemic infections in immunosuppressed patients.
These infections are commonly treated with fluconazole, an antifungal agent targeting
the ergosterol biosynthesis pathway. Current Antifungal Susceptibility Testing (AFST)
methods take time and are often subjective. An alternative to the classical AFST methods
could use Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) Mass
spectrometry (MS). This tool, already used in clinical microbiology for microbial
species identification, has already offered promising results to detect antifungal
resistance and could be used to detect specific peaks associated with fluconazole
resistance.
Methods: We analyzed MALDI-TOF MS spectra acquired, with the Bruker microflex device,
from 21 C. albicans clinical strains isolated from 9 patients. Those strains were
exposed for 3h to 3 fluconazole concentrations (256, 16, 0μg/mL). We optimized a protein
extraction protocol that allowed the acquisition of high-quality spectra. Two steps
of quality controls were implemented. First, the quality of the spectrum was assessed
through its identification score. Second, the variability of the biological and technical
replicates was evaluated, and outliers were removed. We then developed an R pipeline
to identify MALDI-TOF MS signature peaks associated with fluconazole resistance or
with TAC1 or ERG11 mutations known to affect C. albicans susceptibility level, and
we designed score-based diagnostic tests with these signature peaks. Finally, we evaluated
the effects of adding a protein standard (Bacterial Testing Standard, BTS, Bruker)
to the proteins extracts to reduce the variability of the peaks position. and then
we tested the effect of the tolerance inhibitor cyclosporin A on the susceptible strains’
spectra quality.
Results: Up to now, in absence of cyclosporin A or BTS, we identified a 4480 m/z peak,
whose intensity after exposition to 16 μg/mL fluconazole, was inversely proportional
correlated with fluconazole resistance. The intensity of the peak seems to be more
inversely proportional correlated with TAC1 gain-of-function mutation than with ERG11
mutations. Since only a single peak was identified, it is likely that the trailing
effect of fluconazole might hide other interesting peaks. We thus decide to inhibit
fluconazole tolerance using cyclosporin A in further experiments. In addition, the
main challenge of this first analysis was to reproducibly align the spectra. To circumvent
this issue, we decided to introduce a protein standard (BTS) to the fungal proteins
extracts to be analyzed. Effects of BTS or cyclosporin A are currently under investigation.
Conclusion: This study acts as a proof-of-concept for the use of MALDI-TOF MS for
the typing of fluconazole resistance in C. albicans and currently offer promising
results.
Risum
M.
1
Hare
R.
1
Rosenvinge
F.S.
2
Gertsen
J.B.
3
Kristensen
L.
3
Bangsborg
J.
4
Røder
B.L.
5
Marmolin
E.S.
6
Sulim
S.
7
Astvad
K.M.T.
8
Pedersen
M.
8
Datcu
R.
1
Arendrup
M.C.
1
9
10
1Mycology Unit, Statens Serum Institute, Copenhagen, Denmark
2Department of Clinical Microbiology, Odense University Hospital, Odense, Denmark
3Department of Clinical Microbiology, Århus University Hospital, Århus, Denmark
4Department of Clinical Microbiology, Herlev Hospital, Herlev, Denmark
5Department of Clinical Microbiology, Slagelse Sygehus, Slagelse, Denmark
6Department of Clinical Microbiology, Vejle Sygehus, Vejle, Denmark
7Department of Clinical Microbiology, Ålborg University Hospital, Ålborg, Denmark
8Department of Clinical Microbiology, Hvidovre Hospital, Hvidovre, Denmark
9University of Copenhagen, Dept. of Clinical Medicine, Copenhagen, Denmark
10Department Og Clinical Microbiology, Rigshospitalet, Copenhagen, Denmark
P018. Azole resistance in Aspergillus. The first six months data from the Danish nationwide
surveillance study
Objectives: Azole resistance (azole-R) in Aspergillus has been increasingly reported
worldwide. The use of azole fungicides has been proposed to contribute to the emergence
of azole-R A. fumigatus, and isolates harbouring the resistance mechanisms TR34/L98H
or TR46/Y121F/T289A have been dominant among azole-R A. fumigatus in azole naïve patients
and environmental samples. These two resistance genotypes have been sporadically found
in clinical and environmental samples in Denmark, but the size of the problem remains
unknown. The purpose of this study was to establish a nationwide surveillance programme
of azole-R Aspergillus in Denmark and to report the data from the first six month’s
study period.
Methods: Unique Aspergillus isolates were included from all Danish clinical microbiological
departments in the period October-2018 to March-2019. Isolates from same patients
were defined as unique if 1) found >30 days apart, 2) confirmation of another species
or 3) different susceptibility pattern. Inclusion criteria were: a) Aspergillus isolates
regarded clinically significant, or b) any Aspergillus isolate detected on a Monday
regardless of clinical significance. Referral practices varied depending on local
mycology skills. Most laboratories referred all moulds or all Aspergillus isolates
which then underwent species ID and susceptibility testing at the reference lab (SSI).
One laboratory (Aarhus University Hospital) reported the species identification for
Aspergillus (n = 214) and only referred part of the isolates. Thus susceptibility
was performed for 46/138 A. fumigatus isolates from AUH at the time of writing. Referred
Aspergillus isolates were identified to the complex level except A. fumigatus which
was identified sensu stricto using thermotolerance testing. A. fumigatus isolates
underwent screening for azole-R following the EUCAST E.Def 10.1 method and using VIPcheck
azole agar plates (Mediaproducts BV, Grönningen, NL). Screening positive A. fumigatus
isolates and all non-fumigatus Aspergillus isolates underwent EUCAST E.Def 9.3.1 susceptibility
testing. Isolates were deemed intermediate when classified as such for >1 azole. Isolates
with azole MIC(s) above the EUCAST BP(s) underwent CYP51A sequencing. Duplicates within
30 days were excluded.
Results: A total of 695 Aspergillus isolates were included of which 503 were A. fumigatus.
Susceptibility testing was performed for 411 A. fumigatus isolates from 319 patients
at time of writing (Table 1). For A. fumigatus, 92.7% isolates were azole susceptible,
1.7% intermediate and 5.6% azole-R. Nineteen out of 319 patients harboured an A. fumigatus
which was azole-R (6%). Among these 18/19 (95%) harboured an isolate with a CYP51A
mutation (Table 2), and 14/19 (73.7%) a CYP51A mutation derived from the environment.
Furthermore, 2/9 patients harboured three azole resistant A. terreus isolates, each
with a CYP51A mutation (Table 2).
Conclusion: We report a nationwide azole-R rate of 6% in A. fumigatus at the patient
level. The underlying resistance mechanisms was target gene mutations in all but one
case and notably, the vast majority were of environmental origin linked to the use
of azole fungicides. The fact that such isolates have increasingly been found in Denmark
since 2009 is concerning and suggests that a one-health approach involving human and
environmental azole management is necessary to limit further rise in azole-R A. fumigatus
Salse
M.
1
Gangneux
J.P.
2
Cassaing
S.
3
Delhaes
L.
4
Fekkar
A.
5
Dupont
D.
6
Botterel
F.
7
Costa
D.
8
Bourgeois
N.
9
Bouteille
B.
10
Houzé
S.
11
Dannaoui
E.
12
Guegan
H.
2
Charpentier
E.
3
Persat
F.
13
Favennec
L.
8
Lachaud
L.
9
Sasso
M.
1
1Laboratoire De Microbiologie, CHU Nîmes, Nîmes, France
2Laboratoire De Parasitologie-mycologie, CHU de Rennes, Rennes, France
3
Jean-pierre.gangneux@chu-rennes.fr, CHU de Toulouse, Toulouse, France
4Laboratoire De Parasitologie-mycologie, CHU de Bordeaux, Bordeaux, France
5Laboratoire De Parasitologie-mycologie, APHP La Pitié Salpétrière, PARIS, France
6Laboratoire De Parasitologie-mycologie, Hospices civils de Lyon, Lyon, France
7Laboratoire De Parasitologie-mycologie, APHP Hôpital Henri Mondor, Créteil, France
8Laboratoire De Parasitologie-mycologie, CHU de Rouen, Rouen, France
9Laboratoire De Parasitologie-mycologie, CHU de Montpellier, Montpellier, France
10Laboratoire De Parasitologie-mycologie, CHU de Limoges, Limoges, France
11Laboratoire De Parasitologie-mycologie, APHP Hôpital Bichat, Paris, France
12Laboratoire De Parasitologie-mycologie, APHP Hôpital Pompidou, Paris, France
13Parasitologie Mycologie Médicale, Hospices Civils de Lyon, Lyon, France
P019. Multicentre study to determine the Etest® epidemiological cut-off values of
antifungal drugs in Candida spp. and Aspergillus fumigatus SC
Objectives: To determine the specific Etest®-based epidemiological cut-off values
(ECVs) for the main antifungal agents used in clinical practice, against the most
frequent Candida spp. and Aspergillus fumigatus species complex (SC), in order to
harmonise the interpretation of the Etest® minimum inhibitory concentrations (MICs)
results in daily routine.
Methods: For each antifungal agent, the Etest® MICs in Candida spp. and A. fumigatus
SC isolated from routine specimen cultures were retrospectively collected from 2004
to 2018 from the laboratories of 12 French university hospitals. To ensure that robust
and comparable data were included for the ECV estimation, basic requirements and criteria
have been fulfilled during the selection of MIC data: aberrant individual distributions
were excluded, MIC data were pooled only if there are generated by at least three
independent laboratories, and a minimum of 100 MIC values were required after data
pooling for each species-antifungal agent combinations. The ECVs were then calculated
using the iterative statistical method with a 97.5% cut-off.
Results: Forty-eight Etest® ECVs were determined for amphotericin B, caspofungin,
micafungin, anidulafungin, fluconazole, voriconazole, posaconazole and itraconazole,
after pooling and analysing the MICs of 9,654 Candida albicans, 2,939 Candida glabrata
SC, 1,458 Candida parapsilosis SC, 1,148 Candida tropicalis, 575 Candida krusei, 518
Candida kefyr, 241 Candida lusitaniae, 131 Candida guilliermondii, and 1,526 A. fumigatus
SC isolates. The ECV results were identical (17/32 ECVs) or within one two-fold dilution
(15/32 ECVs) to the previously reported Etest®-based ECVs. They were comparable to
the Clinical and Laboratory Standards Institute (CLSI) ECVs in 76.1% (similar or within
one two-fold dilution: 35/46 ECVs) and strictly identical in 39.1% of cases (18/46
ECVs). They were comparable to the European Committee on Antimicrobial Susceptibility
Testing (EUCAST) ECVs in 81.2% of cases (similar or within one two-fold dilution:
26/32 ECVs) and strictly identical in 28% (9/32 ECVs).
Conclusion: On the basis of these and other previous results, we recommend the determination
of method-dependent ECVs. As Etest® is the most widely used commercial method in French
laboratories, the goal of this study was to calculate specific ECVs for this method
and harmonise the interpretation of the Etest® MICs results in daily routine. Etest®
ECVs should not be used in place of breakpoints but may identify non-WT isolates with
a potential resistance to antifungal agents and suggest that an isolate may not respond
as expected to the standard treatment.
Grela
E.
1
Piet
M.
2
Luchowski
R.
1
Grudzinski
W.
1
Paduch
R.
2
Gruszecki
W.
1
1Biophysics, Maria Curie-Sklodowska University, Lublin, Poland
2Virology and Immunology, Maria Curie-Sklodowska University, Lublin, Poland
P020. Human cell cultures exposed to antibiotic amphotericin B: toxicity and defence
Objectives: Amphotericin B (AmB) is a polyene antifungal antibiotic, which is synthetized
by the bacteria Streptomyces nodosus. AmB is a “gold standard” in treatment of systemic
fungal infections, due to its broad spectrum of action, high effectiveness and rare
pathogens resistant to this drug. Unfortunately due to the low selectivity of the
action of this antibiotic, it is very toxic for patients. Although, the exact mechanism
of action of AmB is still unknown, it is used as a lifesaving medicine. Understanding
the mechanism of action of AmB would enable to minimize its toxic side effects, and
possibly increase its therapeutic effects. The aim of this study was to get to know
the toxicity mechanism of action of AmB.
Methods: To understand molecular mechanism underlying the toxicity of AmB, human normal
colon epithelial cells CCD 841 CoTr (ATCC No. CRL-1807) and human colon adenocarcinoma
cells HT-29 (ATCC No HTB-38) were exposed to the drug and were imaged using Fluorescence
Lifetime Imaging Microscopy (FLIM) and Raman scattering spectroscopy. Cultures were
incubated for 24 h with AmB. Fluorescence lifetime imaging was carried out on MicroTime
200 (Picoquant GmbH, Germany) linked with Olympus IX71 inverted microscope. The cell
samples were excited by 405 nm pulsed laser. Fluorescence lifetimes and fluorescence
anisotropy values were analyzed. Raman spectral analysis and imaging on a microscale
was carried out with inVia Reflex confocal Raman microscope (Renishaw, UK) with Cobolt
o8-NLD 405 nm laser (power at a sample 0.2 mW). Obtained results were analyzed by
DCLS spectral deconvolution.
Results: The results confirmed high toxicity of antibiotic AmB for both human cell
lines but lower for HT-29 cells. The results show that AmB incorporates to the lipid
membrane containing cholesterol. The cell defense mechanism against the action of
antibiotic was also observed, which is based on removing AmB from the membrane in
the form of sterol-deficient exosomes.
Conclusion: The lower toxicity of AmB observed for HT-29 cells, may be associated
with high proliferation rate or different membrane composition of adenocarcinoma cells
compared to CCD 841 CoTr cells. AmB binds to the bilayer in the form of small aggregates,
which can cause uncontrolled ion-leakage and lead to the cell death.
Bellet
V.
1
Roger
F.
1
Gouveia
T.
1
Drakulovski
P.
1
Kassi
F.
2
Menan
H.
2
Krasteva
D.
1
Bertout
S.
1
1Laboratoire De Parasitologie Et Mycologie Médicale Umi 233, University of Montpellier,
Montpellier, France
2CEDRES CHU de Treichville, Abidjan, Côte d’Ivoire
P021. African ST173 Cryptococcus deuterogattii strains are commonly less susceptible
to fluconazole: a tricky mechanism of resistance
Objectives: Fluconazole, alone or in association, is often administered during cryptococcosis
treatment especially in Sub-Saharan Africa. Its intensive use leads to the emergence
of resistant strains. The mechanisms underlying resistance are poorly documented for
yeasts belonging to the C. gattii species complex. Literature suggests that resistance
could be due to mutations and/or surexpression of ERG11 gene and overexpression of
efflux pumps, like MDR or AFR.
Methods: In Ivory Coast, we highlighted the presence of strains with a rare subtype
in the molecular type VGII associated with high minimum inhibitory concentrations
to FCZ compared to VGII strains originating from the Pacific Northwest. We investigated
the mechanisms of fluconazole resistance of 18 ivorian clinical strains of C. deuterogattii
with high MIC to FCZ compared to 10 strains with low MIC to FCZ.
Results: We demonstrated that (i) these strains exhibited no mutation in the ERG11
gene, (ii) some strains had an increased in ERG11 and MDR1 mRNA expression while AFR1
and AFR2 were not overexpressed in high MIC to FCZ strains versus the low MIC to FCZ
strains and (iii) exposure to FCZ in strains with high MIC to FCZ induced AFR1 mRNA
overexpression.
Conclusion: We demonstrated that the FCZ-resistant mechanism commonly described in
Cryptococcus neoformans or in Cryptococcus gattii from the Pacific Northwest were
not responsible for the resistance to FCZ in this rare sub-type strains.
Frenkel
M.
1
Yunik
Y.
1
Segal
E.
2
1Tel Aviv University, Tel Aviv, Israel
2Sackler School of Medicine, Clinical Microbiology and Immunology, Tel Aviv University,
Tel Aviv, Israel
P022. Novel Technique to Measure Susceptibility to Lipid-Associated Antifungals
Objectives: Lipid associated antifungals, such as polyenes, are extensively used for
therapy in patients with invasive fungal infections. Thus, susceptibility of fungi
to the lipid- associated antifungals is of importance. The most reliable method used
in susceptibility testing is the broth micro-dilution technique. However, visual-
or spectral- assessment of the antifungal activity is difficult, and possibly unreliable,
in lipid-associated drugs, due to turbidity caused by the lipid. Hence, the objective
of this study was to adapt a method for spectral measurement of susceptibility of
fungi to lipid-associated antifungal drugs.
Methods: The technique was adapted for Amphotericin B- Intralipid (AMB-IL) and Nystatin-
Intralipid (NYT-IL) in comparison to AMB and NYT. Drug preparation: AMB-IL was prepared
by adding to 5 mg/ml of AMB 20% Intralipid in DDW to a final concentration of 0.2
mg/ml AMB and shaken at 280 rpm for 18 h at 240 C. Intralipid in DDW was used as control.
NYT-IL was prepared similarly, by adding to 20 mg/ml nystatin 20% Intralipid in DMSO
to a final concentration of 0.8 mg/mL NYT and shaken at 280 rpm for 18 h at 240 C.
Intralipid in DMSO was used as control. Susceptibility test: Susceptibility of the
Candida albicans strains to AMB, NYT, AMB-IL, and NYT-IL was assessed by the micro-broth
dilution assay using micro-titer plates. Susceptibility was evaluated using spectral
readings in an EMax+ Microplate Reader at 560nm and 650nm, for the standard (AMB,
NYT) and experimental Intralipid-associated polyene formulations (AMB-IL, NYT-IL),
respectively. For AMB-IL or for NYT-IL the drug solutions and the relevant controls
were diluted in RPMI. Each well contained the same concentration of RPMI, IL, DDW
or DMSO, and serial dilutions of the antifungal drugs (AMB, NYT).
Results: The validity of the test was evaluated by testing susceptibility of 40 C.
albicans isolates: 20 from human mucosal infections (M strains) and 20 from candidemia
patients (S strains) vs. a control strain C. albicans CBS-562, for which susceptibility
data were available. We determined 50, 90 & 100% MIC values by spectral readings (see
an example of tests with the control stain – CBS 562–Figure 1). The spectral readings
were compared to visual readings of the micro-titer plates using an inverted microscope.
In addition, minimal fungicidal concentration (MFC) for each strain was determined
(Figure 2).
Figure 1: Spectral readings of Control strain –CBS 562 treated with AMB-IL & NYT-IL.
Figure 2: Spectral readings vs. Visual assessment of M and S Candida albicans strains.
Conclusion: This study presents a spectral method for measuring susceptibility of
yeasts to lipid associated polyenes which deals with the difficulties of visual assessment
of susceptibility to lipid associated antifungals.
Ballard
E.
1
Yucel
R.
2
Melchers
W.
3
Brown
A.
1
Verweij
P.E.
3
Warris
A.
1
1Mrc Centre For Medical Mycology, University of Aberdeen, Aberdeen, United Kingdom
2Ifcc, University of Aberdeen, Aberdeen, United Kingdom
3Medical Microbiology, RadboudUMC, Nijmegen, Netherlands
P023. Antifungal activity of antimicrobial peptides against clinical and environmental
Aspergillus fumigatus isolates.
Objectives: Antimicrobial peptides (AMPs) are a key defence against invading microorganisms.
The activity of AMPs against human fungal pathogen Aspergillus fumigatus remains poorly
understood. We characterised the anti-Aspergillus activity of specific human AMPs
and determined whether in-host adaption could lead to resistance to specific AMPs.
Methods: The antifungal activity of lysozyme, histones, lactoferrin, β-defensin-1
and LL-37, was tested against 15 clinical isolates (from patients with cystic fibrosis,
chronic and acute invasive aspergillosis), 5 environmental isolates and 13 isogenic
A. fumigatus isolates obtained from a single patient over 2 years showing in-host
acquired azole resistance. Hyphae grown from 5x104 conidia and incubated with individual
AMPs at various concentrations for 2 h at 37oC, after which metabolic activity was
determined by XTT. Aspergillus conidia (1x105 per well) were incubated for 10 h at
37oC with specific AMPs and fixed in 4% paraformaldehyde fixing solution. Analysis
of hyphal length was performed using the Amnis ImageStreamX MK II Imaging Flow Cytometer
(Luminex, USA). Data analysis was performed using IDEAS software.
Results: Dose-dependent decreases in hyphal metabolic activity in all isolates were
shown for lysozyme (20-80 µM; 31-65% and 5-25% decrease respectively; p<00001) and
histones (50-100 ug/ml; 35-66% and 6-45% decrease respectively; p<0.0001). No effect
was observed for lactoferrin (10-40 µM), β-defensin-1 (1-10 µM) and LL-37 (5-50 µM).
Imaging flow cytometry revealed that incubation with histones (100 µg/ml), β-defensin-1
(10 µM) or lactoferrin (40 µM) resulted significantly fewer cells in the hyphae gate
(p<0.0001, p = 0.04 and p = 0.003, respectively). No significant differences were
observed with lysozyme (80 µM) or LL-37 (12.5 µM).
Conclusion: Lysozyme and histones show dose dependent antifungal activity against
A. fumigatus hyphae, irrespective of the isolate’s azole resistance profile. In addition,
histones, lactoferrin and β-defensin-1 display inhibitory activity against germinating
conidia. No differences were observed in the susceptibility to AMPs between the groups
of A. fumigatus isolates used. We did not identify any trends indicating increased
resistance to AMPs as a result of in-host adaptation.
Accoceberry
I.
1
El-Kirat-Chatel
S.
2
Quilès
F.
2
Biteau
N.
1
Noël
T.
1
1Cnrs Umr5234, University of Bordeaux, Bordeaux, France
2Cnrs Umr 7564, Université de Lorraine, Villers-lès-Nancy, France
P024. Characterization of I1380T a new FKS1 polymorphism in Candida parapsilosis that
confers high-level echinocandin resistance when associated to P660A
Objectives: The aim of this study was to determine the impact on echinocandin resistance
of a new mutation identified in a FKS1 allele of a clinical isolate of C. parapsilosis.
Methods: To assess the role of mutations potentially implicated in echinocandin resistance,
a C. lusitaniae strain specially engineered for the expression of a single chromosomal
copy of FKS1 was used for the expression of I1380T and P660A FKS1 alleles. The echinocandin
susceptibility of the mutants, as well as their growth rates and phenotypes of interaction
with murine macrophages, were evaluated in the absence or in the presence of caspofungin.
Atomic force microscopy (AFM) was used to observe the morphological changes induced
by caspofungin exposition. Finally, infrared spectroscopy in attenuated total reflection
mode (IR-ATR) spectroscopy was used to detect and quantify the biochemical signatures
of the cell wall before and after caspofungin treatment.
Results: A caspofungin-resistant C. parapsilosis bloodstream isolate was recovered
from a patient who underwent allogeneic bone marrow hematopoietic stem-cell transplantation
(HSCT) for an idiopathic medullary aplasia with positive PNH clone. Full-length nucleotide
sequencing of the FKS1 gene showed that the strain harboured the heterozygous mutation
I1380T (isoleucine at position 1380 replaced by threonine) located 4 amino acids beyond
the HS2 region (amino acids 1369 to 1376), compared to Fks1p of the C. parapsilosis
reference strain CBS 604, in addition to the naturally occurring P660A polymorphism
responsible for increased echinocandin MICS in this species. To characterize the impact
of each mutation on caspofungin resistance, FKS1 alleles bearing I1380T associated
or not to P660A were genetically engineered and expressed in Candida lusitaniae. When
compared to a wild type allele bearing no mutation, I1380T conferred a 12-fold MIC
increase, P660A a 6-fold MIC increase and the combination of both mutations a ≥ 256-fold
MIC increase. Measuring the cellular interactions between C. lusitaniae FKS1 mutants
and murine macrophages showed that, in the presence of caspofungin, I1380T enhanced
yeast phagocytosis but had no effect on intramacrophagic yeast survival rates. AFM
analysis of FKS1 mutants showed that caspofungin treatment impacts the cell surface
topography in both susceptible and resistant strains, and that the single I1380T mutation
leads to morphological changes even in the absence of caspofungin treatment. Infrared
spectroscopy in attenuated total reflection mode (IR-ATR) showed that under high drug
concentration exposure, the FKS1 I1380T mutants exhibited a massive β-glucan synthesis
inhibition that was not proportionally compensated by chitin synthesis.
Conclusion: We have identified I1380T, a new mutation located close to the HS2 region
of the FKS1 gene of a clinical isolate of C. parapsilosis. We demonstrated that the
high caspofungin MIC for this isolate was depending on the combination of I1380T with
the naturally occurring P660A polymorphism. Further characterization at the cell wall
level suggested a potential fitness cost caused by I1380T.
Godeau
C.
Scherer
E.
Laboissière
A.
Léchenault-Bergerot
C.
Reboux
G.
Millon
L.
Rocchi
S.
Mycology - Umr6249 Cnrs Chrono-environnement, University Hospital Besançon, Besançon,
France
P025. Azole resistant Aspergillus fumigatus: surveillance from flowered patios to
departments with at-risk patients
Objectives: The University Hospital of Besançon is geographically located in a highly
agricultural area (wood, vegetables, cereals, fruit and vines), and is very concerned
by the emergence of azole resistance in Aspergillus fumigatus (ARAf), having an increasing
number of cases especially amongst cystic fibrosis patients. For more than 20 years,
the University Hospital of Besançon has been taking weekly samples (n = 25) to assess
fungal air contamination in the 3 departments of hematology and corridors. Since 2015,
the number of ARAf found in corridors has been increasing. This study aimed to determine
whether plant, tree and flowerbeds within or near the hospital are sources of ARAf
and to assess their spread into the hospital.
Methods: Four soil sampling areas within the hospital and in the surrounding environment
have been identified. Zone A corresponds to an outdoor relaxation area within the
hospital, zone B consists of flowerbeds in front of the hospital entrance, zone C
(inside) corresponds to pots containing trees below the hospital reception area and
finally zone D corresponds to an outdoor area at level -1 below the zone A. From January
2019, external samples were taken using a homemade dust vane sensor equipped with
2 wipes, placed on a terrace at second floor, to capture the prevailing wind. Dust
aspiration was also achieved using a filter placed at the end of a vacuum cleaner,
towards the emergency door of the department of hematology, in the corridor along
zone A, then in hematology consultation department. Soils and dust (wipes and aspiration)
samples were seeded on medium containing itraconazole and voriconazole (2mg/L). The
usual weekly air samples taken in the hematology departments and corridors from January
2019 were also analyzed. Resistance of A. fumigatus growing on azole media was tested
using EUCAST method and cyp51A gene of ARAf were sequenced.
Results: From the 85 soils samples, 137 colonies were isolated on azole medium from
zones A, B and D, grew on azole media. Resistance of 90 A. fumigatus isolates was
evaluated with EUCAST method. Other strains (zone B) were Neosartorya fisheri. Moreover,
71% (64/90) of A. fumigatus isolates were resistant to at least one azole. So far,
20 strains have been sequenced and all harbored the TR34/L98H mutation. 59 ARAf were
found in pots containing tulips coming from Netherlands and 5 in soil pots containing
trees. Three isolates were detected on azole media with dust vacuum and 2 (hematology
department and corridor along zone A) were resistant with TR34/L98H mutation. No resistant
strains were collected with the external sensor. Four ARAf with the TR34/L98H mutation
were also isolated from air of corridors.
Conclusion: Patios containing Dutch tulips in particular, had a significant number
of ARAf. On the other hand, samples taken upstream of the esplanades, in relation
to the prevailing wind, did not present any ARAf. Addionally, ARAf were also found
in hospital corridors and to emergency exits, but not in hematology departments. Genotyping
analyses will be carried out to confirm that tulips can be a source of ARAf found
inside the hospital.
Rocchi
S.
Scherer
E.
Laboissière
A.
Léchenault-Bergerot
C.
Godeau
C.
Bellanger
A.-P.
Reboux
G.
Millon
L.
Mycology - Umr6249 Cnrs Chrono-environnement, University Hospital Besançon, Besançon,
France
P026. Triazole-resistant Aspergillus fumigatus: Evaluation of the Fungiplex® Aspergillus
and Azole-R kits
Objectives: Cases of Aspergillosis with azole resistant Aspergillus fumigatus are
increasingly described. Some resistance mechanisms, with two major mutations on the
cyp51A gene (TR34/L98H and TR46/Y121F/T289A), are linked to the unintended impact
of triazole fungicides in agriculture. At the University Hospital of Besançon, 216
resistant strains have been isolated since 2012 and 142 sequenced for beta-tubulin:
52% from professional environments, 28% from clinical samples, 10% from hospital corridors,
7% from the inside garden of the Hospital and 3% from patients’ homes. Given the seriousness
of resistant infections, it is important to detect cases of resistance as soon as
possible. It is in this context that we proposed to test to Fungiplex ® (Aspergillus
et Azole R) kits (Bruker).
Methods: The Fungiplex® Aspergillus Azole-R kit was retrospectively evaluated on DNA
extracts from a collection for which cyp51A gene sequencing and genotyping was previously
performed. According to the instructions, the Azole-R kit is made for the identification
of azole resistance markers in Aspergillus positive species directly from serum, plasma
and bronchoalveolar lavage. We are evaluating the kits for the routine testing of
cystic fibrosis patients working from isolated strains and directly on sputum. We
also are testing the performance of the kits to detect 1) Aspergillus fumigatus strains
2) resistant strains directly in soil samples. To do this, we worked on inoculated
soils with increasing concentrations of resistant strains (from 5 to 50 000 spores/mL
with 200µL sprayed on 250 mg of soil) and directly on soils containing resistant strains.
Results: 97 resistant strains previously characterized by sequencing techniques (beta-tubulin
and cyp51A) were analyzed: 82 TR34/L98H, 2 TR34/L98H/S297T/F495I, 9 TR46/Y121F/T289A
and 4 other resistant strains. Results showed that 99% of strains carrying one of
the two mutations are detected. The first prospective results (22 resistant strains
in Etest, analysed directly with the kits) made it possible to detect 22 A. fumigatus
with the TR34/L98H mutation. Verification sequencing is in progress. Sputum analysis
and tests with cryptic species are also in progress. Tests on inoculated soils show
that resistant strains can be detected even for the smallest inoculated quantities
(200 µL of solution concentrated at 5 spores/mL). Real soil tests provide a signal
for Aspergillus but not for the TR34 and TR46 mutations. Internal reaction controls
are also not detected, suggesting a problem with DNA extraction and inhibitors rather
than poor kit performance. Different methods for extracting DNA directly from the
soil are under evaluation.
Conclusion: The kits show ease of use and very good reliability on retrospective analyses
with 99% of the strains tested validated. The performance of the Aspergillus kit will
also be evaluated on cryptic species of A. fumigatus (Neosartorya fisherii, A. oerlinghausenensis,
Neosartorya hiratsukae, A. lentulus). If performance is equally good in ongoing prospective
analyses, these kits will be promising to improve early diagnosis.
Scroferneker
M.L.
1
Heidrich
D.
2
Vettorato
R.
2
3
Eidt
L.
4
Ribeiro
A. Carvalho
1
Pagani
D.
5
Hellwig
A.H. Da Silva
2
Amaro
T.
3
1Department of Microbiology, Immunology and Parasitology, Universidade Federal do
Rio Grande do Sul, Porto Alegre, Brazil
2Postgraduate Program In Medicine: Medical Sciences, Universidade Federal do Rio Grande
do Sul, Porto Alegre, Brazil
3Serviço de Dermatologia do complexo Hospitalar Santa Casa de Misericórdia de Porto
Alegre, Porto Alegre, Brazil
4Ambulatório de Dermatologia Sanitária de Porto Alegre, Porto Alegre, Brazil
5Postgraduate Program In Agricultural and Environmental Microbiology, Universidade
Federal do Rio Grande do Sul, Porto Alegre, Brazil
P027. Relation between hanseniasis and superficial mycoses: prevalence, fungal identification
and antifungal susceptibility.
Objectives: The aim of this study was to evaluate superficial mycoses in patients
with hanseniasis in relation to the prevalence of fungal species causing mycoses and
susceptibility to antifungals.
Methods: A cross-sectional study was carried out with patients who were seen between
May and October 2017 at the Hanseniasis Service of the Sanitary Dermatology Ambulatory
in the city of Porto Alegre, Brazil. The samples collected were submitted to direct
(DME) and cultural (CME) mycological examinations at the G post of the Santa Clara
Hospital in Porto Alegre, were identified by region sequencing specified for each
genus of fungus and traced sensitivity profile to clinical antifungals using protocols
M38-A2 and M27-A3 from the Clinical and Laboratory Standards Institute.
Results: A total of 91 hanseniasis patients were evaluated and 37 presented suspicion
mycosis. of these, 23 had DME positive and 14 CME cultures were identified, with eight
dermatophytes (seven Trichophyton interdigitale and one Epidermophyton floccosum),
two isolates of Fusarium keratoplasticum, two Acremonium sp., one Candida albicans
and one Arthrinium arundinis. Three patients had claws, caused by hanseniasis, at
the mycosis site, indicating a relation of the two diseases (3/5 patients with claw).
Terbinafine presented the lowest minimum inhibitory concentrations (MICs) for dermatophyte
isolates (0.0078-0.06 μg/ml), while fluconazole MICs were the highest (4-> 64μg/ml).
Fusarium keratoplasticum and Acremonium sp. have higher MICs than all antifungal agents
than dermatophytes.
Conclusion: This is the second onychomycosis caused by Arthrinium arundinis from the
literature and showed low sensitivity to antifungals. Itraconazole had higher MICs
for dermatophytes isolated from patients with hanseniasis (0.25-1μg/ml) than without
the disease cited in the literature, indicating a relation of susceptibility to antifungals
between mycosis and hanseniasis.
Simon
L.
1
Kramer
R.
2
Dupont
D.
3
Déméautis
T.
4
Garnier
H.
5
Lina
B.
6
Rabodonirina
M.
7
Wallon
M.
3
Persat
F.
8
Menotti
J.
4
1Parasitology and Medical Mycology, Hospices Civils de Lyon / CHU de Nice, Nice, France
2Parasitology and Medical Mycology, and Virology, Hospices Civils de Lyon / EUPHEM,
Lyon, France
3Parasitology and Medical Mycology / Umr 5292, Université Lyon 1 / Hospices Civils
de Lyon, Lyon, France
4Parasitology and Medical Mycology / Ea7426 Inflammation and Immunity of The Respiratory
Epithelium, Université Lyon 1 / Hospices Civils de Lyon, Lyon, France
5Parasitology and Medical Mycology, Hospices Civils de Lyon, Lyon, France
6Virology / U1111, Université Lyon 1 / Hospices Civils de Lyon, Lyon, France
7Parasitology and Medical Mycology / U1111, Université Lyon 1 / Hospices Civils de
Lyon, Lyon, France
8Parasitology and Medical Mycology, Université Lyon 1 / Hospices Civils de Lyon, Lyon,
France
P028. Prevalence of azole-resistant Aspergillus fumigatus isolates from respiratory
specimens of patients from Lyon and exploration of resistance mechanisms
Objectives: Resistance of Aspergillus fumigatus strains to triazole antifungals is
increasingly reported in Europe. The main mechanisms of resistance described are mutations
in the cyp51A gene encoding a lanosterol 14-α-demethylase or its promoter. As few
data are available in Southern France, our objective was to assess the burden of Aspergillus
isolates with azole resistance from clinical specimens in Lyon University Hospitals
Methods: In this retrospective cross-sectional study, 203 consecutive A. fumigatus
isolates were identified during a seven-month period from February to September 2017
from respiratory specimens from 182 patients attending the inpatient and outpatient
wards of the Pulmonary Medicine Departments of Lyon University Hospitals. Morphological
identification was confirmed by sequence analysis of the β-tubulin gene. Minimum inhibitory
concentrations were determined using E-test reagent strips for itraconazole, voriconazole,
posaconazole, and isavuconazole. Resistance was defined according to the 2018 EUCAST
clinical breakpoints. The molecular resistance mechanisms were searched for by sequence
analysis of the cyp51A gene and its promoter region, as well as by gene expression
analysis of the cyp51 genes and genes encoding several efflux transporters.
Results: PCR and sequence analysis of the β-tubulin gene confirmed the identification
of Aspergillus fumigatus for the 203 isolates. Four isolates presented with azole
resistance: two isolates against itraconazole/posaconazole/isavuconazole and another
two against all four triazoles. Out of these four strains, three presented silent
polymorphisms in an intronic part of cyp51A and one presented simultaneously the F46Y,
M172V and E427K mutations. No mutation was identified in the cyp51A promoter, but
significant inductions of cyp51A and cyp51B gene expression were observed for all
four isolates and three isolates, respectively. Significant inductions of atrF and
crd1B gene expression were observed for two and three isolates, respectively. No significant
induction of MDR1-4, MFS56 and M85 gene expression was observed.
Conclusion: The prevalence of azole resistance in our study population was 2.2% (95%
CI 0.9-5.6). Only polymorphisms were found in the cyp51A gene and no mutation was
found in its promoter. Nevertheless, significant inductions of the expression of the
cyp51 genes and two genes encoding efflux transporters were evidenced, underlying
the diversity of resistance mechanisms to be explored.
Singulani
J.
1
Galeane
M.C.
1
Ramos
M. Dorisse
1
Gomes
P.C.
1
Santos
C.
1
Souza
B. Monson De
2
Palma
M.S.
2
Almeida
A.M. Fusco
1
Giannini
M.J. Soares Mendes
1
1Clinical Analysis, UNESP, Araraquara, Brazil
2Biology, UNESP (Sao Paulo State University), Rio Claro, Brazil
P029. Antifungal activity of a mastoparan analog peptide: mode of action and in vivo
evaluation in a Galleria mellonella model
Objectives: The purpose of this work was to evaluate the antifungal activity and toxicity
of a peptide analog of mastoparan class from wasps (MK58911) under both in vitro and
in vivo conditions.
Methods: Firstly, MK58911 was tested against Cryptococcus neoformans ATCC 90112 using
the microdilution susceptibility test according to the CLSI M27-A3 (2008). In addition,
the mechanism of action on fungal membrane, death cell events (apoptosis and necrosis)
and reactive oxygen species (ROS) was evaluated by flow cytometry. Secondly, the peptide
toxicity tests on lung fibroblasts (MRC5) and glioblastoma cells (U87) were performed.
Finally, the efficacy and toxicity of the peptide were evaluated in vivo using a Galleria
mellonella model.
Results: The results showed a promising activity of the peptide with minimal concentration
inhibitory (MIC) of 31.2 μg/mL and low toxicity in MRC and U87 cells (IC50 > 500 μg/mL).
Taken together, these results demonstrated that the peptide has a high selectivity
by fungal cells compared to mammal cells (selectivity index - SI > 16). The peptide
presented a mechanism of action on plasma membrane, leading to death of the fungus
mainly by the necrosis process and without production of reactive oxygen species.
Finally, the peptide showed no toxic effects on G. mellonella and significantly enhanced
the survival rates and decreased the fungal burden of larvae infected with C. neoformans.
These effects were independent of an immunomodulatory activity.
Conclusion: The results showed the peptide as a potential new antifungal drug against
cryptococcosis. "This study was supported by the Fundação de Amparo à Pesquisa do
Estado de São Paulo (FAPESP grants no. 2017/06658-9 and 2016/16212-5)."
Zafar
A.
Moin
S.
Jabeen
K.
Farooqi
J.
Laiq
S.
Pathology and Laboratory Medicine, Aga Khan University, Karachi, Pakistan
P031. Screening for Triazole resistance in clinically significant Aspergillus species
from Pakistan
Objectives: To determine the frequency of triazole (itraconazole, voriconazole, posaconazole)
resistance in clinically significant Aspergillus species isolated at a tertiary care
centre, Karachi, Pakistan.
Methods: A descriptive cross-sectional study was conducted in the Department of Pathology
and Laboratory Medicine, Microbiology Section of the Aga Khan University Clinical
Laboratories, Karachi, Pakistan from September 2016 to May 2019. One hundred and fourteen,
clinically significant Aspergillus isolates [A.fumigatus (38; 33.3%), A.flavus (64;
56.1%), A.niger (9; 7.9%) and A.terreus (3; 2.6%)] were included in the study. They
were assessed for their clinical significance. The clinical spectrum ranged from Invasive
Aspergillosis (IA) (n = 25; 21.9%), further divided into proven invasive extrapulmonary
aspergillosis (n = 8; 7%), proven invasive pulmonary aspergillosis (IPA) (n = 6; 5.3%),
putative/probable IPA (n = 11; 9.6%) according to the AspICU and 2008 EORTC criteria,
to Chronic Pulmonary Aspergillosis (CPA) (n = 58; 50.9%), Allergic Bronchopulmonary
Aspergillosis (ABPA) (n = 4; 3.5%), Severe Asthma with Fungal Sensitization (SAFS)
(n = 4; 3.5%) and saprophytic tracheobronchial aspergillosis (n = 23; 20.2%). Screening
for triazole resistance was performed by antifungal agar screening method as described
by Mortenssen et al. The minimum inhibitory concentration (MIC) of 41 representative
isolates [A.flavus (n = 15; 13.2%), A.fumigatus (n = 15; 13.2%), A.niger (n = 8; 7%),
A.terreus (n = 3; 2.6%)] representing a clinical spectrum of extrapulmonary IA (n
= 7; 6.1%), IPA (n = 4;3.5%) putative/probable IPA (5; 4.4%), CPA (n = 18;15.8%),
ABPA (n = 3; 2.6%), SAFS (n = 1; 0.9%), and saprophytic tracheobronchial aspergillosis
(n = 3; 2.6%) were tested according to the CLSI broth microdilution method.
Results: All the isolates were categorized as triazole-susceptible based on the triazole
antifungal agar screening. The MICs of the three azole antifungals for 41 representative
isolates tested were found to be ≤1 ml/L and hence according to CLSI breakpoints,
all the isolates tested were found to be triazole-susceptible. The MIC 90 of itraconazole,
voriconazole and posaconazole of the representative aspergillus isolates was 1mg/L,
1mg/L and 0.5mg/L respectively.
Conclusion: This study may set precedence for future periodic antifungal resistance
surveillance studies in our region on Aspergillus isolates causing invasive disease,
as well as other syndromes requiring long term antifungal therapy.
Farooqi
J.
1
Memon
S.
2
Laiq
S.
1
Naqvi
F.
1
Zafar
A.
1
Jabeen
K.
3
1Pathology and Laboratory Medicine, Aga Khan University, Karachi-KHI, Pakistan
2Microbiology, Karachi University, Karachi, Pakistan
3Pathology and Laboratory Medicine, Aga Khan University, Karachi, Pakistan, Pakistan
P032. Antifungal susceptibility profile of invasive Candida glabrata isolates (2009-2019)
from a tertiary care hospital laboratory in Pakistan
Objectives:
Candida glabrata invasive infections are increasingly associated with antifungal resistance
and poor clinical outcomes. The objective of this study was to evaluate antifungal
resistance and distribution of minimum inhibitory concentrations (MICs) against invasive
C. glabrata isolates (200-2019) at Aga Khan University (AKU) at Karachi, Pakistan.
This laboratory, through its network of satellite collection centers receives specimens
from more than 100 major cities and towns of the country.
Methods: 162 Candida glabrata strains were isolated from blood (101), body fluids
(26), pus and wounds (21) tissue (4) and others (10). Isolates were identified by
conventional method using API 20C AUX, gross morphology on chromogenic and microscopic
morphology on corn meal agar. MICs were determined using colorimetric broth microdilution
(YeastOne Sensititre, Trek diagnostics). Susceptibilities were interpreted according
to Clinical Laboratory standard Institute breakpoints mentioned in “Performance Standard
for Antifungal Susceptibility Testing of Yeasts M60-ED1:2017”.
Results: Out of 1917 archived invasive candida, 162 (8.4%) were C.glabrata 76% of
these isolates were from patients admitted at AKU. Male to female ratio was 1.4 and
62% of the isolates were from ages 18-64 year. MIC90 of these strains against fluconazole
was 64 µg/ml, voriconazole 2µg/ml, itraconazole 1µg/ml and posaconazole 2µg/ml. Among
echinocandins, MIC90 was 0.12µg/ml for caspofungin, 0.06µg/ml for anidulafungin and
0.03µg/ml for micafungin. MIC90 for amphotericin B was 0.5µg/ml.
Conclusion: Antifungal sensitivity testing for invasive candidiasis is essential in
the face of emerging resistance as empiric therapy may not have reliable outcomes.
Surveillance data of antifungal resistance among common Candida species with potential
for developing antifungal resistance, like C. glabrata, should be monitored closely
for identifying resistant strains in circulation.
Seidel
D.
1
Cornely
O.A.
2
Köhler
P.
1
3
Meis
J.
4
Zarrouk
M.
3
Salmanton-Garcia
J.
3
Arenz
D.
5
Blennow
O.
6
Buchheidt
D.
7
Vehreschild
J.-J.
3
Alakel
N.
8
Bergeron
A.
9
Desoubeaux
G.
10
Falces-Romero
I.
11
Klimko
N.
12
Lagrou
K.
13
Lass-Flörl
C.
14
Govic
Y. Le
15
Maertens
J.
16
Ostojic
A.
17
Prattes
J.
18
Racil
Z.
19
Sharpe
A. Reséndiz
16
Schalk
E.
20
Stanzani
M.
21
Steinmann
J.
22
Melchers
W.
23
Vehreschild
M.J.G.T.
24
Verweij
P.E.
23
1Department I of Internal Medicine, Ecmm Excellence Centre of Medical Mycology, Cologne
Excellence Cluster On Cellular Stress Responses In Aging-associated Diseases (cecad),
University Hospital Cologne, Cologne, Germany
2Department I of Internal Medicine, Ecmm Excellence Centre of Medical Mycology, Cologne
Excellence Cluster On Cellular Stress Responses In Aging-associated Diseases (cecad),
German Centre For Infection Research, Partner Site Bonn-cologne, Clinical Trials C,
University Hospital Cologne, Cologne, Germany
3Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany
4Department of Medical Microbiology and Infectious Diseases, Excellence Center For
Medical Mycology (ecmm), Canisius Wilhelmina Hospital, Nijmegen, Netherlands
5Department I For Internal Medicine, Excellence Center For Medical Mycology (ecmm),
University of Cologne, Cologne, Germany
6Karolinska University Hospital, Stockholm, Sweden
7Mannheim University Hospital, Heidelberg University, Mannheim, Germany
8University Hospital Dresden, Dresden, Germany
9Université Paris Diderot, APHP Saint-Louis Hospital, Paris, France
10Cepr Inserm U1100, Université de Tours, Tours, France
11Hospital Universitario La Paz, Madrid, Spain
12Department of Clinical Mycology, Allergology and Immunology, North-Western State
Medical University n.a. I.I. Mechnikov, Saint-Petersburg, Russian Federation
13Clinical Bacteriology and Mycology, Katholieke Universiteit Leuven, Leuven, Belgium
14Div Hygiene & Med. Microbiology, Med. Univ. Innsbruck, Innsbruck, Austria
15Groupe d’Etude des Interactions Hôte Pathogène (GEIHP), Université d’Angers, Angers
Cedex, France
16KU Leuven, Leuven, Belgium
17University Hospital Centre Zagreb, Zagreb, Croatia
18Section of Infectious Diseases and Tropical Medicine, Medical University of Graz,
Graz, Austria
19Department of Internal Medicine - Hematology and Oncology, University Hospital Brno,
Brno, Czech Republic
20Otto-von-Guericke University, Magdeburg, Germany
21“Lorenzo e Ariosto Seràgnoli” S’Orsola-Malpighi Hospital, University of Bologna,
Bologna, Italy
22Institute of Clinical Hygiene, Medical Microbiology and Clinical Infectiology, Klinikum
Nuernberg, Nuremberg, Germany
23Medical Microbiology, RadboudUMC, Nijmegen, Netherlands
24Uniklinikum Frankfurt, Med. Klinik II, Infektiologie, Frankfurt, Germany
P033. Clinical implications of azole-resistant vs. azole-susceptible invasive aspergillosis
in hematological malignancy (CLARITY) – a multicenter study
Objectives: In recent years, survival of patients with invasive aspergillosis (IA)
has improved mainly due to availability of extended spectrum triazoles. These advances
are jeopardized by the emergence of azole resistance in Aspergillus fumigatus, the
most common causative pathogen of IA. Despite several studies suggesting high probability
of azole treatment failure in patients with azole-resistant isolates, the clinical
implications of azole-resistant IA compared to azole-susceptible IA remain unclear.
Thus, we seek to describe the epidemiology and determine the efficacy of antifungal
therapy in patients with documented azole-resistant IA compared to azole-sensitive
IA in patients with hematological malignancy.
Methods: In patients with hematological malignancies, cases of proven or probable
IA (EORTC/MSG 2008) caused by A. fumigatus are registered. Retrospective data are
documented, comprising demographics, diagnosis, treatment, response and outcome. Participating
sites provided susceptibility results or isolates. Provided isolates were analyzed
in a central laboratory.
Results: Since January 2018, 51 sites in 15 countries worldwide enrolled 154 cases
diagnosed with IA between 2010 and 2019, of which 23 (14.9%) had azole-resistant IA.
of 44 cases, the respective clinical fungal isolate was analyzed in the central laboratory.
A mixed fungal infection was reported for 34 patients (22.1%), 1 (2.9%) in the azole-resistant
group; most were related to non-fumigatus Aspergillus species (n = 12, 35.3%) and
non-Aspergillus molds (n = 10, 29.4). Most patients were male (n = 98, 63.6%); 19
(82.6%) in the azole-resistant group, 79 (60.3%) in the azole-susceptible group. Age
was documented in categories instead of the exact age. Median age group was 50-69
years in both groups (ranging from 7-11 to 70-89 years for azole-resistant cases,
1-12 months to 70-89 years for azole-susceptible cases). Underlying disease and survival
are shown in the table.
Conclusion: A worldwide network of investigators contributes to the CLARITY registry
study. Completion of recruitment and subsequent data analysis are planned for 2019.
Further sites may be added if azole resistant cases are encountered.
Manoyan
M.
Sokolov
V.
Gursheva
A.
Gabuzyan
N.
Panin
A.
Veterinary Mycology, The All-Russian State Center for Quality of Animal Medicines
and Feeds, Moscow, Russian Federation
P034. Sensitivity of isolated dermatophyte strains to antifungal drugs in the Russian
Federation
Objectives: The spread of dermatophytosis is a veterinary problem; the spread of dermatophytosis
pathogens in the human population is a social problem. In the Russian Federation immunobiological
preparations, as well as external agents in the form of suspensions or shampoos are
used for a treatment of animals dermatophytosis. As a rule, such agents contain azoles
(clotrimazole, miconazole, enilconazole) or terbinafine as an active substance. They
are often used unsystematically and can trigger the emergence of resistant strains.
Recently, there have been increasing reports about dermatophytoses strains, resistant
to antifungal drugs: M. canis strains resistant to terbinafine and azoles, and T.
rubrum strains resistant to terbinafine.
Methods: For comparison, the strains of dermatophytes - M. canis and T. mentagrophytes,
earlier collected and freshly isolated from cats and dogs, were chosen. Collection
strains, stored in a lyophilized form, were isolated from animals between 1970 and
2000. For the study there were selected ten strains of M. canis and T. mentagrophytes.
Freshly isolated strains were collected from animals between 2015 and 2018, they also
were stored in the laboratory’s collection in a lyophilized form; we also selected
ten strains of M. canis and T. mentagrophytes. The choice of antifungal drugs spectrum,
for which MIC was determined, was dictated by the data on the use of antifungal drugs
in the Russian Federation: for terbinafine there was evidence of resistant strains;
ketoconazole and enilconazole were used for animal fungal infections treatment and
prevention. Cultures were isolated on Saburo medium with chloramphenicol and cycloheximide,
pure cultures for determining MIC were cultivated on Saburo medium without additives
for 7 days at +28°C. A method based on EUCAST E.def 9.3.1 was used to determine the
MIC (inoculum density was 1.0E+5 CFU/ml, the incubation time was 4days.
Results: MIC studies of terbinafine, ketoconazole and enilconazole for strains isolated
between 1970 and 2000 showed following. One of ten M. canis strains was resistant
to the action of terbinafine (MIC > 32 μg/ml), there was no detected any strain resistant
to ketoconazole and enilconazole. None of the ten T. mentagrophytes strains was resistant
to the action of terbinafine, ketoconazole or enilconazole. MIC studies of terbinafine,
ketoconazole and enilconazole for strains isolated between 2015 and 2018 showed following.
Four of the ten M. canis strains were resistant to the action of terbinafine (MIC
> 32 μg/ml); one strain was resistant to the action of enilconazole (MIC > 16 μg/ml);
and we did not find a ketoconazole resistant strain. Four of the ten T. mentagrophytes
strains were resistant to the action of terbinafine (MIC > 32 μg/ml); one strain was
resistant to ketoconazole (MIC > 16 µg/ml); 2 strains were resistant to the action
of enilconazole (MIC > 16 µg/ml). We did not find strains of M. canis or T. mentagrophytes,
resistant to two or three drugs.
Conclusion: The number of antifungal resistant dermatophyte strains was higher among
those isolated for a later period. This may indicate the emergence of resistant dermatophytosis
pathogens strains in the Russian Federation.
Markantonatou
A.-M.
Samaras
K.
Zachrou
E.
Vyzantiadis
T.-A.
First Department of Microbiology, Medical School, Aristotle University of Thessaloniki,
Thessaloniki, Greece
P035. Evaluation of the in vitro susceptibility testing of dermatophytic isolates:
preliminary comparison of four methods
Objectives: Superficial infections caused by dermatophytes affect a high percentage
of the population. Antifungal susceptibility testing (AST) of these pathogens may
offer information about their susceptibility profiles, documentation of the appropriate
treatment and reduction of the cost. However, there are two factors that could delay
and affect the performance of the AST: the slow growth rate of these fungi and their
poor sporulation. The proposed methods by the CLSI or the EUCAST are both laborious
for the everyday routine. Though, there are alternative applications that propose
the use of an inoculum consisting of a conidia-mycelium mixture or even from plain
mycelia, as well as the use of resazurin in order to facilitate the reading. The aim
of this study was to compare these approaches to the EUCAST method in order to evaluate
their performance.
Methods: Three alternative methods of dermatophytic AST were compared to the EUCAST
proposed methodology for conidia forming moulds. The methods under evaluation were
a) a fragmented mycelia method, b) the EUCAST method with the addition of resazurin
sodium salt solution and c) the fragmented mycelia method with the addition of resazurin
sodium salt solution. The susceptibility of twenty dermatophytic isolates (8 Trichophyton
interdigitale, 6 T. rubrum and 6 M. canis) was tested against the antifungal agents
of griseofulvin, terbinafine, fluconazole and itraconazole.
Results: After the measurement of the MICs by all four different methods, the essential
agreement between them was calculated in percentages. Data analysis revealed sufficient
overall essential agreement of the methods with the addition of resazurin to the initial
“uncoloured” ones (98.5% and 97.2% for the EUCAST and the fragmented mycelia method
respectively). The fragmented mycelia method exhibited a relatively sufficient overall
essential agreement to the EUCAST method (88.9%) but not a satisfactory correlation
between the MIC values. The mean MICs (by the EUCAST method, in μg/mL) for the twenty
isolates were 1.78 for griseofulvin, 0.034 for terbinafine, 25.2 for fluconazole and
0.57 for itraconazole.
Conclusion: The addition of resazurin sodium salt solution facilitates the reading
and provides a more objective evaluation. The fragmented mycelia method could serve
as an alternative in cases of poor or no sporulating dermatophytes.
Kordalewska
M.
1
Healey
K.R.
2
Jimenez-Ortigosa
C.
1
Singh
A.
3
Williamson
B.
2
Wilk
A.
2
Berrio
I.
4
Chowdhary
A.
3
Perlin
D.S.
1
1Center For Discovery and Innovation, Hackensack Meridian Health, Nutley, United States
of America
2Department of Biology, William Paterson University, Wayne, United States of America
3Department of Medical Mycology, Vallabhbhai Patel Chest Institute, University of
Delhi, Delhi, India
4Hospital General de Medellín “Luz Castro de Gutiérrez” ESE, Medellin, Colombia
P037. Distribution of azole minimal inhibitory concentration (MIC) values and Erg11
amino acid substitutions among Candida auris isolates from different geographic clades
Objectives:
Candida auris isolates are often characterized with reduced susceptibility to fluconazole
(FLC) and other azole class drugs: voriconazole (VRC), posaconazole (POS), itraconazole
(ITC), and isavuconazole (IVC). Such a situation is extremely worrying, since azoles
are a mainstay in the treatment of Candida infections and antifungals other than fluconazole
might be unavailable in resource-limited countries. Moreover, no antifungal clinical
breakpoints are available for C. auris. CDC provides guidance for C. auris MIC interpretation,
based on information gathered for related Candida species and expert opinion. Tentative
epidemiological cut off values (ECVs) were proposed only for C. auris isolates from
India. The aim of this study was to analyze distribution of azole minimal inhibitory
concentration (MIC) values for isolates belonging to different geographic clades together
with the data regarding molecular resistance determinants.
Methods: A total of 108 C. auris isolates representing four geographic clades (I –
South Asian; II – East Asian; III – South African; IV – South American) were investigated
in this study. Antifungal susceptibility testing (AFST) with azoles (FLC, ITC, IVC,
POS, VRC) was performed in accordance with CLSI document M27-A3. The ERG11 gene, encoding
the target for azole antifungal drugs, was amplified and sequenced. All identified
ERG11 alleles were cloned onto a low-copy vector (pRS416) and expressed within a haploid
strain of Saccharomyces cerevisiae (BY4741). Azole susceptibilities were then determined
for the transformed strains of S. cerevisiae.
Results: The results of C. auris AFST and ERG11 sequencing are presented in Table
1. Expression of ERG11 alleles in S. cerevisiae revealed that triazole resistance
was induced only upon expression of pCauErg11-V125A/F126L, pCauErg11-Y132F, and pCauErg11-K143R,
but not pCauErg11-WT, pCauErg11-K177R/N335S/E343D, pCauErg11-I466M, or pCauErg11-Y501H.
Conclusion: Differences in the distribution of MIC values and resistance-conferring
ERG11 mutations between isolates from different geographic clades underscore the need
for an extreme caution when categorizing isolates as sensitive/resistant on a basis
on AFST results. Only V125A/F126L, Y132F and K143R Erg11 amino acid substitutions
identified in C. auris isolates directly mediate resistance and may be used as diagnostic
markers for C. auris azole resistance. Mechanisms other than ERG11 mutations may also
contribute to reduced azole susceptibility in C. auris, although this remains to be
determined.
Kindo
A.J.
1
Veena
H.
1
Subramanian
A.
2
Krishnan
A.
2
1Microbiology, Sri Ramachandra Medical College and Research Institute, SRIHER, Chennai,
India
2Dermatology, Sri Ramachandra Medical College and Research Institute, SRIHER, Chennai,
India
P038. To study the profile of conventional and newer antifungal agents against dermatophytes
in a tertiary care hospital in South India
Objectives: 1.Speciation of dermatophytes from the clinical isolates 2.To study the
antimicrobial pattern of frequently used antifungal agents 3.To compare the antifungal
profile with the newer antifungal agents
Methods: Clinically diagnosed dermatophytic patients were sampled for microscopy and
culture, grown dermatophytes were subjected to phenotypic identification by conventional
method. Antifungal susceptibility testing was done by broth microdilution method according
to CLSI M38-A2 document guidelines. Trichophyton mentagrophytes ATCC 4439 was included
in each run of susceptibility testing as a quality control. Final drug concentration
in the microdilution plates ranged from 64 to 0.25 µg/ml for fluconazole and Amphotericin
B and from 16 to 0.06 µg/ml for Itraconazole, Voriconazole, Griesofulvin, Terbinafine,
Sertaconazole, Luliconazole, Fenticonazole tested.
Results:
Out of the 100 clinical isolates tested, 58 of them were identified as Trichophyton
mentagrophytes, 40 were Trichophyton rubrum. and the remaining two isolates were identified
as Epidermophyton Floccosum and Microsoprum nanum Antifungal susceptibility testing
was carried out for 100 isolates of Trichophyton species against 9 antifungal agents.
All isolates showed detectable growth after 4-5days of incubation. MICS for Trichophyton
mentagrophytes ATCC 4439 were within the established range.
Conclusion: In this study Trichophyton mentagrophytes was the most common cause of
dermatophytosis. The most challenging task is not the diagnosis but the treatment,
compliance and recurrence. Since it requires a long term treatment, the management
with appropriate and responsive drug is the need of the hour. Currently this study
suggests that the overall activity of Luliconazole, Fenticonazole, Sertaconazole,
as topical agent and Itraconazole, Voriconazole (systemic) are significantly effective
against dermatophytes. Current solution to this could be to maintain skin hygiene,
prudent use of antifungals appropriate dosing and duration, performing antifungal
susceptibility testing, usage of conventional drugs or topical keratolytic agents
in combination with newer drugs in appropriate dosages and combination therapy.
Järv
H.
1
Uhrlass
S.
2
Simkin
T.
1
Nenoff
P.
2
Ramirez
E. Alvarado
3
Chryssanthou
E.
3
Monod
M.
4
1SYNLAB Estonia, Tallinn, Estonia
2Laboratory For Medical Microbiology, Mölbis, Mölbis, Germany
3Clinical Microbiology, Karolinska University Hospital, Stockholm, Sweden
4Department of Dermatology, Lausanne University Hospital, Chexbres, Switzerland
P039. Terbinafine resistant Trichophyton mentagrophytes genotype VIII, Indian type,
isolated in Finland
Objectives: Objective We are reporting first four isolates of Trichophyton mentagrophytes
representing novel Indian genotype VIII in Finland potentially resistant to terbinafine.
Our study points out the need for antifungal susceptibility testing of dermatophytes
as a new issue in microbiology laboratories. The roles of classical mycological culture
vs DNA-based diagnostics for laboratory diagnostics of dermatophytosis will be discussed.
Methods: Primary fungal cultures were made on Sabouraud dextrose and 2% malt extract
agar. Identification of fungal isolates was made by micromorphological appearance
in SYNLAB laboratory in Estonia and by sequencing the ITS region of rDNA and TEF1-alpha
gene in clinical microbiology laboratory in Mölbis, Germany. The genotyping of squalene
oxidase gene was made in dermatology department of Vaudoise, Lausanne, Switzerland.
The minimal inhibitory concentration (MIC) to terbinafine was determined by broth
microdilution method in microbiology laboratory of Karolinska Hospital, Sweden. MIC
was detected spectrophotometrically after 5 days incubation at 28 °C.
Results: We describe four cases of extensive tinea cutis glabrae caused by T. mentagropytes.
All cases were diagnosed from January to April of 2019 and occurred in geographically
distinct regions of Finland. Three of four of T. mentagrophytes sub-type VIII (Indian
sybtype) strains have high MIC values of terbinafine from 4 mg/L to >8 mg/L.
Conclusion: Dermatophytosis has taken epidemic dimensions in India during the last
5 to 6 years. Unusual clinical presentation of dermatophytosis and corticosteroid
modified lesions are making diagnosis challenging. Indian experts panel ECTODERM India
has published new treatment guidelines in 2018. Nenoff et al have demonstrated in
a recently published study from different geographical locations from India that 93.25%
of the dermatophytes isolated from patients suffering from dermatophytosis correspond
to a special genotype (VIII) of T. mentagrophytes. Singh with co-workers have published
data about alarming terbinafine resistance of T. interdigitale strains in India. Trichophyton
spp isolates with Indian origin have demonstrated high MICs to terbinafine, up to
32 mg/L, and also to azoles. Terbinafine is considered to be the most effective drug
for treatment of Trichophyton spp infections. In general, we know that pathogens and
resistant strains follow the human migration routes. The lack of data about presence
of this novel genotype in European countries is because most clinical laboratories
do not use multilocus sequencing for routine identification of dermatophytes. SYNLAB
Estonia uses classical culture-based diagnostics and DNA-based diagnostics (in house
PCR) for dermatophytes, handling approx. 10000 sample per year. We are not able to
identify 1/5 of dermatophytes on species level. Most of commercial diagnostic DNA
panels for dermatophytes do not discriminate T. mentagrophytes and T. interdigitale.
In the light of new data diagnostic laboratories need to put more effort to identify
all dermatophytes on species level by molecular methods. It will help to map the changing
epidemiological situation of dermatophytosis in Europe.
Chen
P.-Y.
Chuang
Y.-C.
Wu
U.-I.
Sun
H.-Y.
Wang
J.-T.
Sheng
W.-H.
Chen
Y.-C.
Chang
S.-C.
National Taiwan University Hospital, Taipei, Taiwan
P040. An emerging clone of Candida tropicalis bloodstream isolates with ERG11 gene
mutations and overexpression conferring reduced activity of isavuconazole
Objectives: In Asia, in vitro activity of isavuconazole (ISA) against clinical Candida
isolates are seldomly reported, especially a rising concern that azole-resistant Candida
tropicalis is common (≥10%) in this region. Also, few studies characterized molecular
mechanisms of reduced isavuconazole activity against C. tropicalis.
Methods: Sixty-four non-duplicated C. tropicalis bloodstream isolates were prospectively
collected in National Taiwan University Hospital in 2017. In vitro susceptibility
to antifungal agents was performed by using the microdilution colorimetric Sensititre
YeastOne panel and the results were interpreted by the Clinical and Laboratory Standards
Institute. ISA MICs were determined using the EUCAST E.def 7.3.1 method. Wild-type
upper limit (wtUL) was defined as two two-fold dilutions above the MIC50. Isolates
with reduced ISA activity (RIA) were defined as their ISA MICs above wtUL. The ERG11
gene was sequenced for all isolates, and the results were compared to the ERG11 wild-type
sequence from C. tropicalis reference strain ATCC 750 (Genbank accession M23673).
Quantitative realtime reverse transcription-polymerase chain reaction (ERG11, CDR1,
and MDR1) were performed. Multilocus sequencing types were analyzed by the eBURST
to determine putative relationships between isolates.
Results: Among sixty-four C. tropicalis bloodstream isolates, the resistant/ non-wild
type rates of fluconazole, voriconazole, posaconazole or itraconazole were 21.5%,
30.7%, 64.6%, and 13.9%, respectively. The proportions of RIA was 18.8% (n = 12).
ISA MICs correlated with other azoles MICs by calculating with nonlinear regression
fits. The R
2 values were in the following orders: posaconazole (0.74) < itraconazole (0.82) <
voriconazole (0.86) < fluconazole (0.88). The mean percentage of ISA trailing was
27.4%. A total of 8 nucleotide mutations were detected in ERG11 gene of all isolates.
Only 2 (A395T/W and C461T/Y) belonged to missense mutations resulting in amino acid
substitutions (Y132F and S154F). There were fourteen isolates with one or two these
mutations all resistant to fluconazole. Twelve of them had RIA, all of which belonging
to the same clone. Gene relative expression levels among three groups (isolates with
low and high ISA trailing, and those with RIA) are shown Figure. Only ERG11 expression
levels demonstrated significant difference (P < 0.001), and neither did CDR1 nor MDR1
(P > 0.05). Those with RIA showed the highest mean ERG11 relative expression level
(3.51 ± 0.59 [S.D.]) with statistically significance, while the mean ERG11 levels
between high and low ISA trailing group were not statistically different (1.08 ± 0.69
and 0.93 ± 0.66, respectively).
Figure
Conclusion:
C. tropicalis bloodstream isolates in our report showed an emerging clone with pan-azole
resistance, including RIA. Fluconazole MICs best correlated to ISA MICs. Combined
ERG11 gene missense mutations and ERG11 overexpression conferred RIA.
Barber
A.E.
1
2
3
Born
J.
4
Sae-Ong
T.
2
Schliebner
I.
4
Kang
K.
2
Walther
G.
2
3
Panagiotou
G.
2
Deising
H.B.
4
Kurzai
O.
1
1University of Wuerzburg, Wuerzburg, Germany
2Leibniz HKI Jena, Jena, Germany
3National Reference Center for Invasive Fungal Infections, Jena, Germany
4Martin Luther University Haale-Wittenberg, Halle (Saale), Germany
P041. Comparative genomics of Aspergillus fumigatus and the influence of agriculture
on ecology and azole resistance
Objectives:
Aspergillus fumigatus is an environmentally ubiquitous saprophyte capable of causing
life-threatening invasive infections and allergic bronchopulmonary disease and current
management strategies rely on the azoles. Unfortunately, over the last decade there
has been a global emergence in resistance to the azoles in A. fumigatus and the dominant
resistance mechanism is of environmental origin, suggesting that resistance is emerging
through a selective pressure applied by the widespread usage of azoles in agriculture.
Methods: To examine the link between the use of azoles in agriculture and the emergence
of clinical resistance, systematic soil sampling was performed over a three year period
on seven conventional agricultural sites in Germany utilizing azole fungicides and
on six organic sites withholding these compounds. Conventional sites were evaluated
both before and after azole application. We also performed WGS on 250 environmental
and 50 clinical isolates.
Results: In total, 2875 soil samples were analyzed between 2016 and 2018. We observed
a high degree of variation in the abundance of A. fumigatus across sites, however,
fields with high A. fumigatus density tended to be consistently so from year to year.
Strikingly, we observed a significant reduction in the abundance of A. fumigatus on
conventional fields following azole treatment – a finding that was not repeated on
an organic agriculture control field – indicating that the application of azoles is
imposing a bottleneck on A. fumigatus. We detected a low overall resistance frequency
amongst agricultural isolates, with only 1-5% of isolates from 2016-2018 showing medical
azole resistance – a rate lower than the 15-20% resistance frequency observed by the
German National Reference Center for Invasive Fungal Infections during the same time
period. Susceptibility to commonly-applied agricultural azoles was also assessed and
isolates resistant to medical azoles almost always had elevated MICs to these agricultural
azoles, suggesting cross resistance. Importantly, we observed an increased tolerance
to both agricultural and medical azoles in the samples taken after the growing season
and application of azoles. At the genome level, isolates from different regions and
types of agriculture did not cluster separately, indicating a lack of population structure
in the fungus. Comparison of environmental isolates with clinical isolates revealed
several subgroups present in the environment that were not represented among clinical
samples. We also observed differences in the distribution of resistance mutations
between environmental and clinical isolates. Resistant environmental isolates were
exclusively either wild type at the cyp51a loci or carried the environmentally-derived
TR34/L98H mutation. Clinical isolates, in contrast, showed a much wider range mutations,
including substitutions at positions F219, M220, S297, F495, and G448.Ongoing work
is focused on defining fungal determinants critical for human infection as well as
genetic mechanisms associated with cyp51a-independent azole resistance.
Conclusion: We observed a marked reduction in A. fumigatus fields following azole
application and increased azole tolerance after the growing season and azole exposure.
No population structure was observed among environmental isolates, but whole genome
phylogeny identified genetic backgrounds enriched for among clinical isolates.
Borman
A.
Muller
J.
Walsh-Quantick
J.
Szekely
A.
Patterson
Z.
Palmer
M.
Fraser
M.
Johnson
E.
Public Health England UK National Mycology Reference Laboratory, Bristol, United Kingdom
P042. MIC distributions for amphotericin B, fluconazole, itraconazole, voriconazole,
flucytosine and anidulafungin and thirty-five uncommon pathogenic yeast species from
the UK determined using the CLSI broth microdilution method
Objectives: Epidemiological cut-off values and clinical interpretive breakpoints have
been developed for a number of antifungal agents with the most common Candida species
which account for the majority of infections due to pathogenic yeasts species. However,
less common species for which susceptibility data are limited are increasingly reported
in high risk patients and breakthrough infections.
Methods: The UK National Mycology Reference Laboratory performs routine antifungal
susceptibility testing of clinical isolates of pathogenic yeast submitted from across
the United Kingdom. Between 2002 and 2016, in excess of 32 000 isolates representing
94 different yeast species were referred to the laboratory. Here we present antifungal
susceptibility profiles generated over this 15 year period for amphotericin B, fluconazole,
voriconazole, itraconazole, anidulafungin and flucytosine against 35 species of uncommon
yeast. Minimum inhibitory concentrations (MICs) were obtained using CLSI broth microdilution
methodologies, and MIC data was interpreted against epidemiological cut-off values
and clinical breakpoints developed with Candida albicans, in order to identify species
with unusually skewed MIC distributions that potentially indicate resistance.
Results: Potential resistance to at least one antifungal agent (>10% of isolates with
MICs greater than the epidemiological cut-off or clinical breakpoint) was evidenced
for 29/35 species examined here. Four species exhibited elevated MICs with all of
the triazole antifungal drugs against which they were tested, and 21 species exhibited
antifungal resistance to agents from at least 2 different classes of antifungal agent.
Conclusion: The current study highlights the importance of rapid and accurate yeast
identification, and provides data to aid clinicians in deciding which antifungal regimens
are appropriate when confronted with infections with rarer yeasts.
Coste
A.T.
1
Li
J.
1
Khanna
N.
2
Goldenberger
D.
3
Garzoni
C.
4
Zhender
Z.
5
Boggian
K.
6
Neofytos
D.
7
Riat
A.
8
Bachmann
D.
1
Sanglard
D.
1
Lamoth
F.
9
10
1Microbiology Institute, University Hospital Lausanne, University of Lausanne, Lausanne,
Switzerland
2Division of Infectious Diseases and Hospital Epidemiology, University Hospital of
Basel, Basel, Switzerland
3University Hospital Basel, Clinical Microbiology Laboratory Medicine, Basel, Switzerland
4Riva Caccia 1b, Lugano, Switzerland
5SYNLAB Suisse SA, Bioggio, Switzerland
6Department of Infectious Diseases, Cantonal Hospital of Saint Gallen, Saint Gallen,
Switzerland
7Infectious Diseases, University Hospital of Geneva, Geneva, Switzerland
8Geneva University Hospitals, Service of Laboratory Medicine, Geneva, Switzerland
9Microbiology Institute, University Hospital Lausanne, Lausanne, Switzerland
10Infectious Diseases Service, CHUV, Lausanne, Switzerland
P043. Case-series of Candida albicans and glabrata echinocandin-resistant candidemia
in Switzerland
Objectives: Echinocandin drugs (anidulafungin, caspofungin, micafungin) represent
first-line therapy of candidemia. Echinocandin resistance, due to hotspot mutations
in the target FKS gene, has emerged among C. glabrata and C. albicans, which is associated
with poor outcome. Therefore, national surveillance programs of echinocandin resistance
are mandatory. The aims of this study were: i) to assess the epidemiology of echinocandin
resistance among C. albicans/glabrata in Switzerland, ii) to describe the characteristics
of candidemic episodes due to echinocandin-resistant isolates, and iii) to assess
the efficacy of high-dose echinocandin against echinocandin-resistant C. albicans
in an invertebrate model of infection.
Methods: Cases of candidemia attributed to C. albicans/glabrata resistant to echinocandins
(CLSI criteria) were identified by: i) screening of a collection of Candida bloodstream
isolates from the Fungal Infection Network of Switzerland (FUNGINOS) including all
candidemic episodes from 26 hospitals in Switzerland from 2004 to 2013, and ii) screening
of the microbiology databases of the most important university hospitals from 2014
to 2018. Echinocandin-resistant Candida isolates were sent to the reference laboratory
for antifungal susceptibility testing (CLSI protocol) and sequencing of FKS hotspots.
Clinical data of candidemia were collected. In order to assess a possible efficacy
of high-dose echinocandins against these isolates, we tested the efficacy of escalating
anidulafungin doses in a Galleria mellonella model of invasive candidiasis with a
C. albicans FKS mutant (S645P) compared to the wild-type SC5314 strain.
Results: Seven cases of candidemia due to echinocandin-resistant C. albicans/glabrata
were identified: 2 from the 2004-2013 cohort (incidence 0.1%) and 5 from the microbiological
database screening from 2014 to 2018. Four infections were due to C. albicans and
3 to C. glabrata. Candidemia originated from urine (2 cases), intravascular catheter
(2), gastro-intestinal tract (1) and primary bloodstream infection (1). All patients
received previous echinocandin therapy with a median duration of 18 days (range 11-60).
Overall mortality was 43%. One patient (C. glabrata infection) was cured by caspofungin
therapy despite in vitro resistance. Minimal inhibitory concentrations (MIC) ranges
for anidulafungin, caspofungin and micafungin were: 2-4, 1->16 and 1-4 µg/mL, respectively.
The following hotspot mutations were identified in C. albicans: S645P (n = 3) and
R1361G (n = 1). Among C. glabrata, 2 isolates displayed the S663P mutation in FKS2,
while no hotspot FKS mutation was recovered from one pan-echinocandin resistant isolate.
Because anidulafungin displayed some in vitro activity against C. albicans S645P mutant
(MIC 2 µg/mL), we assessed its in vivo activity in a Galleria mellonella model of
infection (Figure 1). While a dose-dependent response was observed in C. albicans
wild-type strain-infected larvae, no effect was observed against the C. albicans FKS
mutant despite high anidulafungin doses (12 mg/kg).
Conclusion: Our analysis suggests an increase in echinocandin resistance among C.
albicans/glabrata in Switzerland with 5 cases identified within the last 5 years,
while only two cases were reported in a cohort including all candidemias from 2004
to 2013. Previous echinocandin exposure was observed in all cases. Our invertebrate
model suggests that high-dose echinocandins are ineffective against C. albicans FKS
mutants despite some level of in vitro activity.
Rautemaa-Richardson
R.
1
2
Moore
C.
1
2
Rawson
K.
2
Novak-Frazer
L.
1
2
Angulo
D.
3
Barat
S.
3
Richardson
M.D.
1
2
1Division of Infection, Immunity and Respiratory Medicine, University of Manchester,
Manchester, United Kingdom
2Mycology Reference Centre, Excellence Center For Medical Mycology (ECMM), Wythenshawe
Hospital, Manchester University NHS Foundation Trust, Manchester, United Kingdom
3Scynexis Inc, New Jersey, United States of America
P044. Aspergillus isolates from patients with chronic pulmonary aspergillosis mycologically
and clinically resistant to azole antifungals are sensitive to Ibrexafungerp (SCY-078)
Objectives: Ibrexafungerp (formerly MK-3118, SCY-078) is a novel and structurally
distinct triterpenoid glucan synthase inhibitor whose oral availability and efficacy
has already been demonstrated against a wide spectrum of Candida species. Currently
the drug is being evaluated for efficacy in invasive and chronic pulmonary aspergillosis.
Ibrexafungerp has been shown to be effective in vitro and in vivo against a broad
range of Aspergillus species, including drug-resistant strains. In vitro activity
is an important efficacy indicator of therapeutic success or failure. The objective
of this study was to determine the activity of Ibrexafungerp against a panel of Aspergillus
strains isolated from the respiratory tract of patients unresponsive to or failing
azole therapy, and which were previously shown to be resistant to one or more azole
antifungals (itraconazole, voriconazole, posaconazole and isavuconazole) using the
EUCAST E.Def 10.1 standard.
Methods: Patients failing azole therapy for chronic pulmonary aspergillosis were identified
in the weekly departmental multi-disciplinary team meetings and their isolates selected
for Ibrexafungerp sensitivity testing. Twenty-two Aspergillus fumigatus complex, three
A. flavus complex and one A. niger complex strains with varying degrees of resistance
to one or more azole antifungals were tested by measuring the minimum effective concentration
(MEC) for Ibrexafungerp following the EUCAST E.Def 10.1 standard. Where required,
isolates were sequenced (using primers to the internal transcribed spacers (ITS),
beta-tubulin (bt) and calmodulin (cal) genes) to identify to the species level.
Results: The Ibrexafungerp MEC range for the 26 Aspergillus spp. isolates was 0.008
to 0.25 mg/l. Isolates that were deemed to be resistant to all azoles had an Ibrexafungerp
MEC of 0.015 to 0.25 mg/l. One isolate that was resistant to all azoles and amphotericin
B was sensitive to Ibrexafungerp (MEC 0.125 mg/l).
Conclusion: The exquisite in vitro activity of Ibrexafungerp against Aspergillus fumigatus,
A. flavus and A. niger isolated from patients with chronic pulmonary aspergillosis
suggests that this new compound has potential for treating patients with this condition
and similar manifestations of pulmonary aspergillosis.
Fathima
U. Almas
1
Kindo
A.J.
1
Kumar
S. Prasanna
2
Thirunarayan
M.
3
Sree
V. Lakshmi
4
1Microbiology, Sri Ramachandra Institute of Higher Education and Research, Chennai,
India
2Ent, Sri Ramachandra Institute of Higher Education and Research, Chennai, India
3Microbiology, Apollo Hospitals, Chennai, India
4Microbiology, Apollo Speciality Hospital, Chennai, India
P045. Analysis of the susceptibility pattern among Aspergillus isolates against the
commonly used Antifungal agents in a tertiary care centre in South India.
Objectives: To do in vitro susceptibility testing and analyse the difference in the
Antifungal susceptibility profile of Aspergillus isolates by Broth Microdilution method.
Methods:
Aspergillus grown from various clinical samples (Ear swab, Bronchial wash, Endotracheal
Aspiration, Paranasal sinus, BAL, Sputum) was subcultured on Sabouraud’s Dextrose
Agar/Oat meal Agar. Tease mount/Slide culture was done to study the morphological
features of the hyphae, size, shape and arrangement of the conidia. Antifungal susceptibility
testing(AFST) was done according to CLSI guidelines M38-A2 2008 documented reference
method for Broth Microdilution Antifungal susceptibility of Filamentous fungi. Antifungal
stock solutions of AmphotericinB, Voriconazole, Posaconazole, Itraconazole and Caspofungin
was prepared in di-methyl sulfoxide and stored at -700 C. RPMI medium was prepared,
adjusted to pH 7 and filter sterilized. Sterility was checked and stored at 40 C.
Dilution of the stock solution was done as per the guidelines. Inoculum was prepared
by adjusting conidial suspension in sterile distilled water to 0.5 McFarland Turbidity
standard; to 100 µl of drug solution, 100 µl of inoculum in RPMI 1640 medium was added.
Test micro-titre plate was incubated for 48hrs at 350 C. Growth and sterility control
wells were included in each test. AFST-READING RESULT: For AmphotericinB, Voriconazole,
Posaconazole, Itraconazole - MIC is the first well without visible growth. For Echinocandins,
eg. Caspofungin- MEC is the point (lowest value) where a visible change in growth
as compared to the positive growth is noted.
Results: A total of 30 Aspergillus isolates were collected-Aspergillus niger(14),
Aspergillus flavus(9), Aspergillus fumigatus(6) & Aspergillus terreus(1). The result
was compiled based on mean MIC values of 21 isolates are as follows.
The MIC values of other isolates and Amphotericin B is yet to be completed.
Conclusion: The common Aspergillus species was Aspergillus niger which was obtained
from Ear swab. The susceptibility profile showed high MIC for Itraconazole in case
of Aspergillus niger and high MIC for Itraconazole, Voriconazole and Posaconazole
in case of Aspergillus flavus. For Aspergillus fumigatus no such high MIC was recorded.
In case of Aspergillus terreus, being resistant in nature the low MIC is recorded
only with Caspofungin. Due to less susceptibility of Aspergillus to the common Antifungal
agents, it is important to perform antifungal susceptibility testing to reduce the
morbidity and mortality in patients especially with Invasive Aspergillosis.
Morio
F.
1
Lombardi
L.
2
Binder
U.
3
Loge
C.
1
Robert
E.
1
Lass-Flörl
C.
3
Butler
G.
2
Pape
P. Le
1
1Ea1155 Iicimed, Nantes University Hospital, NANTES, France
2School of Biomolecular and Biomedical Science, Conway Institute, University College
Dublin, Dublin, Ireland
3Department of Hygiene, Microbiology and Public Health, Division of Hygiene and Medical
Microbiology, Medical University Innsbruck, Innsbruck, Austria
P046. CRISPR-Cas9 as a tool box to investigate antifungal resistance
Objectives: Antifungal resistance in human pathogenic fungi is increasingly reported
and a public health issue. Deciphering the mechanisms underlying drug resistance strongly
relies on genetic manipulation techniques such as generating mutant strains carrying
specific mutations or gene deletions. However, these processes have often been time-consuming
and cumbersome in fungi. Over the last decade, the groundbreaking discovery of Clustered
Regularly Interspaced Short Palindromic Repeats and the associate protein Cas9 (CRISPR-Cas9)
revolutionized genome editing. We evaluated the potential of a CRISPR-Cas9 method,
as a tool to perform precise genome editing of the ERG11 gene in the context of azole
resistance.
Methods: A new plasmid-based and marker-less CRISPR-Cas9 genome-editing method, recently
developed for Candida parapsilosis (1), was used to investigate the potential contribution
to azole resistance of two different single nucleotide polymorphisms (G1126A and G1372A),
leading to amino acid substitutions (L376I and G458S, respectively) in the ERG11 gene,
identified in an azole-resistant clinical isolate of Candida orthopsilosis. These
mutations were introduced by homology directed recombination (HDR) using a donor template
into a fluconazole-susceptible C. orthopsilosis background isolate. In vitro susceptibility
of the transformants displaying the expected mutations (two clones for each engineered
mutation) to azoles was then performed.
Results: This approach allowed us to engineer both mutations in the fluconazole-susceptible
background isolate. Though engineering L376I was straightforward, editing G458S required
the addition of silent mutations within the core of the Cas9 recognition site to prevent
Cas9 from recutting again. Eventually, in vitro susceptibility testing of the corresponding
transformants demonstrated that G458S, but not L376I, confers resistance to fluconazole
(MIC increased by 8-fold by introducing the homozygous mutation) and also affected
voriconazole susceptibility. Importantly, after 3 passages on YPD at 30°C, transformants
were not resistant to nourseothricin (selection marker) anymore, showing the rapid
loss of the plasmid containing all the CRISPR-Cas9 components, thus proving that it
did not integrate in the genome.
Conclusion: Our work supports the contribution of G458S but not L376I in fluconazole
and voriconazole resistance in C. orthopsilosis (2). Besides these findings, this
study highlights CRISPR-Cas9 genome editing as a powerful tool to streamline the study
of antifungal resistance in human pathogenic fungi. Though critical points should
be considered (e.g. HDR drops with cut-to-mutation distance), this approach paves
the way for an easier screening approach of any SNP, occurring in any gene suspected
to be involved in antifungal resistance. 1-Lombardi et al., Plasmid-Based CRISPR-Cas9
Gene Editing in Multiple Candida Species. mSphere. 2019 Mar 13;4(2). pii: e00125-19.
doi: 10.1128/mSphere.00125-19. 2-Morio et al., Precise genome editing using a CRISPR-Cas9
method highlights the role of CoERG11 amino acid substitutions in azole resistance
in Candida orthopsilosis. J Antimicrob Chemother. 2019 May 18. pii: dkz204. doi: 10.1093/jac/dkz204.
Satish
S.
1
Jimenez-Ortigosa
C.
1
Zhao
Y.
1
Lee
M.H.
1
Dolgov
E.
1
Kruger
T.
2
Park
S.
1
Denning
D.
3
Kniemeyer
O.
2
Brakhage
A.
2
Perlin
D.S.
1
1Center For Discovery and Innovation, Hackensack Meridian Health, Nutley, United States
of America
2Leibniz Institute for Natural Product Research and Infection Biology (HKI), Jena,
Germany
3University of Manchester, Manchester, United Kingdom
P047. Changes in the lipid microenvironment of β-(1,3)-D-glucan synthase of Aspergillus
fumigatus is a new clinically important echinocandin resistance mechanism
Objectives:
Aspergillus fumigatus is a leading cause of opportunistic invasive fungal infections.
Resistance to first-line triazole antifungals has led to therapy with echinocandin
drugs. Recently, we identified several high Minimum Effective Concentration (MEC)
A. fumigatus clinical isolates from patients failing echinocandin therapy. Echinocandin
resistance in Candida is known to arise from amino acid substitutions in β-(1,3)-D-glucan
synthase encoded by the fks1 gene. Yet, these clinical isolates did not contain mutations
in fks1, indicating an undefined resistance mechanism. The objective of this study
was to explore a novel mechanism of echinocandin resistance independent of fks1 mutations.
Methods: To explore this new mechanism, we used a lab-derived strain, RG101, which
does not contain fks1 mutations but is resistant to caspofungin (CAS). Inhibition
of glucan synthase enzyme isolated from RG101 grown in the absence and presence of
CAS was determined in vitro using radioactive substrate and IC50 (drug concentration
required for 50% reduction of activity) values were measured. Post-translational modifications
(PTMs) potentially leading to resistance was explored by evaluating enzyme derived
from RG101 grown in the absence and presence of CAS (1 µg/mL) using nano LC-MS/MS.
A comprehensive lipidomics analysis was also performed to compare lipid profiles of
glucan synthase containing fractions derived from RG101 grown in the absence and presence
of CAS (1 µg/mL) using LC-MS/MS. To measure Reactive Oxygen Species (ROS) levels induced
by different echinocandins, a 2’,7’-dichlorofluorescin diacetate (DCFDA)-based fluorescence
assay was used, and ROS levels measured after one hour of drug exposure.
Results: Glucan synthase isolated from RG101 was fully sensitive to echinocandins.
Yet, exposure of RG101 to CAS during growth yielded a modified enzyme that was drug
insensitive (4 log-orders) in kinetic inhibition assays, and this insensitivity was
also observed for enzymes isolated from clinical isolates. To understand this alteration,
we analyzed whole enzyme post-translational modifications, but found none linked to
resistance. However, analysis of the lipid microenvironment of CAS-induced resistant
enzyme revealed a prominent increase in abundance of dihydrosphingosine (DhSph) and
phytosphingosine (PhSph). Exogenous addition of DhSph and PhSph to sensitive enzyme
recapitulated the drug insensitivity of the CAS-derived enzyme. Further analysis demonstrated
that CAS induces mitochondrial-derived ROS, and dampening ROS formation by antimycin
A or thiourea eliminated drug-induced resistance.
Conclusion: We conclude that CAS-induces cellular stress, promoting formation of ROS,
triggering an alteration in the composition of plasma membrane lipids within the local
environment of glucan synthase, rendering it insensitive to echinocandins. We have
discovered a new mechanism of resistance in A. fumigatus independent of the well-characterized
FKS mutation mechanism observed in Candida. This study also identifies an off-target
effect of CAS, ROS production in A. fumigatus, and integrates oxidative stress and
sphingolipid alterations into a novel mechanism of resistance. This stress-induced
response has implications for drug resistance and/or tolerance mechanisms in other
fungal pathogens.
De-La-Fuente
I.
1
Matilla-Gutiérrez
A.
1
Guridi
A.
1
Sevillano
E.
1
Pemán
J.
2
Eraso
E.
1
Quindós
G.
1
1Immunology, Microbiology and Parasitology, University of the Basque Country (UPV/EHU),
Bilbao, Spain
2Servicio De Microbiología, Hospital Universitario y Politécnico La Fe, Valencia,
Spain
P048. In vitro activity of fluconazole in combination with magnolol against Candida
auris
Objectives:
Candida auris is an emerging pathogen that causes outbreaks worldwide. This fungus
is responsible for severe infections such as invasive candidiasis that present a high
overall mortality. Treatment of these infections suppose a clinical challenge due
to the high levels of multidrug-resistance in C. auris, which makes the finding of
new therapeutic alternatives an important issue. Combination of fluconazole with different
natural compounds has shown to be effective against fluconazole-resistant Candida
isolates. For this reason, the aim of this study was to assess the in vitro interaction
between fluconazole and magnolol, a polyphenol extracted from Magnolia officinalis,
or epigallocatechin gallate (EGCG), a polyphenol obtained from Camellia sinensis,
against clinical isolates of C. auris.
Methods: We studied 21 C. auris isolates from different clinical specimens (seven
from blood, seven from oropharynx and six from urine). Candida parapsilosis ATCC 22019
and Candida krusei ATCC 6258 were used as quality controls. Different fluconazole
combinations with magnolol or EGCG were studied. Fluconazole concentrations were selected
according to the minimum inhibitory concentration (MIC) determined by the European
Committee on Antibiotic Susceptibility Testing (EUCAST) EDef 7.3.1 document. MIC was
defined as the lowest drug concentration causing 50% growth reduction after 24 h of
incubation. Magnolol and EGCG concentrations ranged from 0.125 to 64 μg/mL. The interaction
between fluconazole and magnolol or EGCG were assessed by a checkerboard microdilution
method. In vitro interactions of drug combinations were interpreted in terms of fractional
inhibitory concentration index (FICI) as follows: FICI ≤ 0.5, synergistic; 0.5 < FICI
≤ 1, additive; 1 < FICI ≤ 4, indifferent and FICI > 4 antagonistic.
Results: Fluconazole MIC range against C. auris was 64 to > 64 μg/mL and magnolol
MIC range 16 to > 64 μg/mL; however, combination of both drugs reduced the concentrations
necessary for inducing 50% reduction in the growth of the isolates. The effect of
the combination of fluconazole with magnolol against seven isolates (33.3%) was interpreted
as additive. The effect against the other 14 isolates was interpreted as indifferent,
although the fluconazole MICs decreased to 1-16 μg/mL. The combination of fluconazole
with EGCG was indifferent against all isolates and did not have any influence in the
MICs of fluconazole.
Conclusion: The combination of fluconazole with magnolol significantly reduced the
fluconazole MICs against all of the C. auris isolates, showing additive effect in
seven of them. The combination of fluconazole with EGCG showed indifferent effect.
Funding: This work was co-supported by the Consejería de Educación, Universidades
e Investigación (GIC15/78 IT-990-16) of Gobierno Vasco-Eusko Jaurlaritza.
Scroferneker
M.L.
1
Pagani
D.
2
Souza
C. C. Tavares De
2
Vieira
A. M.
3
Hellwig
A.H. Da Silva
4
Rios
I. Da S.
3
Heidrich
D.
4
Fuentefria
A. M.
2
Silva
P. Valente Da
2
1Department of Microbiology, Immunology and Parasitology, Universidade Federal do
Rio Grande do Sul, Porto Alegre, Brazil
2Postgraduate Program In Agricultural and Environmental Microbiology, Universidade
Federal do Rio Grande do Sul, Porto Alegre, Brazil
3Federal University of Rio Grande do Sul, Porto Alegre, Brazil
4Postgraduate Program In Medicine: Medical Sciences, Universidade Federal do Rio Grande
do Sul, Porto Alegre, Brazil
P049. Resistance to medical importance antifungals in environmental yeasts
Objectives: The aim of the study was to investigate the presence of potentially pathogenic
yeasts from a lagoon in South America.
Methods: The lagoon was sampled in the summer of 2019. The water samples were inoculated
in acidified YM agar medium (1% glucose, 0.5 % peptone, 0.3 % malt extract, 0.3 %
yeast extract, 2 % agar) and incubated at 25 ºC for 30 days. Representative yeast
colonies were selected and stored in YM medium with 30 % of glycerol at -20 ºC and
in GYMP agar medium (2 % glucose, 2 % malt extract, 0.5 % yeast extract, 0.2 % monobasic
sodium phosphate, 2 % agar) agar slants coated with mineral oil, maintained at 4 °C.
We tested 8 environmental yeast strains. The antifungal susceptibility test was performed
according to CLSI protocol, to select yeasts with possible resistance to clinical
importance antifungals. The yeasts were tested in the antifungal concentrations established
for Candida species against fluconazole, caspofungin and amphotericin B.
Results: Four isolates had MIC ≥ 64 μg/mL to fluconazole, none of them were susceptible
to caspofungin, with MIC ≥ 2 μg/mL. To amphotericin B, six strains had MIC ≥ 1μg/mL.
Three isolates were considered resistant to all tested antifungals, following the
guidelines for Candida species.
Conclusion: The preliminary findings feature this lagoon environment as a possible
resistance genes reservoir and fungal infection source. Further studies are needed
to have a better understanding of this environment and the fungal resistance.
Menéndez-Manjón
P.
1
Arrieta-Aguirre
I.
1
Cuétara
M.S.
2
López-Soria
L.
3
García-Ruiz
J.C.
4
Moragues
M.D.
1
1Enfermería I, Universidad del País Vasco UPV/EHU, Leioa, Spain
2Microbiologia, Hospital Severo Ochoa, Leganés, Madrid, Spain
3Microbiologia, Hospital Universitario Cruces, Barakaldo, Spain
4Biocruces Health Research Institute, Hospital Universitario Cruces, Barakaldo, Spain
P051. A natural substitution in FksB Hot Spot 2 of Saprochaete capitata probably related
to reduced susceptibility to echinocandins
Objectives:
Saprochaete capitata is an emerging opportunistic pathogen reported in patients with
haematological malignancies. Antifungal clinical breakpoints are not established and
therapy remains empirical. S. capitata shows reduced susceptibility to echinocandins,
which inhibit the enzyme β-1,3-D-glucan synthase (Fks) involved in the cell wall synthesis.
Resistance to echinocandins is linked to mutations in two highly conserved regions
of Fks, Hot Spot 1 (HS1) and Hot Spot 2 (HS2). We already reported a natural substitution
F-to-L in Fks-HS1 that might be linked to this fungus low susceptibility to echinocandins
(Arrieta-Aguirre et al., 2018). Mutations in HS2 have also been related to reduced
susceptibility to echinocandins. Therefore, we resolved to sequence HS2 of S. capitata
and search for mutations that could be related to echinocandin resistance.
Methods: DNA of 11 strains of S. capitata was extracted by mechanical cell disruption
and purified with the DNeasy Plant Mini Kit (Qiagen). Since HS1 and HS2 are around
2500 bp apart, we applied a primer walking strategy of smaller fragments sequencing
to locate them. With a specific primer from the HS1 sequence and a degenerate primer
designed for the HS2 region, we obtained an amplicon for one of the strains that allowed
us to design downstream specific primers that led us to HS2. Finally, we designed
specific primers to sequence HS2 in the rest of the strains and the sequences were
compared with other fungal species.
Results: The alignment of Fks-HS2 of S. capitata and other species revealed a natural
substitution R-to-Q in the fourth position of HS2. The recently published genome of
S. capitata reveals two Fks, FksA and B. Our sequence corresponds to FksB, which is
more similar to Fks2 than Fks1 of other species. In C. glabrata, when R in the fourth
position of Fks2-HS2 is modified it results in a deep decrease in the susceptibility
to echinocandins (Jensen, 2016). Therefore, the natural substitution R-to-Q in FksB-HS2
of S. capitata could be related to resistance.
Conclusion:
S. capitata has at least two natural substitutions (F-to-L in Fks-HS1 and R-to-Q in
Fks-HS2) that may be involved in its reduced susceptibility to echinocandins. However,
the effect of both substitutions still needs to be verified empirically. This work
contributes to understanding the molecular basis of antifungal resistance of this
fungus and may help to improve its treatment. Financial support: Grupos de Investigación
Consolidados of the Basque Government (GIC15/103; IT913-16) and Grant FPI of the Basque
Government to PM-M.
Melo
A.
1
Matta
D. Da
1
Favarello
L.
1
Oliveira
C.
2
Sukiennik
T.
3
Telles
F.
4
Nucci
M.
5
Colombo
A.
1
1Medicine, Federal University of São Paulo, São Paulo, Brazil
2Medicine, Hospital Universitário do Oeste do Paraná, Cascavel, PR, Cascavel, Brazil
3Medicine, Santa Casa de Porto Alegre, Porto Alegre, RS, Porto Alegre, Brazil
4Medicine, Hospital de Clínicas da Universidade Federal do Paraná UFPR, Curitiba PR,
Curitiba, Brazil
5Medicine, Hospital Universitário da Universidade Federal do Rio de Janeiro, Rio de
Janeiro RJ, Rio de Janeiro, Brazil
P052. Fluconazole resistance in Candida tropicalis blood stream isolates collected
from 16 Brazilian Medical Centers
Objectives:
C. tropicalis is highly prevalent among episodes of candidemia in Latin America. The
progressive use of fluconazole and other triazoles in regimens of prophylaxis and
empirical therapy in high-risk patients has led to selective pressure and emergence
of secondary resistance among Candida spp. The aim of this study was to check for
temporal trends in the rates of azole resistance among C. tropicalis blood stream
isolates collected from patients assisted at 16 Brazilian medical centers at any time
within 2007 and 2018.
Methods: We selected isolates of C. tropicalis stored at the culture collection of
Laboratório Especial de Micologia (LEMI) – Universidade Federal de São Paulo, Brazil,
that were obtained from candidemic patients admitted in 16 medical centers along the
period of study. The isolates were identified at species level by Matrix-Assisted
Laser Desorption Ionization-Time of Fligh Mass Spectrometry (MALDI TOF MS). In vitro
antifungal susceptibility tests to fluconazole and voriconazole were evaluated by
the Clinical & Laboratory Standards Institute (CLSI ) microbroth method (documents
M27-A3 and M27-S4). For quality control of in vitro assays we used the reference strains
C. parapsilosis ATCC 22019, C. krusei ATCC 6258 and C. tropicalis ATCC 750.
Results: In order to check for historical trends of azole resistance among C. tropicalis
isolates, we compared the MIC values generated with fluconazole and voriconazole against
200 blood stream isolates collect at any time within two different periods: P1 (2007-2012)
versus P2 (2013-2018). Statistical differences were checked by student T Test. After
testing all 200 isolates, 3.5% of C. tropicalis strains were considered to be non-susceptible
to azoles. As illustrated bellow, we found resistance rates of C. tropicalis against
fluconazole of 1.9% and 3.2% in P1 and P2, respectively (p>0.05). In regard to voriconazole,
resistance rates of 0 and 1% were documented in P1 and P2, respectively (p>0.05).
Conclusion: After testing 200 blood stream isolates of C. tropicalis we identified
a rate of 3.5% of isolates exhibiting limited susceptibility (SDD/R) to fluconazole
and/or voriconazole. Otherwise, we were not able to demonstrate any historical trend
in terms of increasing rates of azole resistance among C. tropicalis isolates collected
along the 2 periods of study.
Jan
G.
1
Kristensen
L.
2
Bentsen
I. Bach
2
Nielsen
M.
2
1Department of Clinical Microbiology, Aarhus University Hospital, Aarhus N, Denmark
2Department of Clinical Microbiology, Aarhus University Hospital, Aarhus, Denmark
P053. Evaluation of spectrophotometric reading of EUCAST antifungal susceptibility
testing of Aspergillus fumigatus
Objectives: The rise in azole resistance in Aspergillus fumigatus
1 highlights the importance of antifungal susceptibility testing. While azole containing
agar plates have been shown to have excellent sensitivity and specificity in detecting
azole resistance2, access to MIC testing is still important. As commercially available
methods have serious limitations reference broth microdilution is the only feasible
option at present. The EUCAST method for antifungal susceptibility testing of moulds
relies on visual reading of MICs which is laborious and time-consuming. A recent study3
suggests that spectrophotometric determination of MICs of azoles may improve objectivity
and allow automatisation of the EUCAST E.Def 9.3 method. The scope of this study is
to evaluate the spectrophotometric metod in our center using seven antifungal drugs.
Methods: 22 clinical isolates from A. fumigatus species complex were selected for
the study. 9 isolates were resistant to at least one azole while 13 were wild type.
All isolates were tested against itraconazole, voriconazole, isavuconazole, posaconazole,
amphotericin B, natamycin and terbinafine using EUCAST E.Def 9.3. Visually determined
MICs were read at complete inhibition of growth.
Spectrophotometric reading was performed using a Thermo Multiskan FC microplate reader
with a 492 nm filter. The OD values of the negative control wells were subtracted
from all wells and MICs were determined as the lowest concentration corresponding
to either 5% growth compared to the drug free control or as 0.02 OD values above background.
Spectrophotometrically determined MICs were compared to visually determined MICs.
For all drugs the quantitative agreement within 0, 1 and 2 two-fold dilutions was
calculated. For itraconazole, voriconazole, isavuconazole, posaconazole and amphotericin
B categorical agreement was calculated using EUCAST Clinical breakpoints for fungi
v. 9.0.
Results:
Results are shown in Table 1. When using the 5% growth endpoint we found a high level
of essential agreement (95-100%) for all drugs and a high level of categorical agreement
(86-95%) for itraconazole, voriconazole, isavuconazole, posaconazole and amphotericin
B. of importance no major or very major errors was observed. When using the 0.02 OD
values above background endpoint however we found only 64% essential agreement for
terbinafine and a categorical agreement of 68% for posaconazole including 5% very
major errors.
Conclusion: Spectrophotometric determination of MICs using the EUCAST E-Def 9.3 for
A. fumigatus species complex appears to be a promising method for all tested drugs.
Highest agreement between visual and spectrophotometric MICs is obtained using the
5% growth endpoint. References 1. van der Linden J et al. Prospective Multicenter
International Surveillance of Azole Resistance in Aspergillus fumigatus. Emerg Infect
Dis. 2015;21(6):1041-1044. 2. Arendrup MC et al; Multicentre validation of 4-well
azole agar plates as a screening method for detection of clinically relevant azole-resistant
Aspergillus fumigatus, Journal of Antimicrobial Chemotherapy, Volume 72, Issue 12,Pages
3325–3333 3. Meletiadis, J. et al., Spectrophotometric reading of EUCAST antifungal
susceptibility testing of Aspergillus fumigatus, Clinical Microbiology and Infection,
Volume 23, Issue 2, 98 - 103
Buil
J.
1
Oliver
J.
2
Law
D.
2
Tehupeiory-Kooreman
M.
1
Rex
J.
2
Hokken
M.
1
Melchers
W.
1
Birch
M.
2
Verweij
P.E.
1
1Medical Microbiology, Radboudumc, Nijmegen, Netherlands
2F2G Ltd, Manchester, United Kingdom
P054. Molecular mechanism and frequency of olorofim resistance in Aspergillus fumigatus
Objectives: Olorofim (OLO) is a new antifungal agent with a novel mechanism of action,
targeting dihydroorotate dehydrogenase (DHODH) in the de novo pyrimidine biosynthesis
pathway. It is active against both azole-susceptible and azole-resistant strains of
Aspergillus fumigatus. Thus, OLO may be an important treatment option in patients
with Aspergillus disease, including azole-resistant cases. Azole resistance selection
occurs in >10% of patients with chronic pulmonary aspergillosis during itraconazole
(ITZ) therapy. In this study, we analysed the frequency of resistance mutation induction
of OLO in A. fumigatus in three different strains using two different methods. The
underlying OLO resistance mechanism was also investigated.
Methods: Method 1: From two A. fumigatus isolates, AZN 8196 and V139-36, 10 single
colonies were separately inoculated onto Sabouraud agar and incubated at 37°C for
96h. From each culture 1 x 108 conidia were applied to 6 RPMI agar plates containing
either 0.5 mg/L OLO or 8 mg/L ITZ.. Plates were incubated at 37°C for up to 7 days.
Colonies growing on drug-containing plates were subcultured on RPMI agar containing
0.5 mg/L OLO or 8 mg/L ITZ to confirm resistance. Method 2: Spore stocks of A. fumigatus
strain Af293 were prepared and inoculated onto yeast nitrogen base with glucose agar
(YNBG) containing 0.25 mg/L OLO. A total of 8 x 109 spores were inoculated into 12
x 100 ml YNBG-OLO agar plates that were subsequently incubated for 5 days at 35°C.
Colonies growing on drug-containing plates were subcultured on YNBG-OLO to confirm
resistance. The mean rate of resistance was calculated for each isolate. The pyrE
gene which codes for DHODH was sequenced for initial OLO-susceptible strains and -resistant
progeny isolates and analysed for mutations.
Results: Method 1: The resistance rate of OLO was 1 in 5 x 107 (frequency of 2 x 10-8)
for AZN 8196 compared to 1 in 5 x 106 (2 x 10-7) for ITZ. For V139-36 the resistance
rate of OLO was 1 in 9 x 107 (1.1 x 10-8) while the rate for ITZ was 1 in 8 x 106
(1.3 x 10-7) Method 2: Resistance to OLO in Af293 occurred at a rate of 1 in 6 x 108
(frequency of 1.7 x 10-9). Sequencing of pyrE revealed a hotspot for resistance mutations
in the pyrE gene for OLO resistance in A. fumigatus at locus Gly119. Four amino acid
substitutions were found; G119V, G119C, G119S and G119A. A single OLO-resistant mutant
was obtained that carried the wild type DHODH sequence, but this mutant was slow growing
compared with parent.
Conclusion: We demonstrate that OLO resistance can be selected in A. fumigatus and
can be mediated by mutations in the pyrE gene at locus G119. The frequency of resistance
varied from approximately 2 x 10-8 to 1.7 x 10-9 for spontaneous mutations. The resistance
rate of OLO was 5 to 10-fold lower compared to ITZ. The low resistance rate found
in these experiments indicates that resistance selection may be less frequent in patients
with Aspergillus diseases treated with OLO.
Tóth
Z.
1
Forgács
L.
1
Locke
J.B.
2
Kardos
G.
1
Nagy
F.
1
Kovács
R.
1
Borman
A.
3
Majoros
L.
1
1Department of Medical Microbiology, University of Debrecen, Debrecen, Hungary
2Cidara Therapeutics, San Diego, United States of America
3Public Health England UK National Mycology Reference Laboratory, Bristol, United
Kingdom
P055. Comparison of killing activity of rezafungin, anidulafungin, caspofungin and
micafungin against Candida auris in the presence and absence of serum
Objectives:
Candida auris is an emerging, difficult-to-treat multiresistant pathogen against which
echinocandins are the recommended standard-of-care treatment. Rezafungin is a next-generation
echinocandin with similar in vitro activity to existing echinocandins yet it attains
much higher in vivo concentrations and exposures due to its extended half-life and
front-loaded dosing paradigm. Because in vitro killing data with existing echinocandins
are limited against C. auris and are lacking for rezafungin, we compared rezafungin
to anidulafungin, caspofungin, and micafungin in time-kill assays against C. auris
isolates in standard RPMI-1640 medium and also investigated the impact of serum on
in vitro killing trends.
Methods: Two C. auris clinical isolates from each clade (Japanese/Korean, South Asian/Indian
and South African, obtained from the National Mycology Reference Laboratory, UK) were
tested. Both South African isolates were autoaggregative. MICs in RPMI-1640 +/-50%
human serum were determined using the standard broth macrodilution method (CLSI M27
Ed4). Time-kill studies with the four echinocandins were performed from 0.25 to 32
mg/L in both media, and killing rates were compared. Positive k values indicate killing;
negative values indicate growth.
Results: MIC values were higher in 50% serum than in RPMI-1640 alone (Table 1). In
RPMI-1640, at 1xMIC or higher concentrations all four echinocandins showed only fungistatic
effect. The highest k value with rezafungin (1.34 1/h) was at 32 mg/L against isolate
209. However, none of the echinocandins produced any CFU decrease against aggregating
isolates (k values in cases of isolates 2 and 204 were always negative). Similar results
were found in cases of caspofungin and micafungin against isolates 12, 15 and 27,
and isolate 15, respectively. Re-growth was frequently observed for all four echinocandins.
In 50% serum, growth rates were significantly lower. At 1xMIC or higher concentrations,
echinocandins showed concentration-dependent killing activity, however, this CFU reduction
never reached the fungicidal threshold. The highest k values were 0.34, 0.33, 0.34
and 0.29 1/h for rezafungin, anidulafungin, caspofungin and micafungin, respectively.
However, k values were always negative at 1-32 mg/L, in the case of isolate 2 (an
autoaggregative isolate) for all echinocandins and in cases of isolates 27 and 204
for caspofungin. Table 1. MIC values for rezafungin and echinocandin comparators against
C. auris strains in the presence and absence of 50% serum.
Conclusion: Killing activity in RPMI-1640 alone was less consistently positive than
in 50% serum and only fungistatic activity was detected. Aggregating isolates were
less susceptible to echinocandins than non-aggregating isolates. Rezafungin showed
the same or better activity than anidulafungin and micafungin at clinically attainable
concentrations. The trend towards stronger killing activity in the presence of serum
helps to account for the disconnect between the modest time-kill activity of echinocandins
in vitro versus their strong efficacy against C. auris in vivo, as rezafungin has
previously demonstrated in animal models.
Forgács
L.
1
Tóth
Z.
1
Locke
J.B.
2
Nagy
F.
1
Balázs
B.
1
Prépost
E.
1
Kardos
G.
1
Kovács
R.
1
Szekely
A.
3
Borman
A.
3
Majoros
L.
1
1Department of Medical Microbiology, University of Debrecen, Debrecen, Hungary
2Cidara Therapeutics, San Diego, United States of America
3Public Health England UK National Mycology Reference Laboratory, Bristol, United
Kingdom
P056. Frequency of paradoxical and trailing effects with rezafungin, anidulafungin,
caspofungin and micafungin against Candida species
Objectives: Currently, echinocandins are the first-line agents for treatment of invasive
Candida infections. Echinocandins demonstrate excellent efficacy and fungicidal activity
against Candida species in vivo. However, echinocandins can exhibit some disconnected
growth phenomena in vitro, such as paradoxical effect (PE) where activity decreases
at higher drug concentrations and the trailing effect (TE) where complete growth inhibition
is never achieved or occurs at dilutions well above the 50% endpoint used to determine
MIC values. PE and TE can significantly alter the determination/interpretation of
MIC values and impact other in vitro assays. Rezafungin is a next-generation echinocandin
capable of attaining high concentrations and exposures in vivo, exceeding those measured
for the three approved echinocandins, due to its long half-life and front-loaded dosing
regimen. Given the relevance of evaluating higher drug concentrations for rezafungin
and that rezafungin PE/TE trends have not yet been characterized, herein we compared
the occurrence of PE and TE for rezafungin with data generated in parallel for caspofungin,
micafungin and anidulafungin against clinically important Candida species.
Methods: We tested 365 non-duplicate Hungarian clinical isolates belonging to 13 species
(see Table). Isolates were collected between 2005 and 2018 and were identified with
MALDI Biotyper (Bruker, Bremen, Germany). C. auris were obtained from National Mycology
Reference Laboratory, UK. MICs were determined by BMD method according to CLSI (M27
Ed4) in RPMI-1640. Concentration ranges of antifungals were 0.06-32 mg/L. PE was defined
as visible growth occurring at higher but not at lower supra-MIC concentrations. TE
was defined when yeasts show reduced but observable growth in supra-MIC concentrations.
Results: The percentages of isolates showing PE or TE after 48 hours are shown in
the Table. Cumulative percentage of PE plus TE was the highest for caspofungin (47.7%),
followed by anidulafungin (35.9%), micafungin (25.8%) and rezafungin (17.7%). PE was
observed most frequently for caspofungin (31.4%) but was uncommon for rezafungin (3.1%).
PE with caspofungin, micafungin or anidulafungin occurred most frequently in cases
of C.tropicalis, C. albicans, C. orthopsilosis and C. inconspicua but was never found
incase of C. krusei. The majority of C. auris and C. dubliniensis isolates showed
TE for all four echinocandins. Table. Percentages of Candida spp. isolates demonstrating
paradoxical effect (PE) or trailing effect (TE).
Conclusion: PE or TE was widely observed among Candida species and were echinocandin-
and species-dependent. PE was frequently found with caspofungin, micafungin and anidulafungin
but not with rezafungin. This study is the first to characterize PE and TE trends
for rezafungin and helps to inform future in vitro work with this novel echinocandin.
Imbert
S.
1
Normand
A.C.
2
Gabriel
F.
3
Bonnal
C.
4
Lachaud
L.
5
Costa
D.
6
Cassaing
S.
7
Hasseine
L.
8
Kristensen
L.
9
Schuttler
C.
10
Raberin
H.
11
Brun
S.
12
Piarroux
R.
1
Fekkar
A.
1
1Laboratoire De Parasitologie-mycologie, APHP La Pitié Salpétrière, PARIS, France
2Ap-hp, Groupe Hospitalier Pitié-salpêtrière, Service de Parasitologie Mycologie,
Paris, France
3Parasitology Mycology, CHU de Bordeaux, Bordeaux, France
4Laboratoire De Parasitologie Mycologie, Hôpital Bichat-Claude Bernard, 46 rue Henri
Huchard, PARIS, France
5Laboratoire De Parasitologie-mycologie, CHU de Montpellier, Montpellier, France
6Laboratoire De Parasitologie-mycologie, CHU de Rouen, Rouen, France
7CHU de Toulouse, Toulouse, France
8Laboratoire De Parasitologie Mycologie, CHU DE NICE, NICE, France
9Department of Clinical Microbiology, Aarhus University Hospital, Aarhus, Denmark
10Laboratoire BioParisOuest, Levallois Peret, France
11CHU Saint Etienne, Saint Etienne, France
12Parasitology-mycology, Avicenne Hospital, Bobigny, France
P057. In vitro antifungal susceptibility testing of cryptic species of Aspergillus:
a multi-centre study
Objectives: In recent years, Aspergillus species taxonomy has been revolutionized
with the introduction of the concepts of cryptic species and species complex. However,
these “new” species remain usually rare and their characteristics, including antifungal
susceptibility, are little known. Thus, the aim of this study was to collect a large
number of isolates per cryptic species and to assess their antifungal susceptibility.
Methods: From September 2017, users of the MSI application, a free online MALDI-TOF
mass-spectrometry database, were asked to send to our University Hospital, isolates
they identified as cryptic species of Aspergillus. Identification at the species level
was confirmed by sequencing a part of beta-tubulin and calmodulin genes. Antifungal
susceptibility for azole drugs (itraconazole, voriconazole, posaconazole and isavuconazole)
and amphotericin B was assessed by EUCAST broth microdilution reference method.
Results: During the study period, 192 isolates were collected from 11 centers in France
and Denmark and were assessed for antifungal susceptibility. According to sequence-based
identification, isolates corresponded to 43 species, belonging to 6 species complexes.
Nidulantes species complex was the most represented (53 isolates, 12 species), followed
by the species complexes Circumdati (43 isolates, 10 species) and Fumigati (40 isolates,
8 species). The last species complexes represented were Usti (23 isolates, 4 species),
Flavi (19 isolates, 5 species) and Terrei (14 isolates, 4 species). Regarding the
3 main species complexes, diversity in antifungal susceptibility between cryptic species
was observed. Indeed, inside the Fumigati complex A. thermomutatus, A. lentulus, A.
udagawae, A. fischeri and (15, 7, 3 and 3 isolates respectively) showed pan-azole
high minimal inhibitory concentrations (MICs), whereas A. hiratsukae (8 isolates)
showed low MICs. All these species had low MICs for amphotericin B, except A. lentulus
with MICs ³4 mg/L. Regarding the Circumdati species complex, A. westerdijkiae (12
isolates) showed the highest MICs for amphotericin B (> 16 mg/L), whereas A. sclerotiorum
(17 isolates) showed lower MICs (MIC50 = 4 mg/L). In contrast A. sclerotiorum showed
pan-azole high MICs, whereas azole MICs where much lower for A. westerdijkiae. Finally,
inside the Nidulantes complex, all species showed low MICs for all drugs, except A.
creber and A. unguis (6 and 2 isolates respectively) which showed high MICs (> 8 mg/L)
for itraconazole only. MICs regarding the 3 other species complexes studied are shown
in Table 1.
Conclusion: This study brings evidence on the diversity of antifungal susceptibility
pattern inside the same species complex. However, including more isolates is needed
to confirm these preliminary results. Nevertheless, this underline the importance
of an accurate and easy-to-perform identification of Aspergillus at the species level
for the management of Aspergillus diseases and the conduct of epidemiological studies.
Imbert
S.
1
Normand
A.C.
1
Brun
S.
2
Ranque
S.
3
Hasseine
L.
4
Costa
D.
5
Cassaing
S.
6
Chryssanthou
E.
7
Bonnal
C.
8
Delhaes
L.
9
Rubio
E.
10
Bourgeois
N.
11
Schuttler
C.
12
Guitard
J.
13
Sautour
M.
14
Jost
G.
15
Brandenberger
M.
16
Kristensen
L.
17
Sendid
B.
18
Seeth
C. Gronfors
19
Laere
E. De
20
Hendrickx
M.
21
Rose
V. Sainte
1
Piarroux
R.
1
Fekkar
A.
1
1Laboratoire De Parasitologie-mycologie, APHP La Pitié Salpétrière, PARIS, France
2Parasitology-mycology, Avicenne Hospital, Bobigny, France
3IHU-Méditerranée Infection, Marseille, France
4Laboratoire De Parasitologie Mycologie, CHU DE NICE, NICE, France
5Laboratoire De Parasitologie-mycologie, CHU de Rouen, Rouen, France
6CHU de Toulouse, Toulouse, France
7Clinical Microbiology, Karolinska University Hospital, Stockholm, Sweden
8Laboratoire De Parasitologie Mycologie, Hôpital Bichat-Claude Bernard, 46 rue Henri
Huchard, PARIS, France
9Laboratoire De Parasitologie-mycologie, CHU de Bordeaux, Bordeaux, France
10Barcelona hospital, Barcelona, Spain
11Laboratoire De Parasitologie-mycologie, CHU de Montpellier, Montpellier, France
12Laboratoire BioParisOuest, Levallois Peret, France
13Parasitology-mycology, APHP, hôpital St Antoine, Paris, France
14Laboratoire Parasitology Mycology, CHU Dijon, Dijon, France
15Dianalabs, Geneve, Switzerland
16synlab, Lucerne, Switzerland
17Department of Clinical Microbiology, Aarhus University Hospital, Aarhus, Denmark
18Laboratory of Mycology and Parasitology, University Hospital of Lille, Lille Cedex,
France
19Clinical Microbiology, Karolinska University Hospital, karolinska Institutet, Stockholm,
Sweden
20AZ Roeselare, Roeselare, Belgium
21Mycology, Scientific Institute of Public Health, Brussels, Belgium
P058. Species identification, in vitro antifungal susceptibility testing and human
pathogenicity of Fusarium species: a European multicenter study
Objectives: Fungi belonging to the genus Fusarium are responsible of a large number
of infections that may be difficult to treat because of a low susceptibility to antifungal
drugs available. However, in recent years, fungal taxonomy has been revolutionized
with the introduction of the concepts of cryptic species and species complex. This
is particularly true for the genus Fusarium, which taxonomy remains partially unclear.
In consequence, pathogenicity and antifungal susceptibility specific to each Fusarium
species is largely unknown. Thus, the aim of this study was to collect a large number
of isolates per species, to collect clinical data on their pathogenicity and to assess
their antifungal susceptibility.
Methods: During 1 year (January-December 2018), users of the MSI application, a free
online MALDI-TOF mass-spectrometry database, were asked when they identified a Fusarium
sp. to send prospectively to our University Hospital laboratory the isolates and to
collect clinical data. Identification at the species level was confirmed by sequencing
a part of TEF1α gene. Antifungal susceptibility for azole drugs (voriconazole, posaconazole
and isavuconazole), terbinafin and amphotericin B was assessed by measuring minimal
inhibitory concentrations (MICs) by EUCAST broth microdilution reference method.
Results: During the study period, 145 isolates from 20 centers in 6 European countries
were collected. According to sequence-based identification, 23 species were identified
and were distributed in 6 species complexes (SC) whose main ones are Fusarium oxysporum
SC (FOSC, 54 isolates), Fusarium solani SC (FSSC, 48 isolates) and Fusarium fujikoroi
SC (FFSC, 36 isolates). Regarding these 3 main species complexes, minimal inhibitory
concentrations which inhibited 50% of our isolates (MIC50) were high for all azole
drugs, FSSC showing the highest MICs for voriconazole. For amphotericin B, MIC50 were
not high (1 mg/l) and no differences were observed between complexes. Finally, terbinafine
susceptibility seemed to be related to the species complex. Indeed, FOSC and FSSC
showed the highest MICs (MIC50 = 16 mg/l) whereas FFSC showed lower MICs (MIC50 =
2 mg/l). MICs results are shown in Table 1.
Among isolates collected, clinical data were available for 117 isolates. They were
responsible for 11 invasive/disseminated infections, 19 keratitis and 40 superficial
infections (34 onychomycosis and 6 intertrigo). Sixteen isolates were considered as
pulmonary airways colonization, whereas 31 were considered as culture contaminants.
The three main species complexes identified in the study were represented in each
category.
Conclusion: This study brings evidence on the diversity of Fusarium species implicated
in human diseases and their differences in terms of in vitro antifungal susceptibility,
mainly for terbinafin. This underlines the importance of an accurate and easy identification
of Fusarium species, at least at the complex level, to improve therapeutic management
of Fusarium-related diseases.
Schwarz
P.
1
2
Dannaoui
E.
3
4
1Department of Internal Medicine - Respiratory and Critical Care Medicine, University
Hospital Marburg, Marburg, Germany
2Center For Invasive Mycoses and Antifungals, Philipps University Marburg, Marburg,
Germany
3Hôpital Européen Georges Pompidou, Laboratoire de Parasitologie-Mycologie, Paris,
France
4Working Group Dynamyc, Faculté de Médecine, Hôpital Henri Mondor, Créteil, France
P059. Tacrolimus and not cyclosporin A or sirolimus interacts synergistically in vitro
with isavuconazole against Aspergillus species
Objectives: Invasive aspergillosis is a devastating disease in immunocompromised patients.
It mostly affects patients with hematological malignancies, especially those with
severe and prolonged neutropenia, but is also encountered in solid organ transplant
recipients. Immunosuppressive agents are used as anti-rejection drugs in organ transplant
patients, but besides their immunosuppressive activity, these drugs also possess intrinsic
antifungal activity. The aim of the present study was to assess the in vitro interaction
of the broad-spectrum azole isavuconazole with the calcineurin inhibitors (e.g. tacrolimus
or cyclosporin A) or the mTOR pathway inhibitor (e.g. sirolimus) against the most
important Aspergillus species responsible for human disease.
Methods: A panel of 30 Aspergillus isolates belonging to 5 species responsible for
human invasive aspergillosis was used for the experiments (10 Aspergillus fumigatus,
5 Aspergillus flavus, 5 Aspergillus terreus, 5 Aspergillus nidulans, and 5 Aspergillus
niger). Combinations of isavuconazole with cyclosporine A, tacrolimus or sirolimus
were tested by a broth microdilution checkerboard technique based on the EUCAST reference
methodology. Plates were read spectrophotometrically, and 90% of inhibition was used
as an endpoint for both, the drugs alone and in combination. Results were interpreted
with the fractional inhibitory concentration index (FICI). Drug interactions were
defined as synergistic (FICI≤0.5), indifferent (FICI>0.5≤4) or antagonistic (FICI>4).
Results: Isavuconazole MICs ranged from 0.25 to 16 µg/ml. When tested alone, immunosuppressive
drugs showed poor activity with MICs < 16 µg/ml for only 2, and 3 isolates for tacrolimus
and cyclosporin A, respectively. Combinations of isavuconazole with tacrolimus, cyclosporine
A or sirolimus were synergistic for 53, 20, and 10% of the tested isolates, respectively.
There were some differences between species (Table). Antagonism was never seen.
Conclusion: Calcineurin is a key enzyme in the calcium-dependent signal transduction
pathways of eukaryotes. Antifungal drug resistance and virulence of several pathogenic
fungi, including Aspergillus species, is regulated by this pathway. Results of the
present study showed synergistic interactions between isavuconazole and tacrolimus
and underline the role of the calcineurin pathway as a target for the development
of new antifungal drugs.
Lemes
T.
1
Almeida
B. De
2
Caetano
M.
3
Polaquini
C.R.
4
Regasini
L.O.
5
Maschio-Lima
T.
3
Brizzotti
N.
1
6
Castilho
E.
6
Siqueira
J.P.
7
Marques
M.
6
Almeida
M.
8
1Microbiologia, Unesp, São José do Rio Preto, Brazil
2Unesp, São José do Rio Preto, Brazil
3UNESP, São José do Rio Preto, Brazil
4São Paulo State University - Institute of Biosciences, Humanities and Exact Sciences
(UNESP - IBILCE), São José do Rio Preto, Brazil
5Chemistry and Environmental Sciences, UNESP (Sao Paulo State University), São José
do Rio Preto, Brazil
6FAMERP, São José do Rio Preto, Brazil
7Infeciosas Disease, FAMERP, São José do Rio Preto, Brazil
8Infectious Disease, Medical School, sao jose do rio preto, Brazil
P060. Synergistic antifungal effect of fluconazole combined with pure culture extract
of Candida albicans against Trichophyton mentagrophytes
Objectives: The present study evaluated the synergistic interaction between a pure
culture extract of Candida albicans and fluconazole against Trichophyton mentagrophytes.
Methods: This study used clinical strains of C. albicans from the Laboratory of Microbiology
of the Medical School in São José do Rio Preto (FAMERP), Brazil. A 500-mL Inoculum
prepared in Sabouraud Dextrose Broth was filtered through a 0.2 μm millipore membrane
and separated using ethyl acetate as a counter-phase. The ethyl acetate phase was
completely dried using a rotary evaporator and subsequently solubilized in sterile
distilled water with 10% dimethyl sulfoxide (DMSO). Minimal Inhibitory Concentration
(MIC) tests were performed for T. mentagrophytes strains following the Clinical and
Laboratory Standards Institute (CLSI) M38-A2 guidelines. After obtaining the extract’s
MIC, a checkerboard trial with fluconazole was performed to evaluate the synergistic
interaction with the extract based on the calculation of the fractional inhibitory
concentration index (ICIF) = (MIC fluconazole in the mix / MIC fluconazole alone)
+ MIC extract in the mix / MIC extract isolated). The synergistic interaction was
classified using the method described by Kumar et al., where values ≤ 0.5 indicate
significant interactions.
Results: The results obtained for the C. albicans extract’s MIC against the T. mentagrophytes
strain and fluconazole in isolation were 1000 μg/mL and 16 μg/mL, respectively. However,
when the extract was used in combination with fluconazole, the MIC value was 0,001
μg/mL and of fluconazole it was 2 μg/mL with an ICIF value of 0,01.
Conclusion: The extract of C. albicans in isolation shows antifungal activity against
T. mentagrophytes, which may influence the laboratory diagnosis of mixed infections.
Furthermore, the action of this extract is greater in association with an azole derivative,
thus proving synergy. In the future, the isolation and identification of extract compounds
may allow new therapeutic approaches in the control of fungal infections.
Hadina
S.
1
Benvin
I.
2
Štritof
Z.
1
Habuš
J.
1
Stevanović
V.
1
Perharić
M.
1
Martinković
K.
1
Mojčec
V.
1
Barbić
L.
1
Milas
Z.
1
Starešina
V.
1
Turk
N.
1
Pinter
L.
1
1Department of Microbiology and Infectious Diseases With Clinic, Faculty of Veterinary
Medicine, The University of Zagreb, Zagreb, Croatia
2Faculty of Veterinary Medicine, The University of Zagreb, Zagreb, Croatia
P061. Screening of antifungal susceptibility of Malassezia pachydermatis isolates
from dogs using disk diffusion test
Objectives:
Malassezia pachydermatis is lipophilic yeast commonly seen in skin and ear diseases
of dogs. Lately, different studies are describing the appearance of reduced susceptibility
of M. pachydermatis strains. In addition, reports about its zoonotic potential and
emerged number of Malassezia yeast resistance in human medicine, highlight the need
for study of its antifungal susceptibility. The problem of surveillance of potential
resistance is lying in no established reference method for antifungal susceptibility
testing and no interpretative criteria for this yeast. Various studies used different
methodologies and interpreted obtained results based on different recommendations.
Modified microdilution method is considered the most accurate, but at the same time
more expensive and technically demanding to perform in clinical laboratories. Recent
study demonstrated good correlation of results obtained by modified CLSI M44-A2 disk
diffusion and broth microdilution method, indicating its suitability for in vitro
susceptibility testing of M. pachydermatis in epidemiological studies (Pasquetti et
al., 2015.). The aim of this study was to screen antifungal susceptibility of M. pachydermatis
for miconazole (MCZ) and clotrimazole (CTZ) using disk diffusion method.
Methods: A total of 96 isolates of M. pachydermatis were recovered from animals with
clinical signs of otitis. Identification was based on their ability to grow on Sabouraud
dextrose agar and typical macroscopic and microscopic appearance. Two antifungal tablets
containing most commonly azole derivatives used for topical therapy were tested: MCZ
and CTZ (10 µg, Neo-Sensitabs, Rosco, Denmark). Disk diffusion test was performed
using Mueller-Hinton agar supplemented with 2% glucose and 0.5 μg/l methylene blue.
Inoculum of M. pachydermatis was prepared in sterile saline from 3 days old cultures,
with addition of the lipid source, containing approximately 1-5x107 CFU/mL. Readings
were performed 72 hours after incubation at 37°C, and interpreted according to guidelines
provided by manufacturer.
Results: Out of 96 tested, all isolates demonstrated good susceptibility to CTZ with
variations of inhibition zones from 34 to 73 mm (mean ± SD: 56 ± 8,06 mm). Susceptibility
testing against MCZ resulted with the range of inhibition zones from 14 to 65 mm (mean
± SD: 41 ± 6,77 mm) and good sensitivity of 95 strains, while one isolate demonstrated
intermediate sensitivity. Comparison of the size of the zones showed that CTZ had
larger zones of inhibition than MCZ indicating its higher antifungal activity.
Conclusion: Screening of antifungal susceptibility of M. pachydermatis using disk
diffusion method resulted with identification of one strain with reduced susceptibility
to MCZ. However, taking into consideration that there are no standardized interpretative
criteria for Malassezia yeast, follow up study using broth microdilution assay is
needed, in order to check if this isolate could be categorized as potentially resistant.
Reference: Pasquetti, M., E. Chiavassa, P. Tizzani, P. Danesi, A. Peano (2015): Agar
diffusion procedures for susceptibility testing of Malassezia pachydermatis: Evaluation
of Mueller-Hinton agar plus 2% glucose and 0.5 µg/mL methylene blue as the test medium.
Mycopathologia 180, 153-158.
Vasilyeva
N.
1
Vybornova
I.
2
Bogomolova
T.
1
1Kaschkin Research Institute of Medical Mycology. Department of Medical Microbiology,
North-Western State Medical University named after I.I. Mechnikov, Saint-Petersburg,
Russian Federation
2Kaschkin Research Institute of Medical Mycology, North-Western State Medical University
named after I.I. Mechnikov, Saint-Petersburg, Russian Federation
P062. Susceptibility to voriconazole of Aspergillus spp. strains, isolated from patients
in Russia
Objectives: Azole resistance of aspergillosis etiologic agents is an emerging problem
in Europe and other parts of the world. The aim of the study: to determine susceptibility
to voriconazole of Aspergillus spp. strains isolated from patients with aspergillosis
in Saint Petersburg, Russia.
Methods: Fungal species identification was done using morphological and molecular
methods. A total of 230 strains of Aspergillus spp. isolated from biological samples
of patients with different clinical forms of aspergillosis (invasive, chronic and
allergic) in Saint Petersburg during 2014 – 2019 yrs. have been tested by standard
disk diffusion method according to CLSI M51A Document. For 34 strains MICs of voriconazole
have been determined according to EUCAST Document E Def 9.3.1.
Results: Species distribution among Aspergillus spp. isolates was as follows: A. fumigatus
– 68%, A. niger – 16%, A. flavus – 13%, rare species – 3% (A. terreus – 4 strains,
A. calidoustus – 2, A. ustus – 1, A. sydowii – 1). By disk diffusion method no voriconazole
resistant isolates were found among common species: A. fumigatus, A. niger, A. flavus.
Only 4 resistant strains were revealed among rare species: A. terreus – 1, A. calidoustus
– 2, A. ustus – 1 (inhibition zone diameter < 17 mm). Voriconazole MICs for A. fumigatus,
A. flavus, A. niger strains ranged from 0,125 to 0,5 mg/L. Only A. calidoustus showed
high MIC – 4 mg/L.
Conclusion: Resistance to voriconazole among etiologic agents of aspergillosis in
Saint Petersburg, Russia was found only among rare fungal species (A. calidoustus,
A. ustus, A. terreus). Future surveillance is necessary to monitor antifungal resistance.
Bogomolova
T.
1
Bogdanova
T.
2
Vasilyeva
N.
1
Alexeyev
A.
1
Spiridonova
V.
3
1Kaschkin Research Institute of Medical Mycology. Department of Medical Microbiology,
North-Western State Medical University named after I.I. Mechnikov, Saint-Petersburg,
Russian Federation
2Department of Medical Microbiology, North-Western State Medical University named
after I.I. Mechnikov, Saint-Petersburg, Russian Federation
3Kaschkin Research Institute of Medical Mycology, North-Western State Medical University
named after I.I. Mechnikov, Saint-Petersburg, Russian Federation
P064. Antifungal susceptibility testing of Malassezia furfur
Objectives:
Malassezia spp. may be etiologic agents of superficial and invasive mycoses. There
are no standard protocols for evaluation of antifungal susceptibility of these yeasts.
The aim of the study was to determine Malassezia furfur isolates susceptibility to
antifungal agents by two different methods.
Methods: Disk diffusion method CLSI M44A2 was modified by addition of lipids to the
Muller-Hinton agar. Commercial test panels Sensititre Yeast One were also supplemented
by fatty acid RPMI 1640.
Results: We tested 11 strains of M. furfur isolated from blood of pediatric patients.
Both methods showed resistance to fluconazole and voriconazole. By Sensititre Yeast
One panels the following MICs were obtained: amphoterecin B – 1 mg/L, fluconazole
> 64 mg/L, caspofungin >8 mg/L, voriconazole – 1-8 mg/L, anidulafungin >8 mg/L, micafungin
>8 mg/L, 5-fluorocytozne - > 256 mg/L, itraconazole - < 0,015, mg/L, pozaconazole
– 0,03-0,06 mg/L.
Conclusion: Disk diffusion method may be used for screening antifungal resistance
in Malassezia spp. isolates. Itraconazole and pozaconazole demonstrated highest activity
against M. furfur.
Sana
J.
1
Melloul
É.
1
Guillot
J.
1
Botterel
F.
1
2
Dannaoui
E.
1
3
18 Rue Du General Sarrail, DYNAMYC, Creteil, France
2Laboratoire De Parasitologie-mycologie, APHP Hôpital Henri Mondor, Créteil, France
3Hôpital Européen Georges Pompidou, Laboratoire de Parasitologie-Mycologie, Paris,
France
P065. Evaluation of the efficacy of antifungals against invasive aspergillosis in
an invertebrate animal model
Objectives: Evaluation of new therapeutic strategies for the treatment of invasive
aspergillosis is needed due to emergence of azole-resistance in Aspergillus fumigatus.
Animal models in rodents are generally used but are costly and their use is limited
by ethical considerations. For these reasons, evaluation of antifungal efficacy in
mini-host models such as Galleria mellonella is a promising alternative. The aim of
our study was to develop an invasive Aspergillus infection in larvae of Galleria mellonella
to evaluate the efficacy of antifungals.
Methods: Three clinical strains of Aspergillus fumigatus (HEGP064, HEGP4017 and HEGP2666)
have been used. Minimum inhibitory concentration for voriconazole (VRZ) was 0.5, 0.5
and 8µg/mL for HEGP064, HEGP4017 and HEGP2666 respectively. Inoculum finding experiments
(with spore suspensions at 105 to 108 conidia/mL) have been performed to determine
the concentration that gave 90% mortality at seven days post-infection (LD90). The
presence of an invasive infection has been demonstrated by direct examination of tissues
stained with Gomori-Grocott (GG) and by histopathology (H&S staining). In a first
set of experiments, groups of 10 larvae were infected with the LD90 and treated with
monotherapy by voriconazole (VRZ, [Vfend®] at 0.5, 1, 2, 4, and 8 μg/larva), amphotericin
B (AMB, [Fungizone®], or caspofungin (CSP, [Cancidas®] at 0.2, 0.4 and 0.8 μg/larva).
Subsequently, HEGP4017 or HEGP2666 infected larvae were treated after two hours by
VRZ at 4µg/ml alone and in combination with CSP at 4, 2 or 1µg/larvae. The mortality
was estimated daily for 7 days.
Results: After infection, an invasive aspergillosis was obtained as shown by the presence
of hyphae in tissues. The LD90 values for the HEGP064, HEGP4017 and HEGP2666 strains
were 4.38×107,9.59×107 and 1×108 CFU/ml respectively. In infected and untreated groups,
mortality was at least 90% on day 7 post-infection. The mortality decreased with increasing
doses of amphotericin B. At 4 µg/larvae, the mortality was on average 20% for the
3 strains. In animals treated with VRZ, the mortality rates on day 7 post-infection
were 35%, 40% and 60% for HEGP064 and were 55%, 65% and 95% for HEGP4017 and 85%,
95% and 95% for 8, 4 and 2 μg/larva, respectively (p = 0.0001 compared to control).
VRZ at 8, 4 and 2 μg/larva was statistically more effective for the treatment of larvae
infected with HEGP064 or HEGP4017 compared to those infected by HEGP2666. Administration
of VRZ 4 did not decreased, significantly, the mortality of HEGP2666 infected larvae
(p = 0.304) in contrast to those infected by HEGP4017 (p = 0.0082). The bitherapy
VRZ 4µg/larvae and CSP 4µg/larvae lead to a more significant decrease in mortality
compared to monotherapy (VRZ 4µg/larvae) and control group infected by HEGP4017(p
= 0.026) and HEGP2666 (p = 0.005) strain.
Conclusion:
G. mellonella is a reliable model for inducing invasive aspergillosis. There was a
clear dose-response when animals were treated with VRZ and AMB. This model could be
used for screening and evaluation of different therapeutic strategies against Aspergillus
spp.
Scroferneker
M.L.
1
Pinheiro
T.R.
2
Seibert
G.
2
Poletto
A.L.R.
3
Prade
J.V.
3
Stopiglia
C.D.O.
3
1Department of Microbiology, Immunology and Parasitology, Universidade Federal do
Rio Grande do Sul, Porto Alegre, Brazil
2Universidade Federal do Pampa, Uruguaiana, Brazil
3Universidade Federal do Pampa, Uruguaiana, RS, Brazil
P066. Analysis of the efflux-pump activity against resistant Sporothrix schenckii
complex isolates
Objectives: The present study aims to assess the efflux-pump activity in the resistant
Sporothrix schenkii complex strains.
Methods: The minimum inhibitory concentration (MIC) of itraconazole (0.03125-16 µg/mL)
was determined through broth microdilution method, as described by protocol M38-A2
of the Clinical and Laboratory Standards Institute (CLSI, 2008). Ten clinical isolates
of Sporothrix schenckii complex were prepared from 7-day-old cultures grown on potato
dextrose agar at 28 °C. The inocula were adjusted to a final concentration of 0.5–2.5×103
cells/mL. The microdilution plates were incubated at 35 °C for 72h. The MIC of itraconazole
was defined as the lowest drug concentration capable of inhibiting total of growth.
For the analysis of the efflux-pump activity, the resistant Sporothrix schenckii complex
strains (04/10) were tested against two efflux-pump inhibitors, promethazine and verapamil
by broth microdilution method. Posteriorly, susceptibility test was performed with
itraconazole and sub-inhibitory concentrations of promethazine (MIC/8 = 24 µg/mL)
and verapamil (MIC/8 = 100µMol).
Results: There were no reduction of MIC for itraconazole in the presence of the verapamil
and prometazine inhibitors.
Conclusion: Thus, the present study suggests that the mechanism of resistance of the
isolated evaluated isn’t the presence of efflux pumps.
Nenoff
P.
1
Süβ
A.
2
Ludes
A.
3
Schuller
A.
4
Fischer
E.
5
Dessoi
S.
6
Hofmann
W.
6
Schmidt
S.
7
Verma
S.B.
8
Monod
M.
9
Salamin
K.
9
Krueger
C.
1
Ebert
A.
1
Uhrlass
S.
1
1Partnership Dr. C. Krueger & Prof. P. Nenoff, Laboratory of medical microbiology,
Roetha OT Moelbis, Germany
2Hautärztin, Gemeinschaftspraxis Allgemeinmedizin und Dermatologie, Wittlich, Germany
3Allgemeinmedizinische Praxis Dr. med. Alfons Ludes und Thorsten Koech, Leiwen, Germany
4Hautarztpraxis, Halle/Saale, Germany
5Hautarztpraxis Dr. med. Gunhild Kratzsch, Leipzig, Germany
6Hautzentrum Nordwest, Frankfurt am Main, Germany
7Hautarztpraxis im MVZ Friedrichstadt, Dresden, Germany
8“Nirvan” and “In Skin” Clinics, Vadodara, India
9Dermatology Service, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland
P067. Terbinafine resistant Trichophyton mentagrophytes ITS genotype VIII from India
in Germany
Case Report: Objectives: In India, a dramatic increase of chronic recalcitrant dermatophytoses
due to Trichophyton (T.) mentagrophytes genotype VIII is on the rise. Due to travelling
and migration, globalization of the mostly terbinafine resistant strains of the causative
agent from India to other parts of the world is assumed. First strains of T. mentagrophytes
genotype VIII have reached Germany, now. Patients: 1) A 6 months-old-female infant
from Bahrain visiting Germany with her family for a holiday was seen for extensive
dermatophytosis of the back, buttocks, chest and groins. Topical treatment by terbinafine
for over 2 months was not successful. 2) A 28 years old male from Libya, living for
3 years in Germany, suffered from tinea cruris and tinea faciei involving the left
upper and lower eyelids. Treatment by oral fluconazole and by terbinafine failed.
His German girlfriend was affected by the dermatomycosis, too. Her child was free
of signs of a dermatophytosis. The patient had no contact to India and Indians or
Arabs, he did not visit Libya in the last years. The man, however, regularly goes
to the gym. 3) After a trip to Saudi Arabia, a pregnant German woman suffered from
tinea cruris and tinea corporis (trunk). Her husband was affected, too. 4) An Indian
male, living in Germany, was treated because of a dermatomycosis of the buttocks and
groins by oral terbinafine, without improvement. 5) A couple from Iraq, living for
long time in Germany, suffered from chronic recalcitrant dermatophytosis of the groins
for at least 1.5 years. Repeated topical treatments by „Combo Creams“ failed. Antifungal
topical preparations (ciclopirox olamine, sertaconazole) for 5-6 weeks acted very
slowly, progression of the dermatomycoses lead to therapy break-off. Oral itraconazole
200 mg daily was started. Results: Mycological examination of skin scrapings from
all 5 patients revealed the detection of T. mentagrophytes. Four out of the five dermatophyte
isolates were subject to sequencing of the “internal transcribed spacer” (ITS) region
of the fungal rDNA. All these 4 strains belonged to the newly described genotype VIII
of T. mentagrophytes. The until now investigated point mutations revealed for patient
1) TTC/TTA mutation of the squalene epoxidase (SQLE) gene which is closely associated
with Phe397Leu amino acid substitution of the enzyme. T. mentagrophytes isolates of
patient 2) and 3) were in vitro sensitive to terbinafine. The T. mentagrophytes strain
of patient 3) showed GCT/ACT mutation (Ala448Thr). The above mentioned Phe397Leu and
Ala448Thr substitutions are indicative of in vitro resistance of the dermatophyte
against terbinafine. Conclusion: Transmission of the here found Indian ITS genotype
VIII of T. mentagrophytes to other countries due to globalization is a serious issue
to be considered. Moreover, a significant percentage of these Indian T. mentagrophytes
strains are resistant to terbinafine both in vitro and due to point mutations in the
SQLE gene. Some strains are also found to be partially resistant against itraconazole
and voriconazole. The here described patients represent the first reports on an infection
due to terbinafine-resistant T. mentagrophytes of the ITS genotype VIII from India
in Germany.
Abdillah
A.
1
Packeu
A.
2
Khelaifia
S.
3
Hendrickx
M.
4
Ranque
S.
1
1Vitrome, Aix Marseille Univ, IRD, AP-HM, SSA, Marseille, France
2Mycology & Aerobiology, Sciensano, Brussels, Belgium
3Culturomics, IHU Méditerranée Infection, Marseille, France
4Service of Mycology and Aerobiology, Bccm/ihem Fungal Collection, Sciensano, Brussels,
Belgium
P068. Comparison of two antifungal susceptibility tests for Malassezia yeasts
Objectives: The lipid-dependent Malassezia spp. yeast are members of the normal human
skin microbiota that are occasionally involved in various skin diseases or in blood
stream infections in immunocompromised and neonates patients. A better knowledge of
the antifungal susceptibility testing (AFST) profiles of these yeast is pivotal to
enhance antifungal treatment strategies. This study aimed to compare two broth microdilution
assays for the in vitro AFST of Malassezia spp.
Methods: A total of 19 strains of the BCCM /IHEM collection, including 4 Malassezia
species: (n = 6) M. furfur, (n = 4) M. pachydermatis, (n = 5) M. sympodialis and (n
= 4) M. slooffiae were tested against ketoconazole, voriconazole, terbinafine and
fluconazole using the broth microdilution method (CLSI - M27-A2). Two different media
were used as diluent, modified Dixon broth (mDIXB) and FastFung broth, a new medium
derived from the Schaedler agar. MIC values were visually read after 48 h of incubation
at 30°C. The essential agreement (EA) (±1 or 2 dilution) of the MIC values between
both media was estimated. The inter-laboratory reproducibility between Marseille and
Brussels was tested.
Results: Ketoconazole, voriconazole and terbinafine MICs (mg/l) were low, with MIC90
values of 0.06 to 0.5, 0.03 to 0.125, and 0.125, in mDIXB, and of 0.03 to 0.25, 0.03
to 0.125, and 0.06 to 1, in FastFung broth, respectively. Fluconazole displayed the
highest MIC values. The EAs varied from 84.2 to 100% for ±1 dilution while EAs of
100% were observed for ±2 dilution. The inter-laboratory EAs (±2 dilutions) ranged
from 73.8 to 100% and 94.7 to 100% in mDIXB or FastFung broth, respectively.
Conclusion: Our findings showed that both media performed similarly, with relatively
higher EAs (>60%), except for terbinafine. The FastFung broth can be used as alternative
medium for Malassezia in vitro AFTS.
Nagy
F.
1
Tóth
Z.
1
Forgács
L.
1
Borman
A.
2
Majoros
L.
1
Balázs
B.
1
Kovács
R.
1
1Department of Medical Microbiology, University of Debrecen, Debrecen, Hungary
2Public Health England UK National Mycology Reference Laboratory, Bristol, United
Kingdom
P075. The effect of farnesol on Candida auris biofilms, virulence and azole susceptibility
Objectives: Farnesol is a fungal quorum-sensing molecule, which has a pivotal role
in regulation of fungal morphogenesis. The aim of our study was to examine the effect
of farnesol on biofilms, azole susceptibility (fluconazole, voriconazole, itraconazole,
posaconazole, isavuconazole) against Candida auris clinical isolates as well as in
vivo tissue fungal burden in a murine bloodstream infection model.
Methods: The effect of farnesol on C. auris cells was tested in three experiments:
i) biofilm forming ability of cells pre-treated with farnesol for 24-hours prior to
biofilm formation, ii) biofilm forming ability of cells continuously exposed to farnesol
for 24-hours during biofilm formation, iii) effect of farnesol on one-day-old biofilms.
Farnesol concentrations tested were 10, 50, 100, 300 μM in all experiments. Metabolic
activity of sessile cells was determined at 0, 2, 4, 6, 8, 10, 12 and 24 hours using
XTT-assay. One-way ANOVA with Dunnett’s post-testing was used to analyze the metabolic
activity change exerted by different farnesol concentrations compared to untreated
control. Interactions between farnesol and azoles were examined against one-day-old
biofilms by broth microdilution checkerboard method. The tested concentrations were
1.17-300 μM and 0.125-512 mg/L for farnesol and azoles, respectively. Fractional inhibitory
concentration index (FICI) was used to assess the nature of interactions. Synergy
was specified if the FICI was ≤0.5, between >0.5 and 4 was indifferent and as antagonistic
if the FICI was >4. MICs were determined as at least 50% reduction of metabolic activity
compared to control in case of individual drugs and isoeffective combinations. Farnesol
pre-exposed (75 μM) cells were inoculated intravenously into immuncompromised female
BALB/c mice to examine the day six fungal burden in kidneys, heart and liver.
Results: The one-day-long farnesol pre-exposure (50-300μM) led to formation of biofilms
with significant metabolic activity inhibition after 24 hours (p<0.01). Biofilms formed
under farnesol exposure showed significantly decreased metabolic activity in first
the 12, 12, 10 and 8 hours for 300, 100, 50 and 10 μM farnesol, respectively (p<0.05-0.001).
In case of one-day old biofilms treated with farnesol after formation, only the highest
farnesol concentration caused significant inhibition at 24 hours (p<0.001).The median
MIC values of azoles and farnesol against sessile C. auris clinical isolates are showed
in Table 1. Based on FICI values, farnesol was synergistic with all tested azoles
(FICIs ranged from 0.061 to 0.375) (Table 1). Fungal tissue burdens caused by farnesol
pre-exposed C. auris were significantly higher in all examined organs compared to
organs infected by farnesol-unexposed fungal cells at sixth day postinoculation.
Conclusion: Farnesol inhibited the biofilm forming ability of C. auris and significantly
reduced the metabolic activity of C. auris biofilms in a concentration-dependent manner;
in addition, it showed a remarkable synergistic effect with azoles. On the other hand,
farnesol pre-exposure led to increased tissue fungal burdens. In summary, though its
systemic usage may be hindered by a potential enhancement of fungal virulence, farnesol
may be a useful addition to therapy where biofilm formation is important, e.g. in
catheter-lock therapy against C. auris.
Kovács
R.
1
Jakab
Á.
2
Tóth
Z.
1
Nagy
F.
1
Emri
T.
2
Pócsi
I.
2
Majoros
L.
1
1Department of Medical Microbiology, University of Debrecen, Debrecen, Hungary
2Department of Molecular Biotechnology and Microbiology, University of Debrecen, Debrecen,
Hungary
P076. Transcriptional and physiological effect exerted by tyrosol on Candida parapsilosis
cells
Objectives: Tyrosol has a potent antifungal activity against Candida species; however,
the background of its antifungal mechanism is still poorly understood. We examined
the effect of tyrosol at supraphysiological concentration on growth, biofilm formation,
redox homeostasis, virulence as well as on fluconazole susceptibility to reveal the
background of its antifungal effect. To gain further insights into the physiological
consequences of tyrosol treatment we examined the caused genome wide gene expression
changes using RNA-Seq.
Methods: CLIB 214 Candida parapsilosis (sensu stricto) strain was used and 15 mM tyrosol
concentration was applied to examine physiological and transcriptional changes. Following
4 hours incubation of C. parapsilosis precultures, we added 15 mM tyrosol to the cultures
then growth was examined at 1-hour intervals by determination of cell density both
by means of measuring of the absorbance (at 640 nm) and by counting the living cells.
Reactive species were measured in the presence or absence of tyrosol by a technique
that converts 2’,7’dichlorofluorescin-diacetate to 2’,7’–dichlorofluorescein. One-day-old
biofilm formation ability in the presence of tyrosol was determined by quantitative
CFU determination of adhered cells. Interaction between fluconazole and tyrosol was
evaluated by two-dimensional broth microdilution chequerboard assay. Afterwards, the
nature of interaction was analyzed using Fractional Inhibitory Concentration Index
determination. Tyrosol induced effect on virulence was examined in immuncompromised
systemic BALB/c mouse model.To obtain global transcriptome data regarding tyrosol
treatment, high-throughput mRNA sequencing was performed on Illumina NextSeq sequencing
platform.
Results: Fifteen mM tyrosol caused significant growth inhibition within two hours
of the addition of tyrosol (p<0.01). Tyrosol increased the production of reactive
oxygen species (p<0.001) and it was associated with elevated superoxide dismutase,
glutathione peroxidase and catalase activities (p<0.05). Biofilm formation ability
was comparable in the presence or absence of 15 mM tyrosol in terms of living fungal
cells.The interaction between fluconazole and tyrosol was antagonistic (FICI 4.5).
Fifteen mM daily treatment decreased the fungal tissue burden in the kidneys compare
with untreated control (p<0.01).Tyrosol exposure resulted in 1,462 differentially
expressed genes. Out of them, 261 and 181 genes with at least 1.5-fold increase or
decrease in expression, respectively, were selected for further investigations (Figure
1). Genes involved in ribosome biogenesis showed down-regulation, while genes related
to later biofilm events, oxidative stress response and ethanol fermentation were up-regulated.
In addition, tyrosol treatment up-regulated the expression of certain fluconazole
efflux pump genes including MDR1 and CDR1 and down-regulated the FAD2 and FAD3 virulence
genes belonging to desaturated fatty acid formation.
Figure 1 Up-regulated (red) and down-regulated (dark blue) genes were defined as differentially
expressed genes (p<0.05) where log2(FC)>0.585 or log2(FC)<–0.585, respectively, and
FC stands for fold change FPKM (fragments per kilobase per million mapped fragments)
value.
Conclusion: Tyrosol exposure enhanced oxidative stress response and efflux pumps,
while inhibited growth and ribosome biogenesis as well as virulence. Metabolism was
modulated towards glycolysis and ethanol fermentation. Primer adherence was inhibited,
and rather later biofilm events was enhanced. Our findings suggest that tyrosol may
be a potential locally active and/or adjuvant agent in the development of alternative
treatments targeting quorum-sensing in the future.
Orlandi
C.
1
Pires
R.H.
2
Bila
N.M.
1
Serafim-Pinto
A.
1
Bonatti
J.L.
1
Scorzoni
L.
1
Singulani
J.
1
Santos
C.
1
Andrade
C.R.
3
Silva
P.
4
Chorilli
M.
4
Regasini
L.O.
5
Almeida
A.M. Fusco
1
Giannini
M.J. Soares Mendes
1
1Clinical Analysis, UNESP (Sao Paulo State University), Araraquara, Brazil
2Health Promotion, UNIFRAN (University of Franca)), Araraquara, Brazil
3Physiology and Pathology, UNESP (Sao Paulo State University), ARARAQUARA, Brazil
4Drugs and Pharmaceuticals, UNESP (Sao Paulo State University), Araraquara, Brazil
5Chemistry and Environmental Sciences, UNESP (Sao Paulo State University), São José
do Rio Preto, Brazil
P077. Nonyl 3,4-dihydroxybenzoate as a potent anti-biofilm compound for the treatment
of dermatophytosis
Objectives: This study aimed to verify the in vitro susceptibility of nonyl 3,4-dihydroxybenzoate
against planktonic cells and biofilms of dermatophytes. In addition, efficacy and
toxicity were tested in a murine model of dermatophytosis. The compound was incorporated
into nanostructured lipid systems (NLS) in order to prove whether the incorporation
was able to maintain its antifungal activity as well as, reduce toxicity in human
cell lines and alternative models. In addition, the probable mechanism of action was
evaluated.
Methods: The preformed biofilms and planktonic cells of Trichophyton rubrum and T.
mentagrophytes were treated with different concentrations of synthetic antifungal
drugs and nonyl 3,4-dihydroxybenzoate. The metabolic activities were determined using
the XTT reduction assay. The effects of the treatments on biofilms structures were
visualized by scanning electron microscopy (SEM). The efficacy of the compound was
evaluated in wild type (WT) C57BL/6 mice by determining the fungal burden and histopathological
analysis. Hepatic and renal toxicity were assessed by blood analysis in the equipment
Vitros 250 chemistry analyzer. After incorporation into NLS, susceptibility tests
were conducted according the document M38-A2, proposed by the CLSI (2008). The toxicity
of incorporation was evaluated in HaCaT cell lines by the sulforhodamine B method
and in Caenorhabditis elegans model. Finally, the damage of cell ultrastructure was
evaluated by transmission electron microscopy (TEM).
Results: Both biofilms and planktonic cells were susceptible to nonyl compound in
concentrations ranging from 125 – 250 mg/L. T. rubrum and T. mentagrophytes biofilms
were significantly more resistant to fluconazole, griseofulvin and terbinafine than
planktonic cells (p<0.05). Fluconazole, griseofulvin, terbinafine and nonyl were able
to prevent biofilm formation in all reference strains and clinical isolates tested
(p<0.05). SEM results revealed that the antifungal drugs caused minor or no damage
to the structure of the biofilms, and in some cases, stimulated the development of
resistance structures. In contrast, the biofilms treated with the nonyl compound were
seriously damaged. The fungal burden was significantly reduced after treatment and
histopathological analysis showed complete tissue regeneration. In addition, there
was no systemic toxicity after treatment in mice. Nonyl incorporated to NSL formed
by the surfactant Brij®98 Epikuron® (98-I) presented the best results of minimum inhibitory
concentration (MIC), with values between 1.98 and 3.9 mg/L. Cytotoxicity tests indicated
that the incorporation resulted in cell viability greater than 80% at all tested concentrations.
These results corroborated with in vivo testing in C. elegans model, which indicated
that the incorporation of nonyl significantly increased the survival of larvae. Finally,
TEM results indicated that the compound might have more than one mechanism of action
in the dermatophytes, causing enlargement of the vacuoles, besides derangement and
formation of pores in the plasma membrane, similar to amphotericin B.
Conclusion: These findings show that nonyl 3,4-dihydroxybenzoate may be a promising
antifungal. Funding: CNPq, Capes and FAPESP Process # 2018/02785-9; 2017/18388-6.
Bila
N.M.
1
2
Orlandi
C.
1
Gonçalves
L.C.
1
Vaso
C.O.
1
Santos
C.
1
Silva
P.
3
Chorilli
M.
3
Regasini
L.O.
4
Primo
F.L.
5
Fontana
C.R.
1
Almeida
A.M. Fusco
1
Giannini
M.J. Soares Mendes
1
1Clinical Analysis, UNESP (Sao Paulo State University), Araraquara, Brazil
2Para-clinics, UEM (Eduardo Mondlane University), Maputo, Mozambique
3Drugs and Pharmaceuticals, UNESP (Sao Paulo State University), Araraquara, Brazil
4Chemistry and Environmental Sciences, UNESP (Sao Paulo State University), São José
do Rio Preto, Brazil
5Bioprocess and Biotechnology, UNESP (Sao Paulo State University), Araraquara, Brazil
P078. Anti-dermatophyte biofilm activity of photodynamic therapy using 2-chalcone
as photosensitizer
Objectives: This study aimed to evaluate the anti-dermatophytic and anti-biofilm activity
of 2-chalcone alone and combined with photodynamic therapy (PDT).
Methods: Strains of Trichophyton rubrum, T. mentagrophytes and one clinical isolate
of Microsporum canis were used. Susceptibility tests were conducted according to the
M38-A2 document proposed by CSLI. Planktonic cells, biofilms with 24 h (initial stage)
and 72- 96 h of growth (mature) were formed in RPMI-1640 medium and placed in contact
with different concentrations of 2-chalcone (1 - 500 mg/L), fluconazole (1,25 - 512
mg/L) and terbinafine (0.002 – 32 mg/L). The metabolic activities after the treatments
were quantified using the tetrazolium salt (XTT) reduction method. The topographies
of initial and mature biofilms with or without treatment were visualized by scanning
electron microscopy (SEM). For the PDT assay, 2-chalcone was used a photosensitizer
and a blue led as a source of light with a dose of 150 J.cm-2.
Results: All the strains, in the planktonic form, were inhibited by treatment with
2-chalcone (MIC = 3.9–7.8 mg/L), terbinafine (MIC = 0.008–0.03 mg/L) and fluconazole
(1–64 mg/L). The substance 2- chalcona eradicated the 24 hour biofilm of all strains
tested at the concentration of 15.6 mg/L, terbinafine ranging from 0.06 to 16 mg/L,
and fluconazole inhibited only the formation of T. mentagrophytes biofilm in the concentration
of 32 mg/L. Regarding mature biofilms, only 2-chalcone was able to reduce the metabolic
activities in the concentration of 31.2 mg/L. Mature biofilms were resistant to both
antifungal drugs tested. When planktonic and biofilms (initial and mature) were treated
with PDT using 2-chalcone as photosynthesizer, the inhibitory concentrations were
reduced by 4 times (2–8 mg/L). The SEM of biofilms treated with 2-chalcone showed
a total collapse of the cell walls, resulting from a probable extravasation of cytoplasmic
content.
Conclusion: The substance 2-chalcone is a promising antifungal drug. Funding: CNPq,
IBE process 150/2017, Capes and FAPESP Process # 2018/02785-9; 2017/18388-6.
Shi
D.
1
Liu
W.
2
1Jining No. 1 People’s Hospital, Jining, China
2Institute of Dermatology, Chinese Academy of Medical Sciences & Peking Union Medical
College, Nanjing, China
P079. Antifungal activity of voriconazole and micafungin against the biofilms and
planktonic cells of Candida albicans and Aspergillus fumigatus in vitro
Objectives:
Candida albicans and Aspergillus fumigatus are among the most common invasive opportunistic
pathogens known to have high morbidity and mortality. Micafungin (MCF) and voriconazole
(VOR) are the most commonly used first-line therapies for these fungi. However, to
our knowledge, there are few data comparing the efficacy of MCF and VOR on biofilm
and planktonic cells on both fungi.
Methods: We have carried out an in vitro study to evaluate the efficacy of VOR and
MCF against biofilm development and inhibition of fungal viability within biofilm
formed by these two pathogenic fungi.
Results: We find that, when compared with planktonic cells, the corresponding fungal
biofilm magnificently decreases drug sensitivities. To inhibit more than 50% of cell
viability within a mature biofilm, the concentration of each drug must increase by
at least 5 to 10 MIC – depending on the specific drug and fungus combination. With
specific regard to biofilm formation itself, we find that MCF is more effective than
VOR against developing biofilm in C. albicans, whereas VOR is more effective at high
concentration against developing biofilm in A. fumigatus. We also find that the earlier
the therapeutic intervention, the greater the biofilm inhibition that will be achieved.
The critical time window for biofilm inhibition is 12h for A. fumigatus and 8h for
C. albicans; beyond those points in time safe and effective drug intervention is almost
impossible.
Conclusion: Our results provide a few clinical hints that may help in choosing antifungal
agents when treating common fungal infections.
Durieux
M.-F.
1
2
Melloul
É.
2
Roisin
L.
2
Woerther
P.-L.
2
3
Risco
V.
2
4
Guillot
J.
2
4
Dardé
M.-L.
1
Dannaoui
E.
5
Decousser
J.-W.
2
3
Botterel
F.
2
6
1Hopital Dupuytren, Laboratoire de Parasitologie-Mycologie, Limoges, France
2Dynamyc, Créteil, France
3Microbiology, Hôpital Henri-Mondor, Créteil, France
4École Nationale Vétérinaire D’alfort, Unité de Parasitologie-Mycologie, Maisons-Alfort,
France
5Hôpital Européen Georges Pompidou, Laboratoire de Parasitologie-Mycologie, Paris,
France
6Laboratoire De Parasitologie-mycologie, APHP Hôpital Henri Mondor, Créteil, France
P080. Contribution of Galleria mellonella model to understand interactions between
Aspergillus fumigatus and Stenotrophomonas maltophilia: study of human, animal and
environmental strains.
Objectives: Clinical and environmental strains of Aspergillus fumigatus (Af) and Stenotrophomonas
maltophilia (Sm) can colonize the respiratory tract of patients with chronic lung
diseases such as cystic fibrosis, specially in biofilm form. The interactions between
these two pathogens are not well known (1, 2). For a decade, insect model Galleria
mellonella (Gm) can be used to screen microorganism pathogenicity. The aim of this
study was to compare several associations of Af – Sm strains of different origin in
a Gm model to determine the virulence of these associations.
Methods: An in vivo infection model with human, animal and environmental strains of
Af and Sm, alone and associated, was developed using larvae of Gm. Several couples
were tested using different strains of Sm and Af, especially human reference clinical
strains (Af13073 and Sm13637). Virulence was analysed by survival curves at 7 days
and histology method.
Results: Mortality rate with Af alone showed no significant difference between human,
animal and environmental strains. For Sm, mortality rates showed variations depending
on the strains. The association between reference clinical strains Sm13637 and Af13073
showed a synergistic effect in Gm (60% of mortality at 7 days) versus Sm 13637 alone
(10% mortality) and Af13073 alone (30% mortality). This effect was not observed with
the other co-infections. Gomori-Grocott coloration of histological sections showed
fungal nodules disseminated in the larvae and a Gram coloration demonstrated the presence
of the bacteria, validating our infection model (3).
Conclusion: For the first time, we described a coinfection of Af and Sm in a Gm model
with an interesting synergistic effect between human clinical strains. This effect
did not exist for environmental and animal strains showing a particular fitness between
the human strains. These preliminary results provide complementary data to the in
vitro data already obtained. The Gm model has demonstrated its relevance for the study
of mixed infections, which remains to be improved (2). Indeed, the extracellular matrix
could not be identified in Gm, which could validate the presence of mixed biofilm.
(1, 2) Melloul et al. 2016 and 2018 (3) Société Française de Mycologie Médicale price
of the best oral communication, 2019.
Vikelouda
A.
1
Simitsopoulou
M.
1
Antachopoulos
C.
1
Skoura
L.
2
Chatzimoschou
A.
1
Stamouli
I.
1
Roilides
E.
1
13rd Department of Pediatrics, Aristotle University of Thessaloniki, Thessaloniki,
Greece
2Department of Microbiology, Aristotle University, Thessaloniki, Greece
P081. Antifungal susceptibility of Lictheimia corymbifera biofilms to Amphotericin
B formulations and Posaconazole
Objectives: Mucormycosis is an emerging life-threatening opportunistic fungal infection
that affects patients with underlying immunosuppressive conditions, such as hematological
malignancies, trauma, hematopoietic stem cell transplantation and diabetes mellitus.
The ability of Mucorales to form biofilms, structures with reduced susceptibility
to antifungal agents, has recently been demonstrated., Lictheimia Corymbifera is the
second most common clinically significant Mucorales species and is related mostly
to pulmonary and trauma infections. We investigated the antifungal activity of deoxycholate
amphotericin B (D-AMB), liposomal amphotericin B (L-AMB), posaconazole (POS) against
planktonic cells and mature biofilms of Lichteimia corymbifera. Additionally, we investigated
the combinational activity of L-AMB with POS against mature biofilms.
Methods: Three L. corymbifera clinical strains, were isolated from bronchial secretions
and incubated at 10^5 cfu/ml in 96-well microtiter plates at 37οC for 48h. Biofilm
formation was assessed by 1% safranin staining and measured spectrophotometrically
at 490 nm. In order to determine the MIC, two-fold dilutions of D-AMB, L-AMB and POS
(0.007-256 mg/l) were incubated with biofilms or planktonic cells (2x10^5 cfu/ml)
for 24h (n = 9). XTT metabolic reduction assay was used to assess biofilm damage compared
to controls. MIC50 was determined as ≥50% biofilm damage. The activity of the combination
of L-AMB (0.5-32 mg/l) with POS (0.125-64 mg/l) against biofilms was determined using
a checkerboard microdilution method (n = 10). Drug interactions were analyzed using
Bliss independence model. The combination effect was defined as synergistic, antagonistic
or indifferent when the observed biofilm damage was significantly higher, lower or
equal to the expected damage, respectively.
Results: All L. corymbifera strains exhibited strong biofilm formation. Biofilm MIC50
of D-AMB, L-AMB and POS were 2, 4 and >256 mg/l, respectively, as compared to 0.007,
0.03 and 0.03 mg/l for planktonic cells. Synergistic effects were observed at 1-2
mg/l of L-AMB combined with 8-16 mg/l of POS (mean ΔE value of significant interactions:19%
[range: 17% to 21%]; mean SE:1% [range: 1% to 3%]). None of the combinations exhibited
antagonism.
Conclusion:
L. corymbifera can produce strong biofilms that possess comparable susceptibility
profiles to D-AMB and L-AMB, but are resistant to POS. The combination of L-AMB with
POS at super-therapeutic concentration ranges exhibits synergistic activity against
mature L. corymbifera biofilms.
Gonçalves
L.C.
1
Orlandi
C.
1
Bila
N.M.
1
2
Vaso
C.O.
1
Silva
R.A. Moraes Da
1
Taylor
M.L.
3
Giannini
M.J. Soares Mendes
1
Almeida
A.M. Fusco
1
1Clinical Analysis, UNESP (Sao Paulo State University), Araraquara, Brazil
2Para-clinics, UEM (Eduardo Mondlane University), Maputo, Mozambique
3Microbiology and Parasitology, UNAM (Autonomous University of Mexico), México, D.F.,
Mexico
P082. Biofilm formation by Histoplasma capsulatum in different culture mediums and
oxygen atmospheres
Objectives: This work aimed to study Histoplasma capsulatum biofilms, by checking
the influence of different culture media and oxygen atmospheres in the development
of these communities.
Methods: The biofilm formation by two strains (ATCC 26029 – G186A and EH315) was characterized
in different nutrient conditions [Brain Heart Infusion (BHI), Roswell Park Memorial
Institute (RPMI) with 2% of glucose, Dulbecco’s Modified Eagle Medium (DMEM) supplemented
with 10% fetal bovine serum and nutrient medium HAM-F12 (HAM-F12) supplemented with
glucose (18.2 g/L), glutamic acid (1 g/L), HEPES (6 g/L), and L-cysteine (8.4 mg/L)]
and atmospheres of oxygen (aerobic and microaerophilic). Optical microscopy techniques,
XTT reduction assay, as well as crystal violet and safranin staining, and scanning
electron microscopy (SEM) were performed.
Results: The results indicated that, although all the culture media stimulated the
maturation of the communities, the nutrient-rich culture media, such as HAM-F12, provided
a better biomass and extracellular matrix development when compared to the others.
In addition, microaerophilic conditions were the most favorable than aerobic. The
topographies observed in SEM showed yeasts surrounded at several points by an exuberant
amount of extracellular matrix. However, the biofilms formed in BHI, RPMI and DMEM
presented a reversal yeast for significant filamentation, which needs better investigation.
Conclusion: The results obtained so far represent significant advances for the field
of biofilms and open new possibilities for the study of virulence and Histoplasma-host
interaction. Funding: FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo
Process # 2016/11836-0); CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico
Process # 130018/2018-0) and Capes (Coordenação de Aperfeiçoamento de Pessoal de Nível
Superior).
Burger
E.
1
Grisolia
J.C.
1
Santos
L.A.
1
Dias
N.A.
1
Malaquias
L.C.C.
1
Rodrigues
A.M.
2
Camargo
Z.P.D.
2
1Department of Microbiology and Immunology, Federal University of Alfenas, Alfenas,
Brazil
2Department of Microbiology, Immunology and Parasitology, Federal University of Sao
Paulo, Alfenas, Brazil
P085. Combined treatment of experimental paracoccidiodomycosis with Itraconazole and
low-level LASER therapy
Objectives: Paracoccidioidomycosis (PCM) is a systemic mycosis caused by the fungus
Paracoccidioides brasiliensis (Pb), requiring long treatment, justifying studies that
amplify the therapeutic options. Itraconazole (Itra) is effective for PCM and requires
shorter therapy than other drugs. We propose to study a shorter treatment model with
fewer side effects through the activation of neutrophils by the simultaneous administration
of the antifungal drug Itraconazole and adjuvant therapy with Low Level LASER Therapy
(LLLT) to provide better control in the early stages of the infection, leading to
a more efficient and protective immune response, resulting in the control or cure
of this disease.
Methods: We used a highly purified PMNs preparation by inoculating Pb in subcutaneous
air pouches of mice. We evaluated the effect of Itraconazole (Itra) therapy and/or
LLLT therapy on their size, cellular composition, mitochondrial activity, protein
metabolism, production of reactive oxygen (ROS) and nitrogen (NO) species, production
of IL-10, INF-y and TNF-α cytokines, and on tissue architecture and number of viable
fungi.
Results: The viability of air pouch cells was always greater than 70%, therefore,
none of the treatments had adverse effects, and about 80% of the cells were PMN. Mitochondrial
activity and cell counts differed significantly between LLLT-treated and controls
and between mice treated with different Itra concentrations, with or without LLLT.
Cells from 10mg/mL Itra-treated mice produced the highest levels of ROS followed by
those of 3mg/mL. When Itra was associated with LLLT treatment, we found an increase
in NO production in the 3mg/mL group. Total protein levels were higher when Itra was
used, compared to the untreated group or to the group treated with LLLT alone, in
which LLLT increased protein production in the 3mg/mL group and decreased in the 10mg/mL
group. LLLT increased the production of cytokines IL-10, INF-y and TNF-α, and the
use of antifungal decreased their production at all doses, both using only Itra treatments
and in combination with LLLT. The highest dose of Itra (50mg/mL) resulted in a significant
reduction of air pouch size, volume and weight. The intensity of the inflammatory
process and the number of vessels and neovas decreased when the concentration of Itra
in combination with LLLT increased. The numbers of viable Pb were significantly reduced
when Itra was administered in a dose-response way and more markedly when used in combination
with LLLT.
Conclusion: Pb numbers decreased significantly when Itra was given at all concentrations,
and this effect increased when used in combination with LLLT. Itra at 50 mg/mL caused
a significant reduction in air pouch, indicating a dose-response control. Our results
strongly suggest that the efficiency of Itra is enhanced by LLLT. Therefore, we could
detect a possible additive or synergistic effect of PMNs, the antifungal drug Itra
and LASER therapy, suggesting a new, more effective treatment for PCM. Grants: CNPq
305216/2016-3 and FAPEMIG APQ 012941-16. J.C.Grisolia and L.A. Santos are recipients
of CAPES scholarships.
Setianingrum
F.
1
2
Rautemaa-Richardson
R.
1
3
4
Shah
R.
4
5
Denning
D.
1
3
4
1Division of Infection, Immunity and Respiratory Medicine, University of Manchester,
Manchester, United Kingdom
2Department of Parasitology, Universitas Indonesia, Jakarta, Indonesia
3National Aspergillosis Centre, Manchester University NHS Foundation Trust, Manchester,
United Kingdom
4Manchester Academic Health Science Centre, Manchester, United Kingdom
5Department of Cardiology and Cardiothoracic Surgery, Manchester University NHS Foundation
Trust, Wythenshawe Hospital, Manchester, United Kingdom
P086. Clinical outcome of chronic pulmonary aspergillosis patients managed surgically
Objectives: Surgery is one of the treatment options for chronic pulmonary aspergillosis
(CPA), especially for single aspergilloma, for those with recurrent haemoptysis despite
bronchial artery embolisation or with the unilateral azole-resistant disease. This
study aims to evaluate the outcomes of patients managed surgically and the correlation
between Aspergillus Immunoglobulin G (IgG) levels and response to treatment.
Methods: This was a retrospective cohort study evaluating CPA patients who were treated
at National Aspergillosis Centre, Manchester, UK over an 11-year period (2007-2018).
Clinical variables including antifungal therapy and Aspergillus-IgG (Thermo Fisher/Phadia)
were recorded and correlated with surgical success or relapse. Aspergillus-IgG were
analysed based on 37 patients with complete serology database.
Results:
61 patients with CPA underwent surgery with a mean duration of follow-up of 36 months.
The mean age was 52.8 (± 14.3) years. The most common presenting symptoms pre-operatively
were cough (n = 41, 67%), haemoptysis (n = 24, 39%), dyspnea (n = 23, 38%) and 5 patients
(8%) were asymptomatic. Failed medical therapy (n = 31, 51%) and presumed malignancy
(n = 23, 38%) were the most common indications for surgery. The most common sign of
failed medical therapy was recurrent haemoptysis (n = 19, 31%). Twenty-six patients
(43%) had single aspergilloma, 23 patients (38%) showed chronic cavitary pulmonary
aspergillosis (CCPA) while 19 patients (31%) had one or more Aspergillus nodule. Main
underlying diseases were chronic obstructive pulmonary disease (n = 19, 31%), asthma
(n = 18, 30%), bronchiectasis (n = 14, 23%) and previous pneumothorax (n = 13, 21%).
The procedures included lobectomy (n = 39, 64%), wedge resection (n = 17, 28%), segmentectomy
(n = 3, 5%), pneumonectomy (n = 3, 5%) and decortication (n = 2, 3%). Main surgical
complications were pleural infection (n = 7, 12%), prolonged air leak (n = 3, 5%)
and persistent pneumothorax (n = 3, 5%). Assessed 6-8 months after surgery, 21 patients
were asymptomatic (34%), 23 experienced cough (38%), 6 patients (10%) had haemoptysis,
and the frequency of these symptoms reduced significantly after surgery (p< 0.05).
Relapse occurred in 25 patients (41%) while the rest of the patients (n = 36, 59%)
showed no sign of relapse. Haemoptysis, cough and dyspnea decreased significantly
in the non-relapse group, whereas only haemoptysis was initially less frequent in
relapse group (p<0.05). In this relapse group, 6 (24%) patients received antifungal
before surgery compared to 23 (64%) in the non-relapse group (p = 0.002). Perioperative
antifungal therapy was administered to 5 (20%) patients who relapsed and 19 (53%)
who did not (p = 0.01). The median Aspergillus-IgG in the non-relapse group (n = 12,
32.4%) before surgery was 75 mg/L decreasing to 52 mg/L in the end of follow-up (p
= 0.003). The median survival time (free-relapse) was 88 months in patients with antifungal
therapy before surgery and 31 months in patients without antifungal therapy before
surgery (p = 0.002).
Conclusion: Surgery in these selected CPA patients resulted in favourable outcomes
with a decrease in clinical symptoms and the Aspergillus-IgG level of the non-relapse
group of CPA. Pre and perioperative antifungal therapy reduced the likelihood of CPA
relapse, but confounding factors may contribute to this. The diagnosis of CPA before
surgery is important to optimise pre and perioperative antifungal therapy in patients
to improve prognosis.
Niazi-Ali
S.
1
Denning
D.
2
3
Atherton
G.
2
3
Walczak
M.
3
4
1Fungal Interaction Trust, Macclesfield, United Kingdom
2University of Manchester, Manchester, United Kingdom
3National Aspergillosis Centre, Manchester University NHS Foundation Trust, Manchester,
United Kingdom
4Manchester Academic Health Science Centre, Manchester University NHS Foundation Trust,
Wythenshawe, United Kingdom
P087. The all new, updated antifungal drug interaction database at www.aspergillus.org.uk
The all new, updated antifungal drug interaction database at www.aspergillus.org.uk
The all new, updated antifungal drug interaction database at www.aspergillus.org.uk
Objectives: A drug-drug interaction describes the influence of one drug upon another
or the change in a drug’s effect on the body when the drug is taken together with
a second drug. A drug-drug interaction can delay, decrease, or enhance absorption
or metabolism of either drug. The antifungal drug interaction database was first launched
in 2012. It is available as web (www.aspergillus.org.uk/content/antifungal-drug-interactions)
and app versions to allow information on potential drug interactions with antifungals
with a version for patients and another for health professionals. New drugs are constantly
being launched and approved. In this era, with the requirement of speed and accurate
information due to pressures on all healthcare professionals, a new and updated database
with apps (on Apple and Android platforms) has been created. This allows all of the
above with the added benefit of real time updates in a more time efficient manner.
Methods: Horizon scanning documents were reviewed from 2017, 2018 and 2019 to date.
Drugs that were not in the current database were identified and interactions determined
using the Summary of Product Characteristics together with further exploration, as
needed, with review of papers. CYP P450 and p-glycoprotein are the major interaction
pathways where antifungal drugs are involved. However other pathways like OATP1B1
and BCRP were also explored, as there is new and emerging information on the effect
of these pathways with drug-drug interactions.
Results:
134 new preparations were identified to April 2019 leading to updated information.
16 classed as red (severe interactions with some antifungals), 29 as amber (moderate
interactions where co-administration is possible and guidance on any management required)
and 89 as green (interaction unlikely). These were added to the database together
with references to ensure the user is able to, refer and, find references with ease
to validate their decisions. of 16,324 possible interactions for drugs licensed by
the end of 2018, 507 are potentially severe (3.1%), 1080 (6.6%) moderate and 809 mild
interactions (4.9%). The antifungals with the least interactions are anidulafungin
(n = 3 mild) and micafungin (n = 4 moderate & 8 mild) which contrasts with voriconazole
with 459 interactions (140 severe) and itraconazole with 404 interactions (134 severe).
Conclusion: The new and revised version of the web and smartphone app-based database
of these drug interactions with antifungal agents is now available. This will allow
clinicians to effectively evaluate the use of antifungals with all the new drugs on
the market and allow the best outcome for their patients. It will also enable them
to make the most effective and evidence-based decisions for those drugs that are co-administered
and require a little more clinical management to provide the most successful treatment
for the wellbeing of their patients.
Nguyen
M.-V.
1
Davis
M.
1
Wittenberg
R.
1
Mchardy
I.
1
Baddley
J.
1
Young
B.
1
Odermatt
A.
2
Thompson
G.
1
1Infectious Disease, University of California at Davis, Davis, United States of America
2Toxicology, University of Basel, Basel, Switzerland
P088. Posaconazole Serum Drug Levels Associated with Hypertension and Hypokalemia:
The Syndrome of Pseudohyperaldosteronism
Objectives: Posaconazole tablets are well tolerated and efficacious in the prophylaxis
and treatment of aspergillosis, mucormycosis, and other invasive fungal infections
There have been case reports of posaconazole-induced pseudohyperaldosteronism, however,
its occurrence and association with serum posaconazole drug levels has not previously
been investigated.
Methods: In this single-center retrospective observational study, we examined the
occurrence of posaconazole-induced pseudohyperaldosteronism (PIPH) in outpatients
newly starting posaconazole and evaluated differences in serum posaconazole concentrations
and clinical characteristics between those with and without this syndrome.
Results: Sixty-nine patients receiving posaconazole were included, of whom 16 (23.2%)
met the definition of PIPH. Patients with PIPH were significantly older (61.1 vs 44.7
years, P = 0.007) and more frequently had hypertension prior to starting posaconazole
(68.8% vs 32.1%, P = 0.009). Patients with PIPH had a significantly higher median
serum posaconazole level than those without PIPH (3.0 vs 1.2 µg/mL, P < = 0.0001).
There was a positive correlation between serum posaconazole levels and changes in
systolic blood pressure (r = 0.37, P = 0.01), a negative correlation between serum
posaconazole levels and changes in serum potassium (r = -0.39, P = 0.006), and a positive
correlation between serum posaconazole levels and serum 11-deoxycortisol (r = 0.69,
P < 0.0001).
Conclusion: Posaconazole is associated with secondary hypertension and hypokalemia,
consistent with pseudohyperaldosteronism, and development is associated with higher
serum posaconazole concentrations, older age, and baseline hypertension.
Kim
S.-H.
1
Song
K.Y.
2
Choi
J.-H.
1
1Department of Internal Medicine, College of Medicine, The Catholic University of
Korea, Seoul, Republic of Korea
2Department of Orthopaedic Surgery, College of Medicine, The Catholic University of
Korea, Seoul, Republic of Korea, Seoul, Republic of Korea
P089. A case of primary joint infection caused by Candida pelliculosa
Case Report:
Candida joint infection is most often due to hematogenous seeding during an episode
of candidemia in immunocompromised patients. Exogenous inoculation after trauma or
surgical procedures also can lead to infection, but primary joint infection caused
by Candida species is extremely rare. Until now, some Candida joint infections by
Candida albicans, Candida krusei, Candida lusitaniae, Candida parapsilosis, and Candida
tropicalis have been reported. Herein, we report the first case of primary knee joint
infection caused by Candida pelliculosa without predisposing factors. A 75-year-old
woman came to our hospital with a 3-year history of pain and swelling of the left
knee, with the pain particularly aggravated for the past 1 month. The patient had
undergone a left total knee arthroplasty (TKA) for incapacitating knee pain at a local
hospital 32 months ago. At that time, C. pelliculosa was isolated from the tissue
taken by intraoperative biopsy, but no antifungal treatment was performed. One year
after TKA, C. pelliculosa was repeatedly isolated from left knee synovial fluid and
the patient was treated with intravenous amphotericin B deoxycholate (0.5 mg/kg/day)
for 4 weeks, then intravenous fluconazole (6 mg/kg/day) for 3 weeks, followed by oral
fluconazole (4 mg/kg/day) for 2 weeks. However, progressive bone loss around prosthetic
components was seen on follow-up radiographs and the patient was referred to tertiary
hospital. The patient had no other significant medical history. The patient was admitted
to our hospital and arthrocentesis of the left knee was performed. Results of synovial
fluid analysis were as follows: leukocyte, 608 cells/μL (neutrophil, 54%); protein,
5.9 g/dL; glucose, 7 mg/dL. Magnetic resonance imaging showed a large abscess around
the antero-lateral aspect of the left knee. Fungal culture of synovial fluid reported
as C. peilliculosa susceptible to amphotericin B (minimum inhibitory concentration
[MIC], ≤0.25 mg/L), caspofungin (MIC, ≤0.25 mg/L), fluconazole (MIC, 2 mg/L), micafungin
(MIC, ≤0.06 mg/L), and voriconazole (MIC, ≤0.12 mg/L) by Vitek 2 yeast identification
system. The isolate was confirmed as C. pelliculosa using Internal transcribed spacer
1 and 4 region PCR and sequencing. At 2 weeks of micafungin (100 mg/day) therapy,
the patient underwent prosthesis resection with debridement of soft tissue and bone,
total synovectomy, and placement of an antibiotic-impregnated spacer. Operative findings
showed infected granulation tissue in whole joint space and bony fistula extended
to the anterolateral aspect of the lateral tibial component. Postoperatively, the
patient was given micafungin for 4 weeks, followed by oral fluconazole (6 mg/kg/day).
There were no signs of infection around the left knee and the patient was discharged
from the hospital. Oral fluconazole will be given for at least 6 months and implantation
of new prosthesis is planned at 3 months after the stage 1 procedure. Rare Candida
species could be a causative organism of primary joint infection. Even a single colony
of Candida on synovial fluid or bone biopsy should be considered as pathogenic, and
the patient should be treated with antifungal agents. A high index of suspicion is
required to establish the diagnosis.
Köhler
P.
1
Reimer
R.
2
Whaba
R.
3
Schömig-Markiefka
B.
4
Cornely
O.A.
1
1Department I For Internal Medicine, European Excellence Center For Medical Mycology
(ecmm), Germany and Cecad Cluster of Excellence, University of Cologne, Cologne, Germany
2Department of Diagnostic and Interventional Radiology, University Hospital Cologne,
Cologne, Germany
3Department of General, Visceral and Cancer Surgery, University Hospital of Cologne,
University of Cologne, Cologne, Germany
4Institute of Pathology, University Hospital Cologne, Cologne, Germany
P090. Transdiaphragmatic Mucormycosis
Objectives: Introduction Mucormycosis is a life-threatening infection in immunocompromised
patients and in haematological malignancy patients in particular. Objectives To report
the phenomenon of transdiaphragmatic mucormycosis and to raise concerns with regard
to the extent of imaging studies in patients with mucormycosis.
Methods: Retrospective chart review was performed on patients with Mucormycosis treated
at the University Hospital of Cologne between January 2011 and January 2019.
Results: In a series of three patients we observed a previously unreported contiguous
disease pattern, where the pulmonary type of disease contiguously spread through the
diaphragm into abdominal organs, mostly liver or spleen. Patient 1, a 53-year-old
women with history of B-NHL, ASCT and chronic renal disease developed mucormycosis
of the lungs with continuous growth through the diaphragm into the liver. Histology
showed invasive growth of fungal hyphae of the Mucorales order in liver and diaphragm.
Patient 2, a 35-year-old man with history of AML and no further underlying conditions
developed mucormycosis of the lungs with continuous growth through the diaphragm into
the spleen. Splenectomy was performed and histology showed invasive growth of fungal
hyphae. Fungal cultures of the surgical sample remained negative, but panfungal PCR
resulted in Rhizomucor spp. Patient 3, a 42-year-old man with history of poorly controlled
IDDM and injection drug use developed mucormycosis of the lungs with continuous growth
through the diaphragm into the liver. Fungal culture of BAL fluid showed growth and
PCR positivity of Rhizopus microsporus.
Conclusion: This is the first report of the clinical observation of transdiaphragmatic
mucormycosis in 3 patients. We observed this phenomenon in 11% (3/27) of patients,
raising concerns with regard to the extent of imaging studies in patients with mucormycosis.
Clinicians should become aware of this new-described entity.
Köhler
P.
1
Salmanton-Garcia
J.
2
Gräfe
S.
3
Köhler
F.
3
Mellinghoff
S.
4
Seidel
D.
1
Steinbach
A.
5
Cornely
O.A.
6
1Department I of Internal Medicine, Ecmm Excellence Centre of Medical Mycology, Cologne
Excellence Cluster On Cellular Stress Responses In Aging-associated Diseases (cecad),
University Hospital Cologne, Cologne, Germany
2Department I For Internal Medicine, European Excellence Center For Medical Mycology
(ecmm), Germany and Cecad Cluster of Excellence, University of Cologne, Cologne, Germany
3Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases
(CECAD), Cologne, Germany
4Department I of Internal Medicine, Ecmm Excellence Centre of Medical Mycology, Cologne
Excellence Cluster On Cellular Stress Responses In Aging-associated Diseases (cecad),
German Centre For Infection Research, Partner Site Bonn-cologne, University Hospital
Cologne, Cologne, Germany
5Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases
(CECAD), German Centre for Infection Research, Partner Site Bonn-Cologne, Cologne,
Germany
6Department I of Internal Medicine, Ecmm Excellence Centre of Medical Mycology, Cologne
Excellence Cluster On Cellular Stress Responses In Aging-associated Diseases (cecad),
German Centre For Infection Research, Partner Site Bonn-cologne, Clinical Trials C,
University Hospital Cologne, Cologne, Germany
P091. Baseline predictors influencing the prognosis of invasive aspergillosis in adults
Introduction: Invasive aspergillosis (IA) is a serious hazard to hematological and
critical care patients.
Objectives: To understand prognostic variables we analyzed original articles identifying
baseline factors that predict treatment outcome in patients with IA.
Methods: PubMed database was searched for publications since database inception until
May 2018. Inclusion criteria were published baseline prognostic factors present at
the diagnosis of IA.
Results: In total, 58 studies from 267 centers reported 7,320 patients with IA, and
40 different predictors. Unfavorable predictors in medical history were kidney (7.4%,
10/136) and liver failure (3.7%, 5/136), ICU admission (3.7%, 5/136), and uncontrolled
underlying disease (3.7%, 5/136). Regarding state of immunosuppression, negative outcome
predictors were prolonged neutropenia (12.5%, 17/136), corticosteroid treatment (8.1%,
11/136), and graft-versus-host disease (3.7%, 5/136). On the pathogen side, relevant
predictors were galactomannan positivity (8.1%, 11/136), Aspergillus terreus infection
(2.2%, 3/136), and lack of amphotericin B susceptibility (1.5%, 2/136). IA-specific
predictors were disseminated disease (5.1%, 7/136) and CNS involvement (2.9%, 4/136).
Imaging results associated with negative outcome were multiple consolidations (2.9%,
4/136), bipulmonary lesions (2.2%, 3/136), and pleural effusion (2.2%, 3/136).
Conclusion: At diagnosis of IA, most frequently identified predictors of outcome were
neutropenia, corticosteroid use, elevated galactomannan, renal failure, and disseminated
disease.
Cornely
O.A.
1
Köhler
P.
2
Arenz
D.
3
Mellinghoff
S.
2
1Department I For Internal Medicine, Excellence Center For Medical Mycology (ecmm),
Cecad Cluster of Excellence, Clinical Trials Centre Cologne (zks Köln), University
of Cologne, Cologne, Germany
2Department I For Internal Medicine, Excellence Center For Medical Mycology (ecmm),
Cecad Cluster of Excellence, University of Cologne, Cologne, Germany
3Department I For Internal Medicine, Excellence Center For Medical Mycology (ecmm),
University of Cologne, Cologne, Germany
P092. EQUAL Aspergillosis Score 2018: An ECMM score derived from current guidelines
to measure QUALity of the clinical management of invasive pulmonary aspergillosis
Introduction: Invasive pulmonary aspergillosis is a serious threat to immunocompromised
and critical care patients. Recent detailed guidelines and treatment algorithms lead
microbiologists and clinicians in diagnosis and treatment of invasive aspergillosis.
Objectives: Currently, there is no tool available that allows to measure guideline
adherence and quality of managing invasive pulmonary aspergillosis.
Methods: To develop such a tool, we reviewed current guidelines provided by 5 scientific
societies: European Society for Clinical Microbiology and Infectious Diseases, European
Confederation of Medical Mycology, the European Respiratory Society, Infectious Diseases
Society of America (IDSA), and Infectious Diseases Working Party of the German Society
for Hematology and Medical Oncology. We selected the strongest recommendations for
management as key components for our scoring tool, broke down guidelines, and grouped
recommendations into 3 groups: diagnosis, treatment, follow-up. First, we selected
“AI” and “AII” recommendations. Then, we added aspects that are part of a complete
work-up, but are either recommended with a lower grade of evidence or not specifically
recommended in current guidelines. In a last step, we allocated score points according
to levels of evidence and clinical importance.
Results: We developed the EQUAL Aspergillosis Score 2018 to measure the quality of
clinical management of invasive pulmonary aspergillosis. We integrated diagnostic
measures (chest computed tomography, bronchoalveolar lavage with galactomannan, fungal
culture, fungal polymerase chain reaction analysis, species identification, and susceptibility
testing; histology with silver stain, Periodic acid–Schiff staining, and molecular
diagnostics), treatment (antifungal choice and therapeutic drug monitoring), and follow-up
computed tomography. For ease of use, we have summarized the EQUAL scores on laminate
pocket cards, which will be translated in 15 different languages. The EQUAL Aspergillosis
Score 2018 can be used to evaluate guideline adherence in daily clinical practice
and thus aims to support antifungal stewardship.
Conclusion: The EQUAL Aspergillosis Score 2018 aggregates and weighs the components
recommended in the ideal management of invasive pulmonary aspergillosis and provides
a tool supporting antifungal stewardship and quantifying guideline adherence.
Köhler
P.
1
Mellinghoff
S.
1
Alanio
A.
2
Arenz
D.
3
Hoenigl
M.
4
Lagrou
K.
5
Lass-Flörl
C.
6
Meis
J.
7
Richardson
M.D.
8
Cornely
O.A.
9
1Department I For Internal Medicine, Excellence Center For Medical Mycology (ecmm),
Cecad Cluster of Excellence, University of Cologne, Cologne, Germany
2Paris-diderot, Sorbonne Paris Cité University, Institut Pasteur, Molecular Mycology
Unit, Cnrs Cmr2000, Parasitology-Mycology Laboratory, Lariboisière Saint-Louis Fernand
Widal Hospitals, Assistance Publique-Hôpitaux de Paris, Paris, France
3Department I For Internal Medicine, Excellence Center For Medical Mycology (ecmm),
University of Cologne, Cologne, Germany
4Division of Infectious Diseases, Department of Medicine, UCSD, San Diego, United
States of America
5Laboratory of Clinical Bacteriology and Mycology, Department of Microbiology and
Immunology, Excellence Center For Medical Mycology (ecmm), KU Leuven, Leuven, Belgium
6Division of Hygiene and Medical Microbiology, Excellence Center For Medical Mycology
(ecmm), Med. Univ. Innsbruck, Innsbruck, Austria
7Department of Medical Microbiology and Infectious Diseases, Excellence Center For
Medical Mycology (ecmm), Canisius Wilhelmina Hospital, Nijmegen, Netherlands
8Mycology Reference Centre, Excellence Center For Medical Mycology (ecmm), Wythenshawe
Hospital, Manchester University NHS Foundation Trust, Manchester, United Kingdom
9Department I For Internal Medicine, Excellence Center For Medical Mycology (ecmm),
Cecad Cluster of Excellence, Clinical Trials Centre Cologne (zks Köln), University
of Cologne, Cologne, Germany
P093. EQUAL Mucormycosis Score 2018: An ECMM Score Derived From Current Guidelines
to Measure QUALity of Mucormycosis Management in Hematology
Introduction: Mucormycosis is a serious infectious threat to immunocompromised patients
in general and in hematological malignancy in particular. Detailed standards and treatment
algorithms guide microbiologists and clinicians in the diagnosis and treatment of
mucormycosis.
Objectives: Currently, there is no tool available that allows the measurement of guideline
adherence and the quality of managing mucormycosis.
Methods: To develop such a tool, we reviewed current guidelines provided by four scientific
societies: the European Society for Clinical Microbiology and Infectious Diseases
(ESCMID), the European Confederation of Medical Mycology (ECMM), the European Conference
on Infections in Leukemia (ECIL), and the Infectious Diseases Working Party of the
German Society for Hematology and Medical Oncology. We selected the strongest recommendations
for management as key components for our scoring tool, broke down guidelines, and
grouped recommendations into three groups: diagnosis, treatment, follow-up. First,
we selected “AI” and “AII” recommendations. Then we added aspects that are part of
a complete work-up, but are either recommended with a lower grade of evidence or not
specifically recommended in current guidelines. In a last step, we allocated score
points to the various levels of evidence and clinical relevance of diagnostic and
therapeutic procedures.
Results: We developed the EQUAL Mucormycosis Score 2018 to measure the quality of
mucormycosis management. We integrated diagnostic measures (chest computed tomography,
bronchoalveolar lavage with fungal culture, fungal polymerase chain reaction analysis,
species identification, susceptibility testing, histology with silver stain, Periodic
acid–Schiff staining, and molecular diagnostics), treatment (antifungal choice, surgical
debridement and therapeutic drug monitoring), and follow-up computed tomography. For
ease of use, we have summarized the EQUAL scores on laminate pocket cards, which will
be translated in 15 different languages. The EQUAL Mucormycosis Score 2018 can be
used to evaluate guideline adherence in daily clinical practice and thus aims to support
antifungal stewardship.
Conclusion: The EQUAL Mucormycosis Score 2018 aggregates and weighs recommendations
for management of mucormycosis and provides a tool supporting antifungal stewardship
and quantifying guideline adherence.
Brown
L.
1
Rautemaa-Richardson
R.
1
1Faculty of Biology, Medicine and Health, University of Manchester, pl, United Kingdom
2Division of Infection, Immunity and Respiratory Medicine, University of Manchester,
Manchester, United Kingdom
P094. Compliance with recurrent vulvovaginal candidiasis guidelines – An audit of
RVVC referrals
Objectives: Recurrent vulvovaginal candidiasis (RVVC) is a debilitating, chronic condition
which affects over 138 million (6%) women of reproductive age annually. It is defined
as four or more episodes in a 12-month period. Symptoms include vulval itch, soreness
and discharge. Diagnosis is often difficult, as it is based on a combination of these
non-specific clinical symptoms and detection of Candida by high vaginal swab (HVS)
microscopy, which is part of the normal vaginal flora in many women. A number of other
infectious and non-infectious conditions can present similarly. Treatment of RVVC
comprises of vulval skin care with the use of emollients for personal hygiene and
antifungal treatment combined with long-term antifungal suppression for 6 months or
longer. The British Association for Sexual Health and HIV (BASHH) has published guidelines
on the management of RVVC. The aim of our study is to evaluate the compliance of referring
doctors to the current guidelines.
Methods: This retrospective audit was undertaken in the Infectious Diseases (ID) department
of Wythenshawe Hospital, Manchester from October 2017 to May 2019. All patients referred
to the tertiary clinic with suspected RVVC were included in the analyses. Electronic
patient records were analysed to determine whether appropriate investigations and
treatments had been offered prior to referral. The type and pattern of recorded symptoms
were assessed against the typical clinical picture of RVVC and whether alternative
differentials should have taken precedence.
Results: Patient records from a total of 47 RVVC referrals were analysed. Patient
age at the time of referral ranged from 17-66 years with a mean age of 37.7 years.
Symptom duration prior to referral ranged from 4 months to 24 years, with an average
duration of 6.7 years. “Dry”, “sensitive” skin or “eczema” were mentioned in 36% of
the cases. RVVC was the final diagnosis in 80% of cases. 19% of referrals were deemed
inappropriate due to repeatedly negative HVS for Candida and lack of response to antifungal
therapy, or reported symptoms not fitting with RVVC. 57% of patients had a secondary
diagnosis, the most common being vulvodynia and vulval dermatitis. 94% of the patients
were appropriately investigated by high vaginal swab (HVS) microscopy or culture prior
to referral. Appropriate antifungal regimens, including long term suppression, were
trialled in 53% of patients prior to referral. Only 11% patients were practicing appropriate
vulval skin care before attending clinic. Excessive or otherwise inappropriate personal
hygiene likely to irritate vulval skin, including the use of soap and shower gel,
was mentioned in 21% of cases. MBL deficiency was diagnosed in 28% of the patients.
Conclusion: RVVC is often complicated by coexisting vulval pathologies and vulval
symptoms labelled as RVVC are often due to alternative diagnoses. Education on vulval
skin care is the mainstay of RVVC management and prevention but healthcare professionals
are failing to deliver this. Also, there is lack of awareness of the efficacy and
safety of long term antifungal suppression. The morbidity related to this debilitating
condition could be easily reduced by following the principles summarised in the BASHH
guidelines.
Peppel
R. Van De
Grootveld
R. Van
Hendriks
B.
Paassen
J. Van
Koopmans
J.
Borne
P. Von Dem
Beek
M. Van Der
Boer
M. De
Infectious Diseases, Leiden University Medical Center, Leiden, Netherlands
P095. Validation of a clinical decision rule for selecting empiric treatment for invasive
aspergillosis in a setting with high triazole resistance.
Objectives: World-wide, increasing triazole resistance complicates treatment of invasive
aspergillosis (IA). In case of detected triazole resistance, the first choice treatment
is replaced by lipid formulations of Amphotericin B (LAmB). In settings with substantial
(>10%) prevalence of triazole resistance, empiric combination therapy with both a
triazole and LAmB has been suggested. However, the expected benefits of this strategy
need to be weighed against a higher rate of serious adverse events associated with
combination therapy, as well as higher costs. To evade unnecessary toxicity while
optimizing outcome, a clinical decision rule guiding to monotherapy with either voriconazole
or LAmB was designed and validated.
Methods: In 2015, all medical specialties involved in the diagnosis and treatment
of patients with IA participated in constructing a treatment algorithm that provided
rationale for empiric monotherapy with either voriconazole or LAmB (Figure 1). The
designed algorithm was approved by the institution’s antimicrobial steering committee.
Protocolized CT-scanning and bronchoalveolar lavage (BAL) were performed upon suspicion
of IA. BAL samples were examined by direct microscopy, culture, galactomannan assay
(cut off at 0.5 OD) and from 2017 onwards also by PCR. Triazole resistance was routinely
tested by four well agar plate. All adult patients diagnosed with IA and treated for
this condition in the Leiden University Medical Center from 2015 to 2019 were included.
Data about underlying diseases, age, gender, results of diagnostic tests and outcomes,
were extracted from the hospital’s electronic patient files and anonymized. Primary
outcomes were 30- and 100-day crude- and attributable mortality rates.
Results: At first evaluation, 102 patients had been treated for IA with monotherapy
according to the designed algorithm and 3 patients received off-protocol treatment
regimens (Figure 2). In the period of study, prevalence of voriconazole resistance
in all Aspergillus isolates varied per year between 16.3% and 24%. of included patients,
65 (64%) were male; the median age was 64 (range 18-82), and 80 (78%) patients were
treated for an underlying hematological malignancy. Fifty-nine patients (58%) were
started on voriconazole and 43 (42%) were started on LAmB. Cultures were positive
in 16 (15.7%) patients and phenotypical voriconazole resistance was detected in 7/16
(44%). Upon clinical evaluation of empiric therapy and/or resistance data if available,
a switch was made to LAmB in 23/59 (39%) of patients started on voriconazole. Crude
mortality rates per treatment stratum are listed in Figure 2. Later adaptation of
antifungal therapy to LAmB was not associated with a higher crude mortality rate (p
= 0.73). Definite therapy consisted of voriconazole in 41 (40%) and of LAmB in 61
(60%) of cases.
Conclusion: By applying a comprehensive clinical decision algorithm in our area with
high triazole-resistance rates, 58% of patients were empirically treated with monotherapy
voriconazole, without excess crude mortality even if a later switch to LAmB was needed.
In 40% of patients therapy with LAmB could be safely avoided during the entire course
of treatment. As treatment allocation was not randomized, and confounding by indication
plays a role, it remains unclear whether patients directed to initial LAmB monotherapy
would have benefited from the addition of a triazole.
Gangneux
J.P.
1
Padoin
C.
2
Michallet
M.
3
Saillio
E.
4
Kumichel
A.
5
Tour
R. Peffault De La
6
Ceballos
P.
7
Gastinne
T.
8
Pigneux
A.
9
1Laboratoire De Parasitologie-mycologie, CHU de Rennes, Rennes, France
2Pharmacy Department, CHU de Martinique, Fort-de-France, France
3Clinical Haematology Department, CH Lyon-Sud, Pierre-Bénite, lyon, France
4MSD France, courbevoie, France
5ClinSearch, Malakoff, France
6Saint-Louis Hospital, APHP, Paris, France
7CHRU Lapeyronie de Montpellier, Montpellier, France
8CHU de Nantes, Nantes, France
9Hospital group Haut Leveque de Pessac, Pessac, France
P096. Multicenter study on antifungal prophylaxis in high risk hematology patients
- The SAPHIR Study
Objectives: The main objective of the SAPHIR study was to describe the diagnostic
and therapeutic management of hematology patients at high risk of Invasive Fungal
Disease (IFD) under antifungal prophylaxis (AFP) in France. The secondary objectives
were the description of (1) parameters and diagnostic procedures concomitant to AF
therapy management and (2) preventive procedures adopted in French hematology services.
Methods: SAPHIR was an observational, prospective, multicenter, non-comparative French
study. The included patients were ≥18 years, in a context of myelosuppressive chemotherapy
for acute myeloid leukemia (AML) and initiated an AF prophylactic treatment.
Results: The 23 participating hematology services included 404 patients, whose mean
age was 56.4±14.0 years and of whom 51.2% were male. Duration between AML diagnosis
and inclusion was 65.4±178 days with 65.6% being newly diagnosed. A previous chemotherapy
had been undergone by 26.7%. The index chemotherapy, was started 1.06±4.49 days before
inclusion and its nature was either induction 79.0%, consolidation 12.6% or relapse
8.4%. Among the 404 patients, 91.6% had experienced a profound neutropenia period
with 1.08±0.51 periods per patient and a duration of 23.3±16.6 days. For 373 patients
(92.4%), the initially prescribed AFP was posaconazole tablets (90.6%) or suspension
(1.73%). Considering all patients, 8.17% changed AFP between 1 and 3 times, with the
first change 17.3±23.9 days after inclusion mostly due to absorption issues (65.0%).
The mean AFP period was 24.2±32.1 days after which 267 patients (66.8%) stopped because
they were at the end of the high-risk period and 126 (31.2%) switched to a non-prophylactic
AF treatment (2/3 empirical, 1/3 preemptive/curative), mainly because of fever (67.1%).
A total of 18/404 patients (4.46%) have received a curative treatment based on positive
mycological exams, imaging, antifungal sensitivity tests and/or blood cultures. Among
them, 9/404 (2.23%) were true probable IFDs: 5 probable invasive aspergillosis, 2
pulmonary pneumocystosis, 1 mucormycose and 1 fungemia due to Saccharomyces sp. At
7-15 days after AFP discontinuation, 365/387 patients (94.3%) showed no signs and
symptoms of infection. In the course of the study, 20 patients (5.0%) had deceased
within 57.6±50.3 days after inclusion. of those, 7 patients (35.0%) showed signs and
symptoms of infection and the death of 3 patients was directly linked to IFD infections
(1 candidiasis and 2 aspergillosis). In order to prevent IFD, additionally to AFP,
French haematology services provide the patient with sterile/protected food (94.3%),
digestive decontamination (35.8%), and accommodation in a sterile/isolated area (93.5%).
To gain a better understanding of the patient characteristics possibly indicating
the necessity for a consecutive empirical/preemptive/curative AF treatment, data of
patients with and without such a consecutive treatment were compared. Several parameters
proved to be significantly different, for instance: cytogenetics and molecular biology
prognosis, AML onset, previous chemotherapy treatment but also duration between chemotherapy
initiation and inclusion and the type of current chemotherapy cycle used.
Conclusion: AF prophylactic treatment is frequently prescribed in French hematology
services. Only few prophylactic treatment changes are necessary which reflects treatment
tolerance and effectiveness regarding decreased numbers of intercurrent infections
and mortality.
Spec
A.
1
Mejia-Chew
C.
1
Powderly
W.
1
Köhler
P.
2
3
Cornely
O.A.
2
4
1Internal Medicine, Washington University School of Medicine in St. Louis, St. Louis,
MO, United States of America
2Department I For Internal Medicine, European Excellence Center For Medical Mycology
(ecmm), Germany and Cecad Cluster of Excellence, University of Cologne, Cologne, Germany
3Department I of Internal Medicine, Ecmm Excellence Centre of Medical Mycology, Cologne
Excellence Cluster On Cellular Stress Responses In Aging-associated Diseases (cecad),
University Hospital Cologne, Cologne, Germany
4German Centre For Infection Research, Partner Site Bonn-cologne, Cologne, Germany
and Clinical Trials Centre Cologne (zks Köln), University of Cologne, Cologne, Germany
P097. EQUAL Cryptococcus Score 2018: A European Confederation of Medical Mycology
Score Derived From Current Guidelines to Measure QUALity of Clinical Cryptococcosis
Management
Objectives: Cryptococcocis is an opportunistic fungal infection with high morbidity
and mortality. Guidelines to aid clinicians regarding diagnosis, management, and treatment
can be extensive and challenging for clinicians. Currently, there is no tool available
that allows the measurement of guideline adherence and the quality of cryptococcosis
management.
Methods: We reviewed current guidelines from the Infectious Diseases Society of America,
the World Health Organization, the American Society of Transplantation and recent
significant publications to select the strongest recommendations as vital components
of our scoring tool.
Results: Items included diagnostic tests (blood, tissue, and cerebrospinal fluid cultures,
Cryptococcus antigen, India ink, histopathology with special fungal stains, central
nervous system imaging), pharmacological (amphotericin B, flucytosine, azoles) and
nonpharmacological treatments (intracranial pressure management, immunomodulation,
infectious disease consultation), and follow-up of central nervous system complications.
For ease of use, we have summarized the EQUAL scores on laminate pocket cards, which
will be translated into 15 different languages.
Conclusion: The EQUAL Cryptococcus Score 2018 weighs and aggregates the recommendations
for the optimal management of cryptococcosis, providing a tool that could measure
guideline adherence or facilitate clinical decision-making.
Chander
J.
1
Bhagat
A.
1
Gulati
N.
1
Singla
N.
1
Punia
R.P.S.
2
Agarwal
D.
3
Attri
A.K.
4
Dass
A.
5
1Microbiology, Government Medical College Hospital, Chandigarh, India
2Pathology, Government Medical College Hospital, Chandigarh, India
3Pulmonary Medicine, Government Medical College Hospitaln, Chandigarh, India
4General Surgery, Government Medical College Hospital, Chandigarh, India
5Ent, Government Medical College Hospital, Chandigarh, India
P099. An ongoing malady, mucormycosis from a tertiary care centre at Chandigarh, India.
Objectives: Mucormycosis is an emerging necrotizing angioinvasive infection caused
by commonly encountered filamentous fungi of the class Mucormycetes. In our institution,
as the awareness is gradually increasing, newer and newer species causing mucormycosis
are being recognized. In this very reference a study was undertaken to further enhance
our knowledge on risk factors, species distribution and their antifungal susceptibility
testing.
Methods: The study was conducted on forty-four cases of mucormycosis over a period
of eighteen months from January 1, 2017 to June 30, 2018. The samples were subjected
to microbiological examination like KOH/CFW wet mount and fungal culture and histopathology.
The isolates obtained were identified phenotypically and confirmed by DNA sequencing
of internal transcribed spacer region. The antifungal susceptibility testingwas done
for amphotericin B, posaconazole, itraconazole and terbinafine by microbroth dilution
as per CLSI Reference Method.Study performed by Almyroudis, et al, was referred to
define susceptibility for some of the breakpoints for Mucorales.
Results: The mean age of patients of mucormycosis was 48.89 ± 17.1 years. The proportion
of female patients (54.5%) was slightly more than male patients (45.5%). Rhino-orbito-cerebral
mucormycosis (ROCM) was the commonest presentation (61.4%, 27) followed by pulmonary
(20.4%, 9) and cutaneous (15.9%, 7). One case of mucormycosis of the middle ear was
also seen. The most common risk factor associated with mucormycosis was diabetes mellitus
(77.3%, 34/44), which was found to be statistically significant (P = 0.003). Tooth
extraction was seen as a risk factor in 18.5% (5/27) of ROCM cases. The commonest
isolate was Rhizopus arrhizus (32.3%, 10), followed by Rhizopus microsporus (29%,
9) and Apophysomyces variabilis (12.9%, 4). Rhizopus homothallicus was isolated from
two cases, one of middle ear and other from pulmonary mucormycosis. A case of cutaneous
mucormycosis caused by Thamnostylum piriforme, which is the second case of this genus
and first of this species, is being reported. Amphotericin B was the most effective
drug with an MIC range of 0.0313-4 µg/ml giving 94.7 % isolates as sensitive. The
only isolate resistant was Rhizopus homothallicus with an MIC of 4 µg/ml. Posaconazole
gave an MIC range of 0.0625-4 µg/ml with 68.4% isolates as sensitive. Dual management
with surgery and antifungals proved to be significantly better (P < 0.001) than amphotericin
B alone. Treatment with liposomal amphotericin B (LAmB) was associated with a higher
survival rate (81%), compared to 28.6% survival when patients were treated with conventional
amphotericin B deoxycholate. Twelve (27.3%) patients either left against medical advice
or were referred. A total of 12 patients expired giving a mortality rate of 27.3%
and 37.5% among the treated patients.
Conclusion: India has second largest diabetic population globally with nearly 70%
being found with uncontrolled diabetes leading to high incidence of mucormycosis.
Due to an ever-growing population at risk and the fatal outcome, need for more prospective
studies in this field are emphasized. Increased awareness among clinicians, timely
diagnosis and prompt treatment are the key to the successful management.
Kumari
A.
1
Singh
K.
2
1Animal Mycology Laboratory, Zoology, Mmv, Banaras Hindu University, Varanasi, India
2Animal Mycology Laboratory, Zoology, Mmv, Banaras Hindu University, VARANASI, India
P100. The Protective Role of Cinnamaldehyde against Tenuazonic acid-induced Mycotoxicity
in Murine model
Objectives: Cinnamaldehyde is a natural product obtained from cinnamon and has been
reported to have insecticidal, antimicrobial, anti-inflammatory, immunomodulatory,
anticancer, anti-angiogenic and potential anti-fungal effects. The present study investigated
the possible protective effect of cinnamaldehyde against tenuazonic acid-induced mycotoxicity
in murine model. Tenuazonic acid (TeA), a toxin produced by Alternaria alternata is
a common contaminant in tomato and tomato based products.
Methods: The in-vivo experimental plan was as follows.
All the experimental groups were administered with TeA for 8 weeks. After 2 weeks,
the prophylactic group was administered with cinnamaldehyde along with TeA for the
next 6 weeks. Haematological (differential leukocyte count), histopathological (haematoxylin
and eosin), biochemical (superoxide dismutase, catalase, malondialdehyde, alanine
aminotransferase, aspartate aminotransferase) and cell apoptotic factor (caspase-3)
analyses of the experimental and control mice were performed.
Results: Behavioral observations- Unlike mycotoxicosis induced groups, no behavioural
abnormalities were observed in the animals who received cinnamaldehyde as prophylactic
agent. Weights of the mice of the prophylactic group showed reduced body weight gain
as compared to that of the mycotoxicosis induced groups. Morphological observations-
Hair loss indicating mycotoxicosis was not observed in the prophylactic groups. Anatomical
observations- Spleenomegaly, hepatomegaly and gross lesions on liver and kidney were
noted on autopsy in the mycotoxicosis induced groups. On the other hand, the prophylactic
groups showed normal weights of liver and spleen. The body:organ weight indices of
all the organs remained at par to control group in the prophylactic groups except
for the lungs and brain where the weights of the organs were significantly high. Haematological
analysis- Neutrophilia was observed in IP (mycotoxicosis induced) while neutropenia
was observed in the oral (mycotoxicosis induced) group while the differential leukocyte
count of the prophylactic groups were found to lie in normal range. Biochemical analyses-
Acute intoxication of mice with TeA showed elevated malondialdehyde (MDA), reduced
catalase (CAT) and superoxide dismutase (SOD) production; abnormal levels of aspartate
transaminase (AST) and alanine transaminase (ALT). Treatment with cinnamaldehyde reversed
TeA-induced alterations of antioxidant defense enzyme activities. Histological observations-
Histopathological changes characterised by non alcoholic fatty liver, nuclear pyknosis
and infiltration of immune cells were observed in the mycotoxicosis induced groups
while cinnamaldehyde significantly prevented the TeA-induced organ damage. Cell apoptosis
factor- Reduced activity of caspase-3 enzyme was noted in the mycotoxicosis induced
groups while significantly high activity of the enzyme was observed in the prophylactic
groups.
Conclusion: Thus, from the present study we concluded that, cinnamaldehyde showed
therapeutic effects and toxicity reduction in TeA induced mycotoxicosis.
Logan
C.
1
Fife
A.
2
Goodman
A.
3
Ward
C.
2
Bicanic
T.
1
1Infection & Immunity, St Georges university, London, London, United Kingdom
2Department of Microbiology, Kings College Hospital, London, United Kingdom
3Department of Infection, Guy’s & St Thomas’ Hospital, London, United Kingdom
P101. Candi-SoL: A multi-site retrospective audit and analysis of Candidaemia across
three South London Teaching Hospitals
Objectives: Candidaemia is associated with high mortality, particularly in critical
care and immunocompromised patients. Emergence of multi-resistant candida species,
notably C. glabrata and C. auris, pose further challenges in treatment. This retrospective
surveillance and audit study reviews both adult and paediatric candidaemia cases between
January 2012- December 2018 across three Tertiary South London Trusts; St George’s
Hospital in London, Kings College hospital London and Guys & St Thomas’ Hospital.
Collectively these trusts have over 3500 beds, including over 220 ICU beds (adult
surgical, neurological, cardiac and general ICUs and paediatric and neonatal ICU)
and onsite specialist surgical, cardiothoracic and medical services, including liver,
bone marrow and renal transplant. The aims of this study were to assess trends in
candidaemia epidemiology across the South London region, categorise at risk groups,
antifungal therapy used and review clinical outcomes.
Methods: Blood cultures for adults and children isolating Candida species between
January 2012-December 2018 were retrospectively identified from the Microbiology database.
Species and antifungal susceptibility were reviewed. Persistent candidaemia (identical
Candida sp. isolated ≤ 30days after the initial blood culture) was analysed as a single
episode. Demographics (age, sex); location; risk factors; focus of candidaemia; antifungal
treatment received and duration; ECHO performed and outcome; ophthalmology performed
and outcome; line removal and culture result; length of stay; 30-day and in-hospital
mortality was collected from electronic patient records. Analyses were conducted in
Microsoft Excel 2013 and GraphPad Prism V7.
Results: Between January 2012 – December 2018, 509 candidaemia episodes were identified
across the three Trusts. In total 42% were C. albicans, 30% C. glabrata, 12% C. parapsilosis
and 1% of cases were due to C. auris following an outbreak in 2016. There was no significant
trend towards an increasing proportion of non-albicans species over the 7-year period
(Chi-squared test for trend, p = 0.22). Regarding age at the time of candidaemia,
4% were neonates (< 1 month), 9% were children (aged 1 month - <18 years), and 86%
were adults (> 18 years). Patients on an intensive care unit (adult, paediatric or
neonatal) accounted for 43% of cases. Clinical data regarding the candidaemia foci,
the ECHO and ophthalmology findings, antifungal therapy and duration, B-D-glucan result
if available, length of stay and mortality will also be presented. Susceptibility
profile of isolates will be interrogated and presented to assess if there has been
a change over time across the three trusts.
Conclusion: This retrospective audit of candidaemia across three large South London
Trusts illustrates the changing regional candida epidemiology, with non-albicans species
accounting for over half of all isolates and highlights the emergence of multi-drug
resistant species such as C. auris in the London. The remaining analyses of this large
dataset will highlight which are the dominant causes of candidaemia across the South
London Trusts and examine clinical outcomes with candidaemia over a diverse group
of patients.
Petraitis
V.
Petraitiene
R.
Naing
E.
Maung
B.B.W.
Garcia
A.
Kavaliauskas
P.
Walsh
T.J.
Infectious Diseases/medicine, Weill Cornell Medicine of Cornell University, New York,
United States of America
P102. Antifungal Efficacy of Isavuconazole and Liposomal Amphotericin B for Treatment
of Experimental Exserohilum rostratum Meningitis and Therapeutic Monitoring with CSF
(1→3)-β-D-glucan: A Preclinical Paradigm for Treatment of CNS Phaeohyphomycosis
Objectives: Phaeohyphomycosis of the central nervous system (CNS) is a life-threatening
infection with serious morbidity and mortality. Treatment options for CNS phaeohyphomycosis
are limited. This severity of CNS phaeohyphomycosis was tragically illustrated in
the contamination of a corticosteroid injection preparation that resulted in epidemic
Exserohilum rostratum meningitis (ERM), resulting in meningoradiculitis, brain abscess,
neurologic deficits, and death despite antifungal therapy with voriconazole with or
without liposomal amphotericin B (LAMB). We hypothesized that isavuconazole may be
an alternative approach for patients with ERM and other causes of CNS phaeohyphomycosis,
particularly those with severe infection or intolerance to initially high dosages
of voriconazole therapy. We therefore investigated the in vitro antifungal activity
of isavuconazole with or without LAMB followed by treatment in a new model of experimental
ERM.
Methods: Clinical isolates of E. rostratum recovered from patients suffering from
ERM were used in all experiments. Broth microdilution methodology was used to prepare
an inoculum for each of five clinical isolates of E. rostratum. Combination checkerboard
plates were used to test the activity of isavuconazole and LAMB, either alone or in
combination. Minimum inhibitory concentrations (MICs), minimal lethal concentrations
(MLCs) and fractional inhibitory concentration (FIC) indices were determined. For
in vivo therapeutic studies, E. rostratum were inoculated intracisternally with 1.0x106
microconidia to anesthetized rabbits. Immunosuppression was produced with cytarabine-induced
profound persistent neutropenia and methylprednisolone. Study groups consisted of
isavuconazole, 60 mg/kg/d, LAMB at 5.0, 7.5, and 10 mg/kg/day and untreated controls
(UC).
Results: As there were no in vitro additive, synergistic, or antagonistic interactions
for combinations of isavuconazole plus LAMB against the E. rostratum isolates, in
vivo studies in the rabbit model of ERM were conducted with isavuconazole and LAMB
as monotherapies. Isavuconazole 60 mg/kg/d and LAMB at 5.0, 7.5, and 10 mg/kg/day
demonstrated significant reductions of the residual fungal burden of E. rostratum
in cerebrum, cerebellum, spinal cord, and paravertebral muscle (p<0.01) in comparison
to those of UC. Reduction of the residual fungal burden correlated with significant
reduction of CSF (1→3)-β-D-glucan levels in comparison to those of UC (P<0.05).
Conclusion: Isavuconazole alone or LAMB alone significantly reduced residual fungal
burden in parallel with reduction of CSF (1→3)-β-D-glucan levels in treatment of experimental
E. rostratum meningitis and may serve as alternative regimens for treatment of CNS
phaeohyphomycosis.
Vasilyeva
N.
1
Bosak
I.
2
Bogomolova
T.
1
Vybornova
I.
2
Stepanova
A.
2
Avdeenko
Y.
2
Chilina
G.
2
Aak
O.
2
Solovyeva
G.
1
1Kaschkin Research Institute of Medical Mycology. Department of Medical Microbiology,
North-Western State Medical University named after I.I. Mechnikov, Saint-Petersburg,
Russian Federation
2Kaschkin Research Institute of Medical Mycology, North-Western State Medical University
named after I.I. Mechnikov, Saint-Petersburg, Russian Federation
P103. Pulmonary mucormycosis caused by Lichtheimia ornata (experimental model)
Objectives:
Lichtheimia ornata (A.K. Sarbhoy) Alastr. – Izq. & Walter due to the prevalence and
virulence is one of three most common species causing mucormycosis. This species was
revealed among the agents of invasive mucormycosis in Russia. The purpose of the present
research was to develop a murine experimental model of pulmonary mucormycosis caused
by L. ornata.
Methods: For the infection of mice we used the strain RCPF-1507 of L. ornata, isolated
from the tissue of accessory sinuses of nose from a patient with lymphatic leukemia.
For modeling the pulmonary mucormycosis we used outbred mice, males, with body weight
18-20 g. For inducing the condition of neutropenia we intraperitoneally injected cyclophosphamide
at the dose 150 mg/kg four times (-3, 0, 4 and 8 days). From fungal culture grown
on Sabouraud glucose agar for 3 days at 37° C we made the spore suspension in sterile
0.85% NaCl solution at the concentration 1·107 CFU/ml. Infection of animals was carried
out by intranasal injection of 50 μl of fungal spores suspension to a narcotized mouse.
Results: Data on mice lethality and results of cultural and histological investiga-tions
of murine lungs with invasive mucormycosis were obtained. Death of animals was observed
from 8 to 18 days after infection. No mice survived. Culturing of lung tissue from
all dead mice revealed growth of L. ornata. During the histological investigation
of murine lungs taken after eight days from the beginning of infection the different
area of localization of hyphal cells were found. Hyphae were wide (10-12 µm), with
rare branching at right angle. Hyphal cells in the lung parenchyma generally were
located densely and in parallel relatively each other that is characteristic of tissue
forms of mucormycetes. In lungs vessels massive aggregation of densely and chaotically
oriented fungal hyphae were revealed. Also we observed the hyphal aggregation in gleams
of bronchial tubes and an interstitium. The inflammatory process due to a mycotic
infection was poorly expressed, provided by poor neutrophilic and lymphocytic infiltration,
alteration processes prevailed.
Conclusion: The scheme of immunosuppression used in this work (intra-peritoneal introduction
of cyclophosphamide) showed efficiency for producing infection in mice by L. ornata.
Data on survival of the infected animals and also results of cultural and histological
investigations confirm existence of mycotic damage of lungs at the infected animals.
The developed model of invasive pulmonary mucormycosis can be used for future scientific
research, testing of new antifungal drugs and diagnostic methods.
Shadrivova
O.
1
Kuznetsov
V.
2
Desyatik
E.
2
Borzova
Y.
2
Ignatyeva
S.
2
Bogomolova
T.
2
Vasilyeva
N.
2
Klimko
N.
1
1Department of Clinical Mycology, Allergy and Immunology, North-Western State Medical
University named after I.I.Mechnikov, St. Petersburg, Russian Federation
2Kashkin Research Institute of Medical Mycology, North-Western State Medical University
named after I.I.Mechnikov, St. Petersburg, Russian Federation
P107. Case of successful treatment of chronic pulmonary aspergillosis in a patient
with polycystic disease
Case Report: Background. Publications onchronic pulmonary aspergillosis (CPA) in patients
with polycystic diseaseare limited.
Objection: We present a case of successful treatment of CPA in a patient with polycystic
disease.
Methods: Diagnosis of CPA was made in accordance with the criteria ESCMID/ECMM/ERS,
2016.
Result: Patient V., 70 years old, was admitted to the mycological clinic with complaints
of productive cough and dyspnea in September 2018. It is known that in 2015 lesions
in left lung lower lobe were detected on chest CT scan. The CPA diagnosis was made
on the basis of chest CT scan (multiple aspergillomas in left lung S6), elevated serum
Aspergillus IgG level, and A. fumigatus and A. niger in BAL culture. The patient was
treated with itraconazole 400 mg/day for 2 weeks. In October 2018, the patient underwent
surgery removal of the left lung lower lobe, and CPA diagnosis was confirmed with
histology. Therapy with itraconazole 400 mg/day was continued for 1 month. At the
end of treatment, there was no data for active CPA: no specific lesions on chest CT
scan, with negative BAL microscopy and culture, and serum Aspergillus IgG level. On
the Pubmed website, we did not find a case reports of CPA in patients with polycystic
disease.
Conclusions: Polycystic disease can be underlying disease of chronic pulmonary aspergillosis.
Combinations of antimycotic therapy and surgical treatment are necessary for the successful
treatment of chronic pulmonary aspergillosis in patients with polycystic disease.
Shadrivova
O.
1
Tarasova
M.
1
Desyatik
E.
2
Nikolaeva
N.
3
Borzova
Y.
2
Ignatyeva
S.
2
Bogomolova
T.
2
Vasilyeva
N.
2
Klimko
N.
1
1Department of Clinical Mycology, Allergy and Immunology, North-Western State Medical
University named after I.I.Mechnikov, St. Petersburg, Russian Federation
2Kashkin Research Institute of Medical Mycology, North-Western State Medical University
named after I.I.Mechnikov, St. Petersburg, Russian Federation
3North-Western State Medical University named after I.I.Mechnikov, St. Petersburg,
Russian Federation
P108. Chronic pulmonary aspergillosis in patients with idiopathic pulmonary fibrosis
Case Report: Introduction: Publications onchronic pulmonary aspergillosis (CPA) in
patients with idiopathic pulmonary fibrosis are limited.
Objective: We present a case of successful treatment of CPA in a patient with idiopathic
pulmonary fibrosis.
Materials and methods: Diagnosis CPA performed in accordance with the criteria EORTC/MSG,
2008.
Results: Patient V., 78 years old, was admitted to the mycological clinic with complaints
of productive cough, exertional dyspnea in February 2018. It is known that the patient
was diagnosed with idiopathic pulmonary fibrosis in 2017. He did not receive basic
therapy. On chest CT scan for the first time was detected a cavity of 43x68x29 mm
with the soft-tissue formation 12x14 mm in the apex region of the right lung. A positive
titer the IgG to Aspergillus fumigatus 1: 3200 was determined. Aspergillus fumigatus
grow in the sputum culture was revealed. The patient received itraconazole with 400
mg per day with a positive clinical effect. Decrease of cough and exertional dyspnea
was noted. Duration of antifungal treatment was 3 months. In July 2018, the chest
CT scan showed a decrease in the size of the cavity and reducing of the soft-tissue
formation in the right lung. The IgG titer to Aspergillus fumigatus was decreased
to 1: 800. No fungi were found in sputum culture. The patient received antimycotic
therapy for another 3 months. In October 2018, the CT scan of the chest showed stabilization
of changes. IgG titer to Aspergillus fumigatus decreased to 1: 400. The culture study
of sputum was negative. Antimycotic therapy was stopped. When analyzing the literature
on the Pubmed site, we found 5 cases of aspergillosis with idiopathic pulmonary fibrosis.
1 case where the disease is associated with the intake of glucocorticosteroids, 1
case associated with primary immunodeficiency, 1 case with the influenza B virus and
2 cases in non-immunocompromised patients. Conclusion: Idiopathic pulmonary fibrosis
can be an additional “background” disease of chronic pulmonary aspergillosis. Prolonged
antimycotic therapy is necessary for successfully treatment of chronic pulmonary aspergillosis
in patients with idiopathic pulmonary fibrosis.
Liu
L.
Wang
Y.
Gu
Y.
Li
S.
Chen
B.
Shen
K.
Su
X.
Jinling Hospital, Medical School of Nanjing University, Nanjing, China, nanjing, China
P109. The performance of the BALF later-flow device test in the rapid diagnosis of
nonneutropenic invasive pulmonary aspergillosis
Objectives: The Aspergillus lateral-flow device (LFD) is a new method to test for
Aspergillus antigen. We performed this study to evaluate the value of the LFD test
in diagnosing nonneutropenic invasive pulmonary aspergillosis.
Methods: A total of 136 bronchoalveolar lavage fluid (BALF) samples from 136 suspected
invasive pulmonary aspergillosis (IPA) patients were finally divided into 2 groups:
the aspergillosis group and the non-aspergillosis group. We performed both the LFD
and glactomannan (GM) tests on 80 BALF samples and only the LFD test on the remaining
58 BALF samples.
Results: After systematic clinical diagnosis, all 136 patients were divided into an
aspergillosis group (n = 41, proven (n = 8) and probable (n = 33) diagnosis) and a
non-aspergillosis group (n = 95, pneumonia, tuberculosis and others). The sensitivity,
specificity, positive predictive value, and negative predictive value of LFD for diagnosing
pulmonary aspergillosis in all 136 BALF samples were 69.7%, 72.6%, 87.3% and 46.9%,respectively.
When comparing the GM and LFD test results for the 80 samples, the sensitivity (75.8%
vs 74.1%, P = 0.769) was similar. The specificity of the GM test was higher than that
of the LFD test (83.0% vs 66.0%, P = 0.058), but there was no significant difference
between the tests. The sensitivity and specificity of the combination of the positive
results of both theLFD and GM tests were 63.6%and 89.4%, respectively, and the sensitivity
and specificity of either LFD or GM positive results or both LFD and GM positive results
were 90.9% and 59.6%, respectively.
Conclusion: LFD is a promising alternative diagnostic method to the GM test fortheearly
detection of patients with nonneutropenic pulmonary aspergillosis.
Liu
W.-L.
1
2
Huang
Y.-S.
3
Wang
F.-D.
4
Hsieh
M.-H.
5
Hii
I.-M.
6
Lee
Y.-L.
6
Ho
M.-W.
7
1School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei City,
Taiwan
2Emergency & Critical Care Medicine, Fu Jen Catholic University Hospital, New Taipei
City, Taiwan
3Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
4Medicine, Taipei Veterans General Hospital, Taipei, Taiwan
5Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University,
Kaohsiung, Taiwan
6Internal Medicine, Changhua Christian Hospital, Changhua, Taiwan
7Internal Medicine, China Medical University Hospital, Taichung, Taiwan
P110. High rates of misidentification of uncommon Candida species fungemia with conventional
phenotypic methods
Objectives: Candidemia caused by uncommon Candida species is increasing. Correct identification
of Candida isolates to species level is important for optimizing antifungal choice.
In this study, we aimed to evaluate the accuracy of species-level identification of
conventional methods and MALDI-TOF MS system in patients with candidemia due to the
uncommon Candida species. Sequencing of the internal transcribed spacer (ITS) was
performed for comparison and determination of accuracy.
Methods: From July 2011 to December 2014, uncommon Candida species identified by conventional
phenotypic methods from candidemic patients were prospectively collected at 6 hospitals
in Taiwan. Identification of theses uncommon Candida isolates were performed using
MALDI-TOF MS and DNA sequencing of the fungal internal transcribed spacer (ITS). Susceptibility
of the non-duplicate isolates were determined using SensititreTM YeastOneTM and were
interpreted with cut-off values recommended by the CLSI.
Results: A total of 85 uncommon Candida isolates reported by conventional methods
were included. By conventional methods, the most frequently reported uncommon Candida
species were C. guilliermondii (n = 39), C. sake (n = 7), and C. famata (n = 4). Using
ITS sequencing analysis as standard, none of the C. sake and C. famata identified
by conventional methods was correct, while MALDI-TOF MS correctly identified 10 of
the 11 isolates. With the exclusion of the one unspecified Candida isolate reported
by ITS sequencing, the accuracy of conventional methods and MALDI-TOF MS in the identification
of 84 Candida isolates were 64.3% and 86.9%, respectively (p = 0.001). Eight isolates
were confirmed to be non-Candida yeast species. Compared with other Candida species,
C. guilliermondii showed elevated MIC values of echinocandins. Based on our results,
an identification algorithm for uncommon Candida species was proposed (Figure 1).
Conclusion: Conventional methods could lead to high rate of misidentification of uncommon
Candida species, especially C. sake and C. famata. MALDI-TOF MS assisted with DNA-sequencing
based methods should be considered for identification of uncommon Candida species.
Waqas
S.
1
2
Dunne
K.
2
Talento
A. Fe
3
4
5
Wilson
G.
1
Martin-Loeches
I.
1
Keane
J.
1
2
Rogers
T. R
1
2
1St. James’s Hospital, Dublin, Ireland
2Trinity College Dublin, Dublin, Ireland
3Our Lady of Lourdes Hospital, Drogheda, Ireland
4Royal College of Surgeons Ireland, Dublin, Ireland
5Beaumont Hospital, Dublin, Ireland
P112. Cross-sectional study of respiratory Aspergillus spp. colonization or infection
in patients with various stages of Chronic Obstructive Pulmonary Disease (COPD) using
culture vs non-culture based technique.
Objectives: (*1st and 2nd Authors are equal contributors) COPD patients are now recognized
to be at increased risk of colonization by Aspergillus spp. which may progress to
invasive pulmonary aspergillosis (IA). Published data on the frequency of Aspergillus
detection in COPD are limited. The objective of this study was to determine frequency
of Aspergillus colonization or infection in COPD patients.
Methods: A cross-sectional study was undertaken to determine Aspergillus colonization
or infection in COPD patients undergoing bronchoscopy for any indication.Culture as
well as galactomannan antigen (GM) and Aspergillus nucleic acid detection (PCR) were
performed on bronchoalveolar lavage fluid (BAL).
Results: One hundred and fifty patients were included (44.7 % female, mean age 68.2
years). 21.3% were inpatients, 74.7% outpatients and 4% were ICU patients. Investigation
of lung masses was the most common indication (43.3%) for bronchoscopy. Most patients
(81.3 %) were either GOLD stage 1 or 2 COPD. Cancer was the most frequent co-morbidity
(60.48%). 12 % and 48.7 % were on systemic and inhaled steroids respectively. Lung
mass was the most common (28.43%) CT imaging finding. Seventeen patients (11.3%) had
a positive result for Aspergillus (Culture ± Galactomannan ± PCR). 76.4% out of these
seventeen were in the early stages (GOLD stage 1 or 2) of COPD.
Conclusion:
Aspergillus sp. was detected in 3.3% of patients by culture, which increased to 11.3%
if culture was combined with either a positive GM or PCR result. Overall the frequency
of Aspergillus detection in this population of COPD patients was low which may reflect
the predominance of Gold stages 1 and 2 among the study population.
Jobstmann
M.
1
2
Prattes
J.
2
Stelzl
E.
1
Zurl
C.
1
Hoenigl
M.
3
Rabensteiner
J.
1
Krause
R.
2
Kessler
H.
1
1Medical University of Graz, Graz, Austria
2Section of Infectious Diseases and Tropical Medicine, Medical University of Graz,
Graz, Austria
3Division of Infectious Diseases, University of California, San Diego, United States
of America
P113. Evaluation of new multiplex PCR-based blood assays for detection of candidemia
Objectives: Blood cultures have been considered as diagnostic gold standard for candidemia.
Compared to cultures, assays based on polymerase chain reaction (PCR) may be useful
to shorten the time to diagnosis of invasive candidiasis and to initiation of antifungal
therapy. Recently, the new CandID® and CandID PLUS® kits (OLM Diagnostics, Newcastle
Upon Tyne, England) for molecular detection of different Candida spp. have been developed.
In this study, the analytical and clinical performance of the new kits was investigated.
Reference material and clinical specimens were used.
Methods: Nucleic acid extraction was performed on the EMAG® platform (bioMérieux,
Marcy-l´Etoile, France) using the specific B protocol. Real-time PCR (qPCR) and detection
with the CandID® and CandID PLUS® kits were performed on the Light Cycler® 480 II
CE/IVD instrument (Roche Diagnostics, Penzberg, Germany). Both of the kits are based
on multiplex qPCR providing detection of three different Candida spp. each and an
internal control: Candida albicans, Candida glabrata, Candida parapsilosis with the
CandID® and Candida tropicalis, Candida krusei, Candida dubliniensis with the CandID
PLUS®. The accuracy of the new kits was determined utilizing the Quality Control for
Molecular Diagnostics (QCMD) 2018 Candida spp. EQA Programme. The panel consisted
of 10 members including Candida albicans, Candida auris, Candida glabrata, Candida
krusei, and vials without Candida spp. The clinical performance of the new kits was
determined with specimens obtained from three groups of patients: patients with culture-proven
candidemia (n = 23; EDTA whole blood samples), patients with bacteremia (n = 31; plasma
samples), and healthy controls (n = 25; EDTA whole blood samples). Whole blood samples
from candidemic and bacteremic patients were drawn at the same day as the corresponding
blood cultures.
Results: With the quality control program, all panel members were correctly identified
by the new PCR assays. With the clinical study, 2 EDTA whole blood samples obtained
from patients with candidemia and 7 EDTA whole blood samples obtained from healthy
controls were found to be inhibited and thus excluded from further analysis. In patients
with candidemia (mean age, 67 years, range 20 to 89 years), 14 of 21 samples (67%)
gave a positive result when employing the new PCR assays. In patients with bacteremia
(mean age, 62 years, range 23 to 91 years), 1 of 31 samples gave a false-positive
result with the CandID assay. All healthy controls (mean age, 57 years, range 45 to
66 years) remained negative with the new PCR assays. All Candida spp. from candidemic
patients were correctly identified with the PCR assays.
Conclusion: When compared to blood culture, two thirds of samples obtained from patients
with candidemia were found positive with the new CandID® or CandID PLUS® kits. While
in samples obtained from patients with bacteremia one false-positive result was observed
with the new PCR assays, results of samples obtained from healthy controls were all
found to be correct.
Godichaud
S.
1
Verrier
J.
1
Boisson
R.
1
Lagardere
G.
1
Flori
P.
2
1ADEMTECH, PESSAC, France
2CHU ST ETIENNE, ST ETIENNE, France
P114. Development of a New Quantitative Real-Time PCR Assay for the Detection and
Identification of Aspergillosis and Mucormycosis Infections
Objectives: Mucormycosis is associated with high mortality rates, especially in haematological
patients, and remains difficult to diagnose. The early distinction with invasive aspergillosis
is of importance because the antifungal treatment for each is different. Unfortunately,
the underlying conditions of both infections are similar. Moreover, detection of circulating
antigens such as galactomannan and β-D-glucan provides no help for diagnosing mucormycosis,
and cultures are often delayed or negative, preventing early management. Delayed directed
antifungal treatment impacts the outcome of mucormycosis. The goal of this study is
to evaluate a new quantitative PCR assay allowing the simultaneous detection of Aspergillus
spp. and Mucorales spp.
Methods: The MycoGENIE Aspergillus-Mucorales spp. real-time PCR kit (Ademtech, France)
is a triplex PCR assay targeting specifically Aspergillus spp., Mucorales spp. (including
Lichtheimia, Mucor, Rhizomucor, Rhizopus and Cunninghamella species), and an exogenous
Internal Control. This study was performed on a reference panel of 30 biopsy specimen,
including 10 positive for Aspergillus spp. and 12 positive for Mucorales spp. previously
identified by direct examination and culture. Twenty negative serum samples were also
included in this study to evaluate the specificity of the assay. DNA extraction was
performed using the MycoGENIE DNA Extraction kit (Ademtech, France) and 10 µL of pure
DNA was used as a template for the PCR assay. Mucorales-infected samples were also
evaluated with other external laboratory-developed PCR tests targeting only Mucor,
Rhizomucor, Rhizopus and Lichtheimia species.
Results: On the 30 samples, the MycoGENIE Aspergillus-Mucorales spp. PCR kit successfully
detected 19 Aspergillus-positive samples including A. fumigatus, A. niger and A. nidulans
identified by culture. Mucorales spp. were also detected in 18 samples. Notably, the
PCR assay efficiently detected Aspergillosis-Mucormycosis co-infections in 9 samples.
All serum samples returned negative.
Conclusion: This new CE-IVD compliant real-time PCR assay, combined with an automated
DNA extraction process is a sensitive and specific molecular diagnostic tool for the
diagnosis of aspergillosis and mucomycosis infections in biopsy samples. The possibility
to efficiently detect aspergillosis/mucormycosis co-infections is highlighted in this
study and is of importance for a better therapeutic management.
Badiane
A.
Sene
A.
Gaye
A.
Diongue
K.
Seck
M.C.
Ndiaye
M.
Gangneux
J.P.
Ndiaye
D.
Parasitology and Mycology, Universite Cheikh Anta Diop de Dakar, Dakar, Senegal
P115. Comparison of multiplex nested PCR and Maldi tof to identify clinically isolated
candida from Sénégal, a preliminary study
Objectives: The objective of this study is to compare the identification of clinically
isolated candida species using Maldi tof and a multiplex nested PCR.
Methods: A total of 38 clinically isolated strains of Candida from superficial mycosis
and vaginal infection were conserved in brain heart broth additioned with 15 % glycerol
and stored at – 20 °C. The strains were then sub cultured in CHROMagar™ candida (Becton
Dickinson), incubate at 37 °C for 24 to 48 hours and identified using Maldi tof (Bruker)
at University of Rennes / France. A multiplex nested PCR were performed directly from
the conservative media at Cheikh anta Diop University of Dakar/ Senegal.
Results: Thirty five (35) samples gave interpretable results for both methods. 51.43
% of the samples showed the same result with Maldi tof and multiplex PCR, among which
Candida albicans (45.71 %) and C. parapsilosis (2.85%). The species identified by
both methods were C. albicans, C. tropicalis and C. parapsilosis. C. albicans alone
or mix with other species was identified 71.73 % by Maldi tof and 61.70% by PCR nested
multiplex. C. tropicalis alone or mix with other species was identified 13.04 % with
Maldi tof and 8.51% with multiplex nested PCR. C. parapsilosis alone or mix with other
species was identified 2.17 % with Maldi tof and 23.40% with multiplex nested PCR.
C. glabrata, C. pintolepesii, C. orthopsilosis and C. pararugosa were identified only
by Maldi tof. C. lusitania and C. kefyr were only identified by Mutiplex nested PCR.
Seven (7) mix species were identified with multiplex nested PCR and five (5) with
Maldi tof. Among the samples, identified as C. albicans using multiplex PCR three
were identified as C. tropicalis by Maldi Tof. C. parapsilosis genotype 2 identified
by multiplex PCR has been identified as C. orthopsilosis with Maldi tof. Threes strains
identified as C. albicans by multiplex PCR were identified as C. tropicalis by Maldi
tof.
Conclusion: The multiplex nested PCR and the Maldi tof identified the most prevalent
Candida species, but there is discordance for the identification of the non albicans
species. The multiplex PCR identified more multiple strains infections than the Maldi
tof. The differences observed between these two methods could be due to the absence
of these strains in the Maldi tof database and the subculture of predominant strains.
Also genetically close species might not be differentiated using PCR.
Vavrova
A.
1
Svobodova
L.
1
Mrazek
J.
2
Hamal
P.
1
1Department of Microbiology, Palacký University, Faculty of Medicine and Dentistry
and University Hospital Olomouc, Olomouc, Czech Republic
2Department of Bacteriology and Mycology, Institute of Public Health in Ostrava, Ostrava,
Czech Republic
P117. Utilization of MALDI-TOF MS for identification of filamentous fungi: a comparative
study
Objectives: Identification of filamentous fungi based on morphological characteristics
is the most simple and available approach used in diagnostic clinical mycology laboratories.
In many cases, however, it requires considerable experience of the rater and the ability
of strains to produce spores. For its rapidity and accuracy, MALDI-TOF mass spectrometry
is invaluable for identification of microorganisms including fungi. It is more challenging
to use this method for identification of filamentous fungi than bacteria or yeasts
given differences in cell wall structures and a limited spectrum of species in the
database. The study aimed to find the optimal way of identifying filamentous fungi
in mycology laboratories using MALDI-TOF MS.
Methods: The tested group of moulds comprised 193 isolates obtained from clinical
specimens. The identification started with morphological assessment (appearance of
colonies, micromorphology from slide culture). Then isolates were identified using
MALDI-TOF, both directly from culture and following culture in liquid media with extraction;
in the latter case, both supernatant and sediment were tested. Finally, all the methods
were compared as to the rate of agreement. Subsequently, a small sample of selected
isolates was identified by sequencing.
Results: Based on morphological criteria, 17 genera were identified; due to a lack
of sporulation, another 5 isolates could not be identified. Further, 13 species were
identified, mostly within the genus Aspergillus. With MALDI-TOF MS performed directly
from culture, nine isolates were identified to the genus level and 184 were identified
to the species level, with a total of 75 species being noted. With MALDI-TOF MS after
culture in liquid media with extraction, 190 isolates were identified to the species
level, with 43 species being noted; in only three cases, identification of filamentous
fungi was limited to genera. Comparison of identification from supernatant and sediment
showed that identical results were always obtained at both the genus and species level
and there were only minimal differences in identification scores. The rates of agreement
between isolate identification using morphology and MALDI-TOF MS from culture were
58.55% at the genus level and 22.24% at the species level. The rates of agreement
between isolate identification using morphology and MALDI-TOF MS after culture in
liquid media with extraction were 84.97% at the genus level and 46.11% at the species
level. Based on disagreeing results of the above identification methods, 20 isolates
from the sample were selected to be identified by sequencing. The highest agreement
rate (70%) was found for identification with MALDI-TOF MS after culture in liquid
media with extraction and only 35% using MALDI-TOF MS directly from culture.
Conclusion: The results suggest that the optimal approach to identification of filamentous
fungi in diagnostic medical mycology laboratories is a combination of morphological
characteristics and MALDI-TOF MS. For species identification of morphologically typical
isolates, assessing the appearance of colonies and slide cultures is sufficient; in
the other cases, MALDI-TOF MS after culture in liquid media with extraction is recommended.
If identification with the above combination is ambiguous or impossible to perform,
molecular genetic methods need to be used, in particular sequencing.
Poissy
J.
1
Damonti
L.
2
Bignon
A.
3
Khanna
N.
4
Kietzell
M. Von
5
Boggian
K.
5
Neofytos
D.
6
Vuotto
F.
7
Coiteux
V.
8
Artru
F.
9
Zimmerli
S.
2
Pagani
J.-L.
10
Calandra
T.
11
Sendid
B.
12
Poulain
D.
12
Delden
C. Van
6
Lamoth
F.
13
Marchetti
O.
11
Bochud
P.-Y.
11
1Intensive Care Department, University Hoospital of Lille, Lille Cedex, France
2Department of Infectious Diseases, Inselspital, Bern University Hospital, University
of Bern, University Hospital of Bern, Bern, Switzerland
3Surgical Intensive Care Department, University hospital of Lille, Lille Cedex, France
4Division of Infectious Diseases and Hospital Epidemiology, University Hospital of
Basel, Basel, Switzerland
5Department of Infectious Diseases, Cantonal Hospital of Saint Gallen, Saint Gallen,
Switzerland
6Infectious Diseases, University Hospital of Geneva, Geneva, Switzerland
7Infectious Diseases Department, University Hospital of Lille, Lille Cedex, France
8Hematological Disorders Department, University Hospital of Lille, Lille Cedex, France
9Digestive Intensive Care Department, University Hospital of Lille, Lille Cedex, France
10Intensive Care Department, University hospital of Lausanne, Lausanne, Switzerland
11Infectious Diseases Department, University Hospital of Lausanne, Lausanne, Switzerland
12Laboratory of Mycology and Parasitology, University Hospital of Lille, Lille Cedex,
France
13Microbiology Institute, University Hospital of Lausanne, Lausanne, Switzerland
P118. Risk Factors for Candidemia: A Prospective Matched Case-Control Study
Objectives: Candidemia is an opportunistic infection associated with high morbidity
and mortality in hospitalized patients, both inside and outside intensive care units
(ICUs). Identification of patients at risk for preemptive approach and early detection
is crucial. Prospective control-matched studies and comparison between ICU and non-ICU
patients are lacking in this field. We aim to identify and compare specfic risk factors
in ICU and non ICU patients.
Methods: This was a prospective multicenter matched case-control study assessing risk
factors for candidemia and death in candidemic patients, both outside and inside ICUs
from 6 teaching hospitals in Switzerland and France. Controls were matched to cases
based on age, hospitalization ward, hospitalization duration and, when applicable,
type of surgery. Risk factors were analyzed by univariate and multivariate conditional
regression models, as a basis for a new scoring system for prediction of candidemia.
Results: The study included 183 cases and 378 matched controls. 44% were hospitalized
inside ICU and 56% outside. Independent risk factors for candidemia within the ICU
population included total parenteral nutrition (TPN) (OR = 9.24, 95%CI 3.39-25.2,
p<0.001), acute kidney injury (OR = 6.48, 95%CI 2.39-17.6, p<0.001), solid cancer
(OR = 4.59, 95%CI 1.23-17.2, p = 0.02), heart disease (OR = 4.26, 95%CI 1.37-13.3,
p = 0.01), invasive mechanical ventilation (OR = 3.37, 95%CI 1.17-9.67, p = 0.02),
exposure to fluoroquinolones (OR = 3.05, 95%CI 1.14-8.16, p = 0.03) and exposure to
aminoglycosides (OR = 2.47, 95%CI 1.07-5.69, p = 0.03). Independent risk factors for
candidemia within the non-ICU population included central venous catheter (CVC) (OR
= 10.20, 95%CI 3.87-27.2, p<0.001), TPN (OR = 3.37, 95%CI 1.53-7.41, p = 0.003), exposure
to glycopeptides (OR = 4.10, 95%CI 1.57-10.7, p = 0.044), and to nitroimidazoles (OR
= 5.14, 95%CI 1.59-16.6, p = 0.006). The weighted ICU-score was as follows: TPN, +2;
AKI, +2; heart disease, +1.5; solid cancer, +1.5; invasive mechanical ventilation,
+1.0; fluroquinolone: +1.0. aminoglycoside: +1. AUC of the ROC curve was 0.773. The
optimal cut-off was ≥5 (sensitivity = 76%, specificity = 64%). The bestl cut-off to
optimize specificity was ≥7 (sensitivity = 23%, specificity = 95%). The weighted non-ICU
score was as follows: CVC: +3.5; nitroimidazole: +2.5; TPN, +2; Glycopeptide: +2.0.
AUC of the ROC curve was 0.717. The optimal cut-off was ≥4 (sensitivity = 83%, specificity
= 49%). The best cut-off to optimize specificity was ≥6 (sensitivity = 49%, specificity
= 82%). Independent factors for death in candidemic patients in the whole population
were septic shock (OR = 7.17, 3.06-16.8, p<0.001), acute kidney injury (OR = 5.04,
2.16-11.8, p<0.001), number of antibiotics (OR = 1.42, 1.15-1.75, per unit, p<0.001).
After stratification between ICU and non-ICU patients, only septic shock (OR = 4.49,
1.53-13.1, p = 0.006), acute kidney injury (OR = 3.13, 1.02-9.17, p = 0.04), the number
of antibiotics to which patients were exposed before candidemia (OR = 1.35, 1.05-1.75
per unit, p = 0.02) remain significantly and independently associated with death in
ICU patients. For non-ICU patients, acute kidney injury (OR = 10.7, 2.16-52.5, p =
0.004) and septic shock (OR = 10.50, 2.80-39.7, p<0.001) were the only variables significantly
associated with death
Conclusion: Risk factors for candidemia are variable in the ICU versus non-ICU setting,
including different patterns of antibiotic exposure. Weighted scores predictive of
candidemia can be built based on these risks, with better performances for ICU-patients.
An improved prediction of the risk of candidemia may contribute to guide targeted
preventive and therapeutic antifungal strategies.
Persat
F.
1
2
Dupont
D.
3
Eynard
J.-C.
2
Poggi
B.
2
Wallon
M.
3
1Parasitology and Medical Mycology, Hospices Civils de Lyon / Université Lyon 1, Lyon,
France
2Association Pro.Bio.Qual, Lyon, France
3Parasitology and Medical Mycology / Umr 5292, Université Lyon 1 / Hospices Civils
de Lyon, Lyon, France
P119. Inter-laboratory robustness of screening tests for the detection of Aspergillus
antibodies for chronic aspergillosis: finding from a French External Quality Assessment
Objectives: Six million people worldwide are estimated to have chronic aspergillosis
or/and allergic broncho pulmonary aspergillosis. Serum Aspergillus assay is an important
parameter to detect and follow the immune host response against this fungus. Reliable
tests are crucial for patients’ care but also to perform much needed multicentre-studies.
However, the current tests are not standardized and may lack in robustness. We have
analysed the results of a French External Quality Assessment (EQA) programme to assess
the inter-laboratory robustness of four Aspergillus antibody techniques among those
recommended in France as first-line screening tests.
Methods: The study was based on the answers provided by 40 laboratories that took
part at least once to the EQA organised by ProBioQual between 2013 to 2018 for Aspergillus
serology. A total of 24 sera were tested over this time period, 18 positive from patients
with chronic or allergic bronchopulmonary aspergillosis and six negative sera from
blood donors. The results of the following techniques were analysed: 1) PlateliaTM
Aspergillus IgG EIA (Bio-Rad, France); 2) ELISA ClassicTM
A. fumigatus IgG (Virion/Serion, Germany); 3) ELISA Aspergillus fumigatus IgG (Bordier
Affinity Products, Switzerland) since 2014; 4) Aspergillose Fumouze indirect hemagglutination
(Biosynex Fumouze, France).
Results: reported by the participants for the Bio-Rad test were all negative for five
of the six negative sera and all positive for 14 of 18 positive sera. Those reported
for the Virion/Serion ELISA were all negative for four of the six negative sera and
all positive for 10 of the 18 positive sera. The results reported for the Bordier
test were all concordant but only based on five positive sera and one negative serum.
Results provided with the hemagglutination test were all negative on all negative
sera, but none of 18 positive sera were found by all laboratories to be positive,
and 10 of the positive sera were even found by all laboratories to be negative.
Conclusion: Hemagglutination technique appears lacking too much in sensitivity to
be used as single screening test, compared to the ELISA techniques, probably explaining
its decreasing use. ELISA Bio-Rad appeared as more robust than Virion/Serion ELISA.
The potentially better performances of ELISA Bordier have to be confirmed.
Chagas
O.J.
1
Buccheri
R.
2
Naves
G.
1
Pinto
P.L. Silva
3
Chioccola
V.L. Pereira
4
Benard
G.
1
Negro
G.M.B. Del
1
1Laboratório De Investigações Médicas 53, Insituto de Medicina Tropical, São Paulo,
Brazil
2Hospital Ward, Instituto de Infectologia Emílio Ribas, São Paulo, Brazil
3Intitution: Intituto Adolfo Lutz - Núcleo de Enteroparasitas, São Paulo, Brazil
4Intituto Adolfo Lutz - Laboratório de Biologia Molecular de Parasitas e Fungos do
Centro de Parasitologia e Micologia, São Paulo, Brazil
P120. rUsefulness of conventional PCR through induced sputum to diagnoses Pneumocystis
pneumonia in HIV infected patients
Objectives: The present study aims to determine the usefulness of conventional PCR
in induced sputum to diagnoses Pneumocystis pneumonia in HIV infected patients.
Methods: Study design was of a prospective-cohort with systematic collection of data.
Ninety-five HIV-infected patients presenting cough and dyspnoea for more than 7 days
were included. They should not have received antiretroviral therapy (ART) or a history
of inadequate treatment. Induced sputum, obtained through concentrated saline solution
(NaCl 3%-5%) inhalation, was obtained at admission and submitted to O-toluidine staining
and conventional PCR for Pneumocystis. Clinical, laboratorial, imaging records and
clinical outcome from PCR positive and PCR negative patients were compared. We also
compared these data in PCR positive patients and confirmed pulmonary tuberculosis
(TB) (presence of bacillus-acid-alcohol-resistant or positive rapid-molecular-test).
Results: PCR was positive in 34/95 (35.7%) of the patients. This group presented higher
rates of dry cough (55.8% X 22.9%, P = 0.003), interstitial infiltrate on chest X-ray
(94.1% X 61%, P = 0.001), ground grass on chest tomography (CT) (89.2% X 42.8%, P
= 0.0002) and higher lactate dehydrogenase (LDH) levels (488U/L x 329U/L, P<0.0001),
when comparing with the PCR negative patients. PCR positive patients had also more
non-invasive ventilation (NIV) (47% X 15%, P = 0.002) and more admissions at the intensive
care unit (ICU) (35.2% X 13.5%, P = 0.02). Both groups did not differ regarding n.
of days with symptoms, PaO2, HIV viral load (VL) and Candida infection during hospitalization.
There were 32 PCR positive patients without TB and 11 patients with confirmed pulmonary
TB. TB was associated with CT budding tree (57,1% X 0, P = 0.0005), CT micronodules
(85,7% X 23%, P = 0.008); trend toward higher CD4 cell count (129 X 47 cell/mm³, P
= 0.01) and lower use of NIV (6,2% X 50%,P = 0.0417), and less CT ground grass (14,2%
X 92,3%, P = 0.0001). There was no difference between LDH, PaO2, HIV VR, and ICU hospitalization
between the two groups. of note, 5/34 (14.7%) of the PCR positive patients did not
receive any antimicrobial treatment for PCP during hospitalization but improved their
initial symptoms, hence the difficult to differentiate between colonization and infection
solely based on the conventional PCR. In addition, only two PCR positive patients
did not display ground grass at CT: both had pulmonary consolidation.
Conclusion: Our PCR assay was useful to define a subgroup of patients with clinical,
laboratorial and imaging aspects related to PCP. Besides it, pulmonary CT is a fundamental
tool, since suggestive alterations in this exam strongly contribute to the diagnosis,
helping to differentiate the pulmonary involvement of PCP from that of TB, the main
differential in our country, where TB is highly prevalen. of note, five PCR positive
patients did not receive specific treatment during hospitalization, pointing to either
the existence of false-positive cases or to P. jiroveci colonization. The possibility
that differentiation between these patients could be achieved by quantitative real-time-PCR
remains an open question, since the literature is controversial, due mainly to the
different primers and techniques employed. Another discriminatory assay would be the
β-D-Glucan test, which, combined with PCR assay, would be able to discriminate colonized
from infected patients.
Gaajetaan
G.
Kampermann
T.
Tegelen
D. Van
Dingemans
G.
PathoNostics, Maastricht, Netherlands
P121. Performance characteristics of the MucorGenius® real-time PCR assay for the
detection of clinically relevant Mucorales species
Objectives: Determine the performance characteristics of the commercially available
MucorGenius® real-time PCR assay for the detection of clinically relevant Mucorales
species. For this purpose, several verification studies were performed including sensitivity,
specificity and EQA evaluation.
Methods: The analytical sensitivity or limit of detection (LOD) was determined by
testing serial dilutions of all reference Mucorales culture extracts including Rhizopus
oryzae, Rhizopus microsporus, Cunninghamella bertholletiae, Lichtheimia corymbifera,
Rhizomucor pusillus, Mucor hiemalis and Rhizomucor miehei on various real-time PCR
instruments. The LOD dilution of a Mucorales species was accepted if at least 19 out
of 20 reactions were detected. This dilution was finally quantified for each species
by using droplet digital PCR (ddPCR). Analytical specificity was determined by testing
multiple high concentrations of bacterial and fungal DNA strains that can be present
in the respiratory tract. The seven serum samples of the FPCRI Muc Panel (2018-1)
were tested with the MucorGenius® PCR after DNA-extraction with the NucliSENS® easyMag
system (bioMérieux).
Results: The LOD was successfully determined for all seven Mucorales targets on 5
different real-time cyclers. All results of the LOD study are listed in Table 1. Table
1. Overview of MucorGenius® LOD results.
No cross-reactivity was observed with any of the clinically relevant fungal or bacterial
strains. Mucorales DNA was successfully detected in 6 out of 7 serum DNA- extracts
from the FPCRI panel. All samples showed an internal control signal indicating reliable
DNA-extraction performance. These results were in agreement with the data of the FPCRI.
Conclusion: The MucorGenius® real-time PCR assay can detect all Mucorales species
at low DNA-concentrations of 1-2 copies/µl which corresponds with high Ct-values (Ct
31-43). This indicates reliable analytical sensitivity. No cross-reactivity was observed
for any of the tested fungal or bacterial strains, reducing the chance of a false
positive result. Although 100% agreement was obtained with the FPCRI Muc Panel, this
is a limited data set and only provides an indication of the performance. Clinical
validation studies are now ongoing on different sample types to determine the clinical
performance and suitability for routine diagnostics.
Gits-Muselli
M.
1
2
Villiers
S.
3
Hamane
S.
2
Berçot
B.
4
Donay
J.-L.
4
Denis
B.
5
Guigue
N.
2
Alanio
A.
6
Bretagne
S.
6
1Molecular Mycology, Institut Pasteur, PARIS, France
2Parasitology-mycology Laboratory, Lariboisière Saint-Louis Fernand Widal Hospital,
Assistance Publique-Hôpitaux de Paris (AP-HP), PARIS, France
3Anesthesiology Department, Lariboisière Saint-Louis Fernand Widal Hospital, Assistance
Publique-Hôpitaux de Paris (AP-HP), PARIS, France
4Microbiology Department, Lariboisière Saint-Louis Fernand Widal Hospital, Assistance
Publique-Hôpitaux de Paris (AP-HP), PARIS, France
5Tropical and Infectious Diseases Department, Lariboisière Saint-Louis Fernand Widal
Hospital, Assistance Publique-Hôpitaux de Paris (AP-HP), PARIS, France
6Paris-diderot, Sorbonne Paris Cité University, Institut Pasteur, Molecular Mycology
Unit, Cnrs Cmr2000, Parasitology-Mycology Laboratory, Lariboisière Saint-Louis Fernand
Widal Hospitals, Assistance Publique-Hôpitaux de Paris, Paris, France
P122. Time to and differential time to blood culture positivity for assessing catheter-related
candidemia: A longitudinal, 7-year study in a single university hospital
Objectives: Central venous catheters (CVCs) significantly increase the risk of candidemia,
and inversely, candidemia is often associated with intravascular catheters. Most guidelines
for candidiasis management recommend removal of existing intravascular catheters,
if feasible. Although the recommendation is strong, various authors have recommended
a re-consideration of CVC removal on an individual basis. Practically, one must consider
the possible deleterious consequence of CVC removal, especially in patients who require
long-term CVC for treatment or nutrition. This raises the question of systematic early
removal of CVC in the management of candidemia since some authors did not report differences,
knowing that patients who cannot tolerate CVC removal have often a worse prognosis.
Time to positivity (TTP) and differential time to positivity (DTTP) between central
and peripheral blood cultures are commonly used for bacteremia to evaluate the likelihood
of CVC related bloodstream infection. Few studies have addressed the application of
these approaches to candidemia. This study aimed to evaluate TTP and DTTP to assess
CVC-related candidemia (CVC-RC).
Methods: We retrospectively analyzed the results from 105 adult patients with incident
candidemia, with CVC removed and cultured, collected from 2010 to 2017. The bottles
were incubated in a BioMérieux bact/ALERT 3D and kept for at least 5 days. Patients
with CVC removed and cultured at the time of fungemia were divided into two groups
as previously proposed: (i) CVC-related candidemia (CVC-RC) when the same yeast species
was recovered from at least one BC and from the CVC culture; (ii) non-CVC-related
candidemia (NCVC-RC) when a yeast species was recovered from at least one BC, and
the CVC culture was negative or yielded a different species.
Results: of the 105 patients included, most were oncology patients (85.7%) and had
of long-term CVC (79.6%); 32 (30.5%) had a positive CVC tip with the same species
as in BC, defined as CVC-RC. The main species involved were C. albicans (46%), C.
parapsilosis (19%), C. glabrata (15%), and C. tropicalis (9%), with no statistical
difference between NCVC-RC and CVC-RC. Considering all species, the median TTP of
the first bottle collected from CVC was statistically shorter in the CVC-RC group
(16.8 h [9.7-28.6]) compared to the NCVC-RC group: median (29.4 h [20.7–41.3], p =
0.001, Figure 1). When analyzing TTP regarding the Candida species, a significant
shorter TPP was only observed in C. albicans with a median of 12.9 h [10.0–22.7] vs.
32.3 h [24.4–41]) for CVC-RC and NCVC-RC, respectively (p = 0.001, Figure 1). A TTP
< 10 h had the best positive likelihood ratio (21.5), although the sensitivity was
only 28%. DTTP was available for 52 patients. A DTTP > 5 h had a sensitivity of 100%
and a specificity of 71%.
Conclusion: Even if some cut-offs supported the diagnosis of CVC-RC, they did not
allow the prompt decision on CVC removal, since the median TTP was 17 h. More rapid
methods for detecting infected catheters should be tested to avoid withdrawal of non-infected
CVC (69.5% of CVCs in our study).
Kozlova
Y.
1
Frolova
E.
2
Uchevatkina
A.
3
Filippova
L.
1
Solovjeva
G.
1
Sobolev
A.
1
Vasilyeva
N.
1
Klimko
N.
1
1Department of Clinical Mycology, Allergology and Immunology, North-Western State
Medical University named after I.I. Mechnikov, Saint-Petersburg, Russian Federation
2Department of Clinical Mycology, Allergology and Immunology, North-Western State
Medical University n.a. I.I. Mechnikov, Saint-Petersburg, Russian Federation
3North-Western State Medical University n.a. I.I. Mechnikov, Saint-Petersburg, Russian
Federation
P123. Diagnostic value of immunological markers of allergic bronchopulmonary aspergilosis
in patients with asthma
Objectives: Allergic bronchopulmonary aspergillosis (ABPA) is a severe lung disease
caused by hypersensitivity to Aspergillus spp. The study of the role of different
immunological mediators in the formation of chronic allergic inflammation in patients
with ABPA is necessary to identify potential targets for therapeutic intervention
and timely diagnosis of the disease. To study of the role of immunological mediators
in the development of allergic bronchopulmonary aspergillosis in patients with asthma.
Methods: A prospective study conducted 58 patients with severe asthma (median of age
- 45 years, men - 13, women - 45). The control group consisted of 16 apparently healthy
people without allergic disease in history (median age - 24 years old, male - 4 women
- 12). All patients underwent allergological (skin tests with fungal allergens, determination
of serum levels of total IgE (“Polygnostus”, Russia) and specific IgE (sIgE) for fungal
allergens (“AlcorBio”, Russia). Computed tomography of the chest was performed according
to the indications. Diagnostic criteria of ABPA 2013 [R. Agarwal et al] were used.
The concentration of TARC (“R&D Systems”, USA), TSLP (“R&D Systems”, USA), IL-8 (“Vector-Best”,
Russia) in the serum were determined by ELISA. All patients were performed respiratory
function and used the AST questionnaire (Asthma Control Test). The obtained data was
processed using the STATISTICA 10 software system.
Results: ABPA patients demonstrated significantly lower scores on the questionnaire
AST and worse lung function FVC and FEV1 compared to patients with asthma. The analysis
of the content of TSLP in serum did not establish statistically significant differences
between patients with ABPA 13.0 (9.70 ÷ 24.70) and asthma 19.0 (15.0 ÷ 29.0), and
data from the control group 10.5 (9.0 ÷ 25.0) pg/ml. It was revealed that the level
of IL-8 in patients with ABPA 35.0 (23.0 ÷ 49.0) was significantly higher than in
patients with asthma 22.0 (14.4 ÷ 28.0; p = 0.002) and in healthy individuals (12.0
(4.5 ÷ 15.5); p = 0.000) pg/mg. The content of TARC was higher in patients with ABPA
733.5 (599.0 ÷ 909.0) compared with asthma patients 336.1 (208.0 ÷ 571.3; p = 0.000)
and control 224.5 (184.0 ÷ 265.0; p = 0.000) pg/mg. The importance of TARC in the
development of allergic inflammation in patients with fungal sensitization was confirmed
by a positive correlation of the content of the proinflammatory chemokine with the
levels of total IgE (r = 0.35, p <0.05), sIgE to A.fumigatus (r = 0.39, p <0.05),
the number of eosinophils (r = 0.34, p <0.05) and a negative correlation with the
indicators of the respiratory function of FEV1 (r = -0.44; p <0.05). The high content
of TARC and IL-8 suggests a mixed eosinophilic-neutrophilic type of inflammatory response
in patients with ABPA.
Conclusion: An increase in serum TARC can serve as a diagnostic biomarker for the
development of ABPA in patients with asthma.
Mareković
I.
Pleško
S.
Vranješ
V. Rezo
Jandrlić
M.
Department of Clinical and Molecular Microbiology, University Hospital Centre Zagreb,
Zagreb, Croatia
P124. Comparison of serum beta-D-glucan levels obtained by Fungitell Assay and Wako
beta-Glucan Test
Objectives: Fungitell Assay (FA) is the most widely used test for determining serum
beta-D-glucan (BDG) levels in Europe. However, the disadvantage of this test is the
need for samples’ cummulation to collect appropriate number of samples assuring optimal
test usability. It poses a problem in laboratories with limited number of samples
because of prolonged turnaorund-time and questionable impact on the diagnostic process
and antifungal treatment of invasive fungal infections (IFI). Wako β-Glucan Test (WBG),
recently launched in Europe, offers the possibility of individual sample testing.
The objective of this study was to compare serum beta-D-glucan levels obtained by
these two assays in patients with suspected IFI.
Methods: The study was performed at the University Hospital Centre Zagreb, Department
of Clinical and Molecular Microbiology. The Fungitell Assay (Associates of Cape Cod
Inc.) and Wako β-Glucan Test (FUJIFILM Wako Chemicals Europe Gmbh) were performed
on patients’ sera and the results were interpreted according to manufacturers’ instructions.
Two to three serum samples were tested in each patient on two or three consecutive
days. Discrepant results between two tests were explained and commented in the context
of patients’ clinical data.
Results: Forty-four serum samples in 24 patients were tested. FA was positive in 13
and WBG in 9 patients. The results obtained with two different tests regarding positivity
and negativity were correspondent in 83,3% (20/24) of patients. In 16,7% (4/24) of
patients, the results were discordant, with positive FA and negative WBG result. Results
for four patients with discordant FA and WBG results are shown in Table 1. When cut-off
value for WBG was lowered from manufacturer’s cut-off value of 11 pg/mL to 3,8 pg/mL,
as suggested in a recent publication, all the results, except one result in Patient
1, were concordant. Moreover, in Patient 1 diagnosis of IFI was excluded after diagnostic
work-up as well as in Patient 2. In Patient 3, with a recent heart transplant, chest
HR-CT was done eight days after last serum sample tested with both tests, demonstrated
pulmonary infiltrates suspected by radiologist to be of fungal etiology and subsequently
determined levels of BDG with FA only showed further increase. Aspergillus fumigatus
grew in a culture of sputum and patient was treated with voriconazole. Patient 4 had
a cystic fibrosis with A. fumigatus isolated in sputum and treated with voriconazole.
In Patients 1, 2, and 4 the highest level of BDG determined with WBG was 5,1 pg/mL,
while in a Patient 3 the highest level was 9.62.
Conclusion: In our study FA was more frequently positive in comparison to WBG test.
Among patients with positive FA and negative WBG was a also a patient with IFI. This
is in concordance with so far conducted studies showing superior sensitivity of FA.
Therefore, cut-off value for WBG should be reconsidered. As the results of any of
these tests should be evaluated in the context of clinical picture and microbiological
and radiological findings, WBG test remains a useful alternative for laboratories
with low number of samples.
Tomás
A.L.
1
2
Cardoso
F.
1
Almeida
M.P. De
3
Pereira
E.
3
Franco
R.
2
Matos
O.
1
1Medical Parasitology Unit, Group of Opportunistic Protozoa/hiv and Other Protozoa,
Global Health and Tropical Medicine, Instituto de Higiene e Medicina Tropical, Universidade
Nova de Lisboa, Lisboa, Portugal
2Departamento De Química, UCIBIO/REQUIMTE, Universidade NOVA de Lisboa, Caparica,
Portugal
3Departamento De Química E Bioquímica, REQUIMTE/LAQV, Faculdade de Ciências da Universidade
do Porto, Porto, Portugal
P125. An immunonanodiagnostic approach for Pneumocystis pneumonia at point-of-care
Objectives:
Pneumocystis jirovecii is an ubiquitous opportunistic fungus capable of causing fatal
pneumonia (PcP) in immunocompromised patients worldwide. The diagnosis relies on microscopic
visualization of the P. jirovecii organisms or on DNA detection, in respiratory specimens
obtained by invasive and costly techniques. Thus, the search for a specific serological
biomarker and for a faster, cost-effective and less-invasive diagnostic approach is
an old ambition of the scientific community. This work aims to develop a point-of-care
strip-based platform for anti-P. jirovecii antibodies detection in human serum specimens,
applying the innovative association of recombinant synthetic antigens (RSA) technology
with functionalized gold nanoparticles (AuNPs).
Methods:
P. jirovecii’s surface proteins such as the major surface glycoprotein (Msg) and kexin-like
serine protease (Kex1) present highly reactive antigenic properties. Thus, newly recombinant
synthetic (multi-epitope) antigens from these proteins were designed based on immunogenicity
of their sequences. After synthesis and purification by immobilized metal affinity
chromatography, these RSA were applied in ELISA tests as biomarkers of the disease
to identify circulating anti-P. jirovecii antibodies in serum specimens of patients
previously classified in distinct clinical groups. After confirming their applicability
as biomarkers of infection, these RSA were conjugated with spherical AuNPs previously
functionalized with mercaptoundecanoic acid. The interaction of such RSA-AuNP bionanoconjugates
with specific anti-P. jirovecii antibodies in patient’s sera, was characterized by
agarose gel electrophoresis (AGE). The same RSA-AuNP bionanoconjugates were then utilized
in two strip-based lateral flow immunoassays (LFIA) prototypes, projected to detect
the presence of human IgM reactive to each of the P. jirovecii’s RSA. These prototypes
were optimized and tested with positive and negative clinical samples.
Results: A synthetic antigen with three reactive Msg epitopes and another with three
Kex1 reactive epitopes were produced. ELISA results with both RSA showed that IgM
anti-P. jirovecii levels were significantly increased in patients with PcP compared
with patients colonized by P. jirovecii (p = 0.002 with Kex1 RSA; p<0.001 with Msg
RSA) or patients without P. jirovecii infection (p≤0.001 with both RSA). AGE assays
with the RSA-AuNPs bionanoconjugates confirmed that these bionanoconjugates reacted
with anti-P. jirovecii antibodies present in patient’s serum. The LFIA prototypes
developed were tested with sera pools from patients with active PcP (positive sample)
and from patients without P. jirovecii infection (negative sample). Both samples showed
the expected performance, namely, test and control colored lines with the positive
sample and only a control colored line with the negative sample.
Conclusion: Our results support the possibility to diagnose PcP and distinguish active
disease from colonization using RSA as biomarkers of the disease and IgM anti-P. jirovecii
antibodies as targets for immunonanodiagnosis. Further improvement and validation
of the LFIA prototypes developed will help in the global effort to reduce high costs
of medical diagnosis and consequently treatment of PcP. This applies not only to more
economically advanced regions, but can also be a huge contribution, in terms of public
health and economy, in communities with low-income and scarce access to technology.
Piarroux
R.
1
Dubus
J.-C.
2
3
Reynaud-Gaubert
M.
3
4
Gouitaa
M.
5
Ranque
S.
6
7
Vitte
J.
8
1Research & Development, LDBIO Diagnostics, Lyon, France
2Pneumo-pédiatrie, Crcm Enfants, Aix-Marseille Université AP-HM Hôpital Timone Enfants,
Marseille, France
3Ihu Méditerranée Infections Mephi, Aix-Marseille Université IRD AP-HM, Marseille,
France
4Pneumologie Crcm Adultes, Aix-Marseille Université AP-HM Hôpital Nord, Marseille,
France
5Clinique Des Bronches Et Allergies Du Sommeil, Aix-Marseille Université AP-HM, Marseille,
France
6IHU-Méditerranée Infection, Marseille, France
7Vitrome, Aix Marseille Univ, IRD, AP-HM, SSA, Marseille, France
8Ihu Méditerranée Infections Mephi, Aix-Marseille Université IRD AP-HM SSA, Marseille,
France
P126. Interest of Aspergillus fumigatus Western Blot assay for differential diagnostic
between IgE sensitization and Allergic Broncho Pulmonary Aspergillosis.
Objectives: Diagnosis of Allergic Broncho Pulmonary Aspergillosis (ABPA) is complex
and based on a multi-criteria definition. One of the major ABPA criteria, the detection
and quantification of immunoglobin E (IgE) responses to Aspergillus fumigatus (Af),
is also associated with Af-sensitization. In the past few years, a novel approach
assaying IgE responses to molecular antigens enhanced the discrimination between Af
sensitization and ABPA, but without allowing for a clear-cut distinction between these
two diseases. We recently reported that a Western Blot detecting Af-specific IgE antibodies
could be helpful in separating those two entities. This work aimed to strengthen the
evaluation of the Af IgE WB assay as a differentiating tool between Af-sensitization
and ABPA.
Methods: 221 sera with known sIgE reactivity (21 ABPA, 200 Af-sensitized) were assayed
with the LDBIO Asp II WB. Sera were collected 2014-2018 and previously assayed with
ImmunoCap® to Af extract and molecular antigens. We evaluated the ability of WB LDBIO
to detect Af sensitization and to discriminate between ABPA and Af-sensitized patients,
relying on ImmunoCap® and clinical chart conclusions as a reference.
Results: Samples displayed 0 to 10 bands in the 10-37 kDa range. Among those bands,
four (16, 18-20, 22 and 30 kDa) were most frequent and therefore considered as major
bands, while the others were considered as minor bands. Af IgE WB positivity was defined
by the presence of at least 2 major bands. The Af IgE WB was positive in 21/21 ABPA
and 115/200 Af-sensitization sera (61% sensitivity). Af IgE WB positivity was strongly
correlated to IgE level and was positive in 95% (75/79) of the sera with IgE to Af
>2kUa/l. Band patterns formed various profiles for discrimination between ABPA and
Af-sensitization. of those profiles, one (5 detectable bands, regardless of minor/major
classification) had 100% sensitivity (21/21) and 91% specificity (201/221) for ABPA
diagnostic. However, by using a more specific profile with 2 major bands and 2 minor
bands outside of the 16-30 kDa range, the WB yielded 90% (19/21) sensitivity and 96%
(211/221) specificity.
Conclusion: This study shows the potential of WB in the work-up of IgE responses to
Af in asthmatics and cystic fibrosis patients: Af IgE WB was able to discriminate
between ABPA and Af-sensitized patients, with a sensitivity of 90% and a specificity
of 96%. In comparison, the ISHAM criteria (tIgE>1000 kUI/l and Af IgE>0.35 kUa/l)
had 48% sensitivity (10/21) and 98.2% specificity (217/221) in the same population
Noteworthy, among the Af IgE WB negative patients, 4 were sensitized to another mould
(Alternaria alternata), thus Af IgE WB could avoid unnecessary diagnostic difficulties
due to such cross-reactivity. A multicenter evaluation is now needed to confirm those
results, but Af IgE WB shows a great potential for second-line diagnostic tool when
ABPA is suspected.
Özenci
V.
Ininbergs
K.
Seeth
C. Gronfors
Jansson
K.
Ullberg
M.
Clinical Microbiology, Karolinska University Hospital, karolinska Institutet, Stockholm,
Sweden
P127. Rapid Identification of Candida spp. from positive blood cultures
Objectives: Candidemia is related to high mortality, morbidity. Rapid and reliable
identification of Candida spp. and antifungal susceptibility testing is essential
for early appropriate antifungal treatment. The aim of the present study was to evaluate
two rapid methods for identification of Candida spp. from blood cultures (BC) using
matrix assisted laser desorption ionization (MALDI-TOF MS). The methods tested were
(i) the Sepsityper kit and (ii) short-term culture followed by MALDI-TOF MS. The effect
of using Bact/ALERT FA Plus and BACTEC Mycosis-IC/F blood culture bottles were compared
for the performance of both of the rapid methods. The performance of short-term culture
on faster antifungal susceptibility testing was also analyzed. Conventional identification
and antifungal susceptibility testing methods were used as reference.
Methods: In total, 52 clinical Candida spp. were inoculated in two types of BC bottles
and incubated in respective BC systems. Each bottle was inoculated with 8 mL human
blood from a healthy donor which had been spiked with ~ 125 colony forming units (CFU)
per mL. When the BC bottles signaled positive, the samples were analyzed directly
from BC bottles by the Sepsityper kit as well as after 6h short-term incubation on
sabouraud agar and chrome plates followed by MALDI-TOF MS if growth in 6h was observed.
Results: Overall, the Sepsityper kit and short-term culture method performed similarly
and could identify Candida spp. in 66/101 (65.3%) and 63/85 (74.1%), respectively
(NS). Mycosis-IC/F had higher identification rates than FA Plus BC bottles using the
Sepsityper kit 39/51 (77%) and 27/50 (54%), respectively (p<0.05). Similarly Mycosis-IC/F
performed better than FA Plus BC bottles using the short-term culture method 38/43
(88%) and 25/42 (60%), respectively (p<0.01). Six hours short term culture followed
by Sensititre YeastOne method was tested on ten positive BC bottles. On average the
MIC levels for antifungal agents were within one titre difference compared to conventional
Sensititre method which was performed after overnight culture plates for both BC bottles
(NS).
Conclusion: The present data shows that Sepsityper kit and short-term culture method
can be used in rapid identification of Candida spp. from blood cultures. Mycosis-IC/F
has higher performance in identification of yeast with both methods compared to FA
Plus BC bottles. Preliminary data suggest that six hours short term culture followed
by Sensititre Yeast One method can be used as a reliable rapid antifungal susceptibility
testing method for positive blood cultures.
Özenci
V.
1
Lopez
N.I.M.
1
Seeth
C. Gronfors
1
Ullberg
M.
1
Dinnetz
P.
2
1Clinical Microbiology, Karolinska University Hospital, karolinska Institutet, Stockholm,
Sweden
2School of Natural Sciences, Technology and Environmental Studies, Södertörn University,
Stockholm, Sweden
P128. Comparison of Bactec Mycosis IC/F and BacT/Alert FA plus bottles in laboratory
diagnosis of candidemia
Objectives: The aim of the present study was to compare the clinical performance of
Bactec Mycosis IC/F and BacT/Alert FA blood culture bottles in detection and time
to detection of Candida spp. over eight years period in a tertiary care hospital setting.
Methods: During a period of eight years, all blood samples that were concurrently
taken from patients with suspected candidemia and cultured paralleled in Bactec Mycosis
IC/F, BacT/Alert FA, and BacT/Alert FN blood culture bottles were included in the
study. Data collection was retrieved from the laboratory information system and from
the respective blood culture system. Bottles that did not signal positive in 10 days
in the blood culture system were regarded as negative. Time to growth was counted
from the time that the incubation occurred until the time the system gave a positive
signal. Results analyzed with use of R, conducting a mixed linear model and Chi-square
test.
Results: In total, 2,748 positive blood cultures with Candida spp. i.e Bactec Mycosis
IC/F, BacT/Alert FA Plus, and BacT/Alert FN Plus bottles from 810 patients were analyzed.
Triplicates of blood culture bottles that were taken in 24 h were included. There
were 274 blood cultures from 261 patients with all three blood culture bottle types.
The BacT/Alert FN bottles had too few positives and excluded from further analysis.
The Bactec Mycosis IC/F Bottles could detect Candida spp. in 216 (78.83%) of 274 samples,
whereas BacT/Alert FA aerobic bottles were able to detect in 233 (85%) of 274 samples.
The Mycosis IC/F and BacT/ Alert FA aerobic Bottles did not differ in detection of
Candida spp. (NS). There was no species-specificity difference between the two bottles
in growth of different Candida spp (NS). There was no difference in to time to detection
between the two bottles (NS).
Conclusion: The present study shows that the performance of Bactec Mycosis IC/F and
BacT/ Alert FA blood culture bottles in detection of Candida spp. are similar. Further
analysis of the discrepant results and the patient characteristics will be performed.
Vannini
M.
Chandemerle
E.
Landreau
A.
Simon
L.
Pomares
C.
Hasseine
L.
Laboratoire De Parasitologie Mycologie, CHU DE NICE, NICE, France
P129. Direct identification of 81% of candidemia from blood culture bottles by MALDI
–TOF MS
Objectives: Candidemia are associated with high mortality rate (40%). Rapid yeast
species identification is crucial to introduce as soon as possible appropriate treatment
which is associated with an increase of patients survival rate. Because of the emergence
of echinocandin resistance, strategies that allow targeted use of antifungals are
essential, notably to reduce the duration of echinocandins exposition. With the conventional
method, 24 to 48 hours are required to obtain enough fungal colonies for species identification.
In this study, we developed a method to directly identify yeast from blood cultures
bottles within one hour. In the context of Laboratory Accreditation we performed method
validation according to the ISO 15189 norm.
Methods: This work was done in the laboratory of parasitology and mycology at the
University Hospital Center of Nice, France. We used 37 positive blood cultures obtained
from 25 patients from 4th January 2019 to 28 Mars 2019. 1.3 ml of positive blood cultures
was transferred into 1.5 ml microtube and each sample was extracted according to the
SDS lysis-based protocol (Jeddi et al 2017). Supernatants were spotted four times
on the target plates that were read by the MADLI-TOF mass spectrometer (Bruker®).
We validated species identification when a log (score) from Bruker either ≥ 1.6 or
≥ 1.1 three-times repeatable was found, since we did not observe any species misidentification
above these thresholds. In case of failure, spectra were analyzed online with the
MSI-Users® database (Mass Spectrometry Identification, Normand et al 2017) and identifications
were validated when the score was ≥ 17%. We compared our results with the conventional
culture and identification methods.
Results: 30 blood cultures (81%) were correctly identified with both databases. Only
68 % with Bruker database and 73 % with MSI User database. This method is reproducible
and reliable. We tested two mixed infections but they were not totally identified.
Conclusion: Our study shows that direct identification of candidemia from blood culture
with the SDS protocol allows rapid identification of the causative yeast in 81% of
cases. It allows early identification of yeast species and thus appropriate antifungal
treatment. This will improve patient’s healthcare in the context of candidemia. Mycological
culture remains necessary when our protocol doesn’t work and in case of mixed infections.
Chidebelu
P.
Nweze
E.
Microbiology, University of Nigeria, Nsukka., Enugu, Nigeria
P130. rThe Use of Coffee Agar As An Alternative Medium For The Presumptive Identification
of Cryptococcus neoformans In Resource – Limited Settings.
Objectives: Cryptococcus neoformans is presumptively identified by its characteristic
brown - black colonies on media containing caffeic acid (Katiyar et al., 2011). In
low income regions, neither the conventional identification media nor their basic
component(s) is readily affordable and available. We proposed an alternative coffee
agar media made of coffee which is rich in caffeic acid, for differential recovery
of C.neoformans colonies from mixed cultures of various sample sources.
Methods: The coffee agar media which is a modification of caffeic acid agar was composed
with coffee as follows: ammonium sulphate (5.0g), glucose (5.0g), yeast extract (2.0g),
dipotasium phosphate (0.8g), magnesium sulphate (0.7g), coffee (18.5g), chloramphenicol
(0.05g), ferric citrate solution (4.0ml), agar (15.0g). The final pH of the solution
in 1 litre of de-ionized water was adjusted to 6.5 ± 0.3. After sterilization at 121
ºC and 15psi pressure for 15 minutes, the solution was cooled at 45ºC, dispensed in
Petri dishes and stored at refrigeration temperature until use. Test isolates (n =
12) of Cryptococcus neoformans VNI (ST32) identified by MALDITOF MS and MLST, were
streaked on the Coffee agar plates and monitored for pigmentation. Reference strains
of Cryptococcus neoformans var. grubii VNI H99 and Candida albicans SC5314 were cultured
parallel as the control. A set of the plates was incubated at 30ºC, and the other
at 37 ºC for up to 1 week.
Results: The colonies of Cryptococcus neoformans (both the test and reference strains)
turned brown - black on the coffee agar plates, while Candida albicans SC5314 remained
as cream – white colonies. Melanization was more conspicuous at 30ºC after 5 days
of incubation.
Conclusion: The brown - black pigmentation observed for C. neoformans indicated that
coffee is rich in caffeic acid and thus, provided the substrate for the phenoloxidase
enzyme laccase, which is absent in C. albicans. Expression of melanin especially at
30ºC remains integral for Cryptococcus’ environmental survival. After the necessary
validation, we suggest that coffee agar can provide an alternative detection media
especially in resource – limited settings where absence and cost of conventional identification
media pose a barrier to research and diagnostic studies of Cryptococcus neoformans
and Candida albicans.
Guigue
N.
1
Gits-Muselli
M.
1
2
Hamane
S.
1
Alanio
A.
3
Bretagne
S.
3
1Parasitology-mycology Laboratory, Lariboisière Saint-Louis Fernand Widal Hospital,
Assistance Publique-Hôpitaux de Paris (AP-HP), PARIS, France
2Molecular Mycology, Institut Pasteur, PARIS, France
3Paris-diderot, Sorbonne Paris Cité University, Institut Pasteur, Molecular Mycology
Unit, Cnrs Cmr2000, Parasitology-Mycology Laboratory, Lariboisière Saint-Louis Fernand
Widal Hospitals, Assistance Publique-Hôpitaux de Paris, Paris, France
P131. Prospective comparison of colometric and turbidimetric assays for diagnosing
invasive fungal infection in a routine laboratory
Objectives: The detection of serum (1→3)-β-D-glucan (BDG), an antigen common to several
fungi, is part of the definition of invasive fungal infections (IFI). The turbidimetric
or the colorimetric detection is the basis of two different commercial kits, the Fungitell
assay (FA) and the Wako assay (WA), respectively. The FA has been used in Europe since
the 2000’s, whereas the WA has been only recently CE approved. We wondered how the
WA compares to the FA on a routine use.
Methods: We prospectively tested 321 serum samples (median: 1[1-11]) from 171 patients
(sex ratio M/F = 60%, mean age 48+/-16 yrs) mainly with hematological conditions (64%;
110/171) using both assays from December 21st 2018 to April 23rd 2019. The tests were
performed according to the manufacturers’ recommendations.
Results: Twenty-three patients had IFI (pneumocystosis n = 12; invasive aspergillosis
n = 4; candidemia n = 3; invasive fusariosis n = 2; Hepato-splenic candidiasis n =
1; and cryptococcosis n = 1). Both assays provided similar AUC around 0.9 (Figure
1).
Sensitivity, specificity, positive and negative predictive values were as follow:
FA (positivity threshold ≥80 pg/ml): 91%, 89%, 55%, 98%; WA (positivity threshold
at 11 pg/ml): 48%, 97%, 67%, 92%; WA (positivity threshold at 4 pg/ml): 83%, 94%,
68%, 97%. When removing the FA negative (<80 pg/ml) and FA overflow values (> 523
pg/ml), the analytical comparison showed a good correlation (R2 = 0.81), the FA values
being 28 higher in mean than the WA values (Figure 2). Among 73 samples from patients
with bacterial sepsis, 21% (15/73) were FA positive, 11% (8/73) were WA positive (positivity
threshold at 4 pg/ml), whose 7 positive with both methods. These possible false positive
results should be taken with caution knowing that extensive work-up for diagnosing
fungal infection was not homogenous between the patients due to the study design and
that bacterial and fungal diseases are often concomitant.
Conclusion: The WA performed similarly compared to the Fungitell assay when a lower
cutoff (4 instead of 11 pg/ml) is used for the diagnosis of IFI, with a better specificity.
The WA is a single sample testing which is clinically relevant when prompt therapeutic
decision is on stake.
Green
J.
Dempsey
K.
Bruker Microbiology, Glasgow, United Kingdom
P132. The development of a real-time PCR assay for the detection of Mucormycosis infections:
Fungiplex Mucorales
Objectives: Mucorales have been increasingly reported as causes of invasive fungal
infections in immunocompromised subjects, particularly in patients with haematological
malignancies, uncontrolled diabetes mellitus or those undergoing dialysis. Mucorales
are now also being reported in Aspergillus-positive patients who are not responding
to first line treatments. Histology and culture are still the most important diagnostic
approaches for mucormycosis because of the lack of molecular diagnostic methods available,
and β-d-Glucan detection is not useful due to the extremely low content of the biomarker
in the Mucorales order. Timely diagnosis of invasive mucormycosis is essential due
to the rapid progression of the disease, and because signs and symptoms of the infection
are consistent with other invasive fungal infections. PCR would improve detection
of Mucorales and complement the current Bruker offering within the area of invasive
fungal disease.
Methods: The Bruker real-time PCR assays are designed in an easy to use format with
minimum hands on time and results generated in around 1 hour after extraction. Universal
primer and probe sequences have been designed to target the internal transcribed spacer
(ITS) region of the rRNA gene for the genera detailed in Table 1. The probes associated
with each pathogen have been labelled with different fluorescent dyes enabling some
differentiation within the multiplex reaction. A specific control material has been
developed to give the users an option of running it as an extraction control or a
PCR inhibition control.
Results: The coverage specified in Table 1 has been confirmed using a range of simulated
samples prepared from plasmid and genomic DNA. The limit of detection for species
representing the highest prevalence of IFD for each genus has been assessed over six
thermocycler platforms to ensure accuracy across a variety of systems.
Conclusion: Bruker have developed a Mucorales assay which identifies a wide range
of clinically relevant genera. The assay is being further developed to produce a kit
to provide rapid detection of the main causative agents in invasive mucormycosis.
Green
J.
Donnelly
T.
Bruker Microbiology, Glasgow, United Kingdom
P133. The development of a real-time PCR assay for the diagnosis of Pneumocystis pneumonia:
Fungiplex Pneumocystis
Objectives:
Pneumocystis jirovecii is the causative organism in Pneumocystis pneumonia (PCP);
a life-threatening pneumonia in immunocompromised patients. Due to a lack of methods
to propagate P. jirovecii in vitro, the most common current practice in diagnosis
of PCP is the microscopic identification of P. jirovecii through staining of bronchoalveolar
lavage (BAL) samples. This method lacks sensitivity to detect low fungal burdens,
therefore PCR methods of detection would improve diagnosis of PCP and complement the
current Bruker offering within the area of invasive fungal disease. Two different
Pneumocystis assays have been designed for initial evaluation against various sample
types, with the intention of selecting the best performing assay for further development.
Methods: Two Pneumocystis real-time PCR assays were initially developed, with specific
primer and probe sequences designed to target different genes of Pneumocystis jirovecii.
Assay 1 targeted the internal transcribed spacer 2 (ITS) sequence between the 5.8S
and 28S rRNA genes, with Cy5 as the fluorophore. Assay 2 targeted the mitochondrial
large subunit (mtLSU) rRNA gene and used Yakima Yellow as fluorophore. During initial
feasibility studies a range of plasmid concentrations were amplified in the presence
of an amplification and extraction control using the standardised Fungiplex assay
real-time PCR conditions. The real-time PCR assays were also tested against QCMD’s
P. jirovecii pneumonia EQA panels.
Results: When assessed against a range of known plasmid concentrations, both assays
were found to perform equivalently, with a limit of detection of 20 ipc and a dynamic
range of 20 – 2x106 ipc as displayed in Figure 1. When tested against the QCMD 2017
PCP panel, the correct result was obtained in duplicate testing of all extracts using
Assay 2, whereas several replicates were undetected using Assay 1. The internal control
was detected in all samples, showing that there was no inhibition. Assay 2 was then
assessed against the QCMD 2018 PCP panel; again, the correct result was obtained in
duplicate testing of all extracts using this assay.
Conclusion: The assay designed to target the mtLSU gene, is therefore most suitable
and will be developed further. The better performance of this assay is likely due
to the presence of multiple copies of the mtLSU gene per organism, compared with just
a single copy of ITS2.
Zarkada
E.
1
Tragiannidis
A.
2
Papageorgiou
T.
2
Hatzipantelis
E.
2
Samaras
K.
1
Markantonatou
A.-M.
1
Zachrou
E.
1
Vyzantiadis
T.-A.
1
1First Department of Microbiology, Medical School, Aristotle University of Thessaloniki,
Thessaloniki, Greece
2Haematology-oncology Unit, Second Department of Paediatrics, Medical School, Aristotle
University of Thessaloniki, Thessaloniki, Greece
P134. Upgrading the level of diagnosis towards a more targeted treatment. A six year
period of laboratory monitoring in a Paediatric Haematoloy-Oncology Unit.
Objectives: Invasive fungal infections have been proved an important cause of negative
prognosis in critically ill patients and notably the immunocompromised, while the
paediatric haematolgy-oncology patients form a high risk group among them. Although
several laboratory methodologies for the diagnosis of fungal infections are not completely
validated in these patients, the criteria provided by the EORTC/MSG, alongside to
the recommendations by ESCMID and ECIL, can be followed in order to upgrade the level
of diagnosis. The main aim of this study was to show that the implementation of laboratory
testing in this group of patients, served as a useful tool towards a more targeted
antifungal approach by managing to upgrade the diagnosis from possible to probable
or maintain the clinical suspicion in just a state of readiness.
Methods: Data was gathered retrospectively, over a period of six years, from the records
of the forty-three patients with haematological malignancies that were hospitalised
in the Paediatric-Oncology Unit and had at least one mycology result during their
follow-up. All mycology studies were performed in the First Department of Microbiology
and included specific cultures, testing for galactomannan, mannan and antibodies against
Candida, as well as PCR for Aspergillus or Candida. Also, sensitivity testing or TMD
for antifungal drugs, when necessary.
Results: Among the above 43 haematology patients the 22 were initially considered
to have a possible diagnosis according to the EORTC/MSG criteria due to the presence
of at least one host factor and a clinical criterion (mainly positive CT imaging).
From the 22 patients, nine proved to have a probable infection by Aspergillus with
positive galactomannan and (in one case) / or (in one case) a positive PCR for Aspergillus.
One patient had a proven diagnosis, based on the isolation of Rhizopus arrhizus from
several cultures of face biopsies and one was considered with a “probable” diagnosis
due to the presence of positive high titers of mannan, positive Candida PCR in blood
and kidney ultrasound imaging of a possible mycotic mass. There were also three “probable”
patients with sequential positive mannan testing (with decreasing to negative titers
after treatment) that although not included in the criteria, they could not be considered
as just possible. Consequently, the diagnosis was considered upgraded, after the mycology
findings, in almost 64% (14/22) of patients with possible diagnosis. Among the 22
patients with possible diagnosis, the rest eight, as well as all other 21 patients
(from the total of 43), remained with a possible or completely negative diagnosis
(respectively) for fungal infection proving the utility of negative and often excluding
results.
Conclusion: The results of this study could argue in favor of a meticulous and well
organized protocol of laboratory monitoring in this group of patients. Taking in consideration
that the here-mentioned monitoring, due to several reasons, is not performed with
the proposed frequency and combination, the laboratory results are already a useful
adjunct towards the documentation or even the exclusion of a fungal infection, helping
the clinical decision and guiding the targeted treatment.
Vasilyeva
N.
1
Riabinin
I.
1
Mihaylova
Y.
2
Borzova
Y.
1
Alieva
L.
1
1Kaschkin Research Institute of Medical Mycology. Department of Medical Microbiology,
North-Western State Medical University named after I.I. Mechnikov, Saint-Petersburg,
Russian Federation
2Laboratory of Biosystematics and Cytology, Komarov Research Institute of Botany,
Saint-Petersburg, Russian Federation
P135. Revision of special microscopic techniques for visualization of aspergilli structural
elements
Objectives: Objective of the study is to select the optimal type of special light
optics for obtaining microphotographs of Aspergillus spp.
Methods: Aspergillus clinical strains belonging to 14 species from the Russian Collection
of Pathogenic Fungi (Saint-Petersburg, Russia) were used: A. fumigatus, A. flavus,
A. tamarii, A. terreus, A. niger, A. nidulans, A. ustus, A. calidoustus, A. sydowii,
A. versicolor, A ochraceus, A. candidus, A. amstelodami, Neosartorya hiratsukae. Original
strains were previously identified by the target DNA-sequencing of ITS and β-tubulin
loci according to the CLSI MM18 protocol (2nd edition). Subcultures were obtained
on Czapek yeast extract agar (28°C, 10 days). Micropreparations were studied by three
microscopic methods: (1) phase contrast (PCM), (2) «dark field» (DFM), (3) water-immersion
light microscopy with oblique illumination (WILMOI) using an «aplanatic» condenser
(Leningrad Optical-Mechanical Association, Russia). Presto T55 (Rekam, Canada) and
PowerShot A2000IS (Canon, USA) cameras were used for taking photo. Digital image processing
was performed in XnView 2.35 and GIMP 2.8.6 software.
Results: Series of microphoto for three described techniques were received. As it
turned out, the phase-contrast microscopy sharply emphasizes the contours of the aspergilli
thallic elements and their conidia, but the most successful microscopic pictures are
obtained only in areas of location of the structural elements in single layer. With
dark-field microscopy in some species (A. fumigatus, A. nidulans, Aspergillus spp.
of Versicolores and Usti sections) structural details of the conidial heads are not
clearly visible at high magnification. In dark-field microscopy, in comparison with
elements of mycelium, vegetative spores and ascospores are better visualized. Dark-field
and phase-contrast microscopy to varying degrees distort the natural color of the
microscopic elements of the Aspergillus spp. cultures. In contrast to the techniques
mentioned above, the use of a condenser with a shiftable iris diaphragm and a water-immersion
diaphragmatic lens made it possible to take microphoto while preserving the natural
pigmentation of aspergilli, sufficient contrast and creating a «relief» of a microscopic
picture. In addition, by changing the setting of the aplanatic condenser, it is easy
to transform the microscope in dark-field microscopy mode. Examples of taken images
are shown in the figure.
Figure. Aspergillus flavus micrographs made by using three special microscopic techniques
(abbreviations are placed in text).
Conclusion: Water-immersion microscopy with oblique illumination allows to obtain
the most natural microphoto of aspergillosis causative agents with the effect of «soft»
contrasting. Moreover, the WILMOI mode is much faster adjusted by a microscopist than
the DFM and PCM modes. We believe high-quality reproduction of the WILMOI technique
is fundamentally available on microscopes of various manufacturers. With the help
of phase-contrast and dark-field microscopy, it is possible to make quite spectacular
images, but for complex representation of the strains they must be supplemented with
the results of traditional light microscopy or WILMOI technique.
Page
L.
1
Wurster
S.
2
Schadt
F.
3
Einsele
H.
4
Ullmann
A.J.
1
Samnick
S.
3
1Internal Medicine Ii, Division of Infectious Diseases, University Hospital of Wuerzburg,
Wuerzburg, Germany
2Ut Md Anderson Cancer Center, 1515 Holcombe, Houston, United States of America
3Nuclear Medicine, University Hospital of Wuerzburg, Wuerzburg, Germany
4Internal Medicine Ii, University Hospital of Wuerzburg, Wuerzburg, Germany
P136. Evaluation of 99mTc-Amphotericin B for nuclear imaging of mould infections in
a transwell in vitro system
Objectives: Several studies have evaluated molecular imaging using positron emission
tomography (PET) and single photon emission computed tomography (SPECT) to diagnose
invasive mycoses. The most promising tracers described in the literature include 68Ga-labelled
Aspergillus-derived siderophores [1] or A. fumigatus-specific antibodies labelled
with 64Cu [2]. However, these PET-tracers are cost-intensive and onsite generation
may not be feasible at regional medical centers. Therefore, we investigated the potential
of Amphotericin B (AMB) labelled by the gamma emitter technetium-99m (99mTc) to visualize
pathogenic moulds.
Methods: Sodium pertechnetate (derived from a 99Mo/99mTc generator, 1000 MBq in 0.35
mL saline) was added to a suspension of 50 µg AMB in 100 µL HBSS, followed by 20 µL
of a stannous chloride solution (10 mg SnCl2 in 1 mL 0.1 N HCl). After 20 min incubation
at room temperature, the 99mTc-AMB solution was neutralized by addition of 3 ml PBS
and sterile filtered. To prepare the transwell assay, 1x105 human pulmonary artery
endothelial cells (HPAEC) were seeded on the lower side of a transwell insert (24-well
format, 3 µm pore size) according to previously published protocols [3-4]. The HPAEC
layer was infected with 2.5x101-2.5x106
A. fumigatus conidia or a bacterial control (Staphylococcus aureus). Additional inserts
were infected with 2.5x105 spores of different Mucorales species. After an incubation
period of 15 h to obtain early mycelial biofilms, 99mTc-AMB was added to the medium
at 50 ng/mL, a concentration that is significantly below commonly achieved therapeutic
serum levels [5]. Transwell inserts were incubated for 120 min in 600 µL of the tracer
solution (initial activity: 30 kBq per well) and were subsequently washed five times
with HBSS. Thereafter, the activity was measured using a gamma counter and normalized
to the activity of 600 µL tracer-supplemented medium to calculate percent uptakes.
Independently prepared inserts were imaged by autoradiography.
Results: Radiochemical yield and purity of 99mTc-AMB, as determined by thin layer
chromatography, reached >98 % and >99 %, respectively. Minimal unspecific tracer uptake
was found in uninfected (0.03-0.14 %) or S. aureus infected inserts (0.05-0.16 %).
An inoculum-dependent increase in tracer uptake was seen in A. fumigatus infected
inserts, with a limit of detection at 2.5x104 fungal cells (Panel A). Low inter-assay
variability was found (mean coefficient of variation = 0.32, n = 3). Quantitative
results were validated by autoradiography imaging, confirming inoculum-dependent tracer
enrichment in A. fumigatus-infected HPAEC monolayers (Panel B). Finally, we tested
the applicability of 99mTc-AMB to detect Mucorales. Mean 4.4 and 6.2-fold 99mTc-AMB
enrichment compared with an uninfected control was found in inserts infected with
2.5x105 spores of Rhizopus arrhizus or Cunninghamella bertholletiae, respectively.
Conclusion: Our in vitro results suggest promising potential of 99mTc-AMB as an easily
producible, cost-efficient alternative for nuclear imaging of pathogenic moulds by
SPECT. These data could encourage further in vivo evaluation of modified antifungal
drugs as nuclear medical tracers. [1] Petrik et al., 2014, Mol Imaging Biol; [2] Rolle
et al., 2016, PNAS; [3] Hope et al., 2007, J Infect Dis; [4] Belic et al., 2019, Front
Microbiol; [5] Hamill, 2013, Drugs.
Savio
J.
Mathew
J.
Microbiology, St. John’s Medical College, Bangalore, India
P137. Detection of circulating DNA in serum by PCR for the diagnosis of invasive mucormycosis
Objectives: Mucormycosis is an invasive fungal infection with increasing incidence
in developing countries like India. Because of its rapid and fatal progression, early
diagnosis is essential. Conventional methods like microbiological and histopathological
investigations have limitations. In the absence of specific biomarkers for diagnosis,
detection of circulating Mucorales DNA in serum by PCR proves a better alternative
to conventional methods. The objectives of this study were to compare detection of
circulating Mucorales DNA in serum by PCR with the conventional microbiological and
histopathological investigations.
Methods: The Institutional Ethics Committee approved this study. 36 patients with
a clinical diagnosis of mucormycosis whose samples were received in the microbiology
and or histopathology laboratories were included. Three patients with laboratory finding
not suggestive of mucromycosis were excluded. Blood samples from all 33 patients were
collected. Serum was separated and stored until further analysis. Microbiological
investigations included microscopy and culture. Isolates were identified by the conventional
techniques. Histopathological examination by microscopy was carried out using H and
E, PAS and GMS stains. DNA was extracted from serum by the salting out and TRIZOL
methods. Conventional Pan-Mucorales PCR was performed on the extracted DNA.
Results: of the 33 patients, 20 were males and 13 were females. Mean age was 52 years.
29 of the 33 patients had diabetes mellitus and 3 were diagnosed following admission.
Clinical presentation included rhino orbito cerebral in 25, cutaneous in 4, pulmonary
in 3 and renal in one. All 32 samples received for microbiological investigations
were positive by microscopy and 22 (68.8%) were culture positive. Isolates included
Rhizopus species 17, Apophysomyces 2, Lichtheimia 1 and one is yet to be identified.
24 of the 25 samples on histopathological examination was suggestive of mucormycosis.
19 of the 33 serum samples were positive for circulating DNA. PCR was positive in
80% of the culture negative patients. PCR was positive in 42.1% of samples with no
angioinvasion. PCR was positive in 50% of ROC and cutaneous manifestation and all
3 patients with pulmonary mucormycosis. 61.9% of PCR positive patients had an unfavorable
outcome.
Conclusion: Most patients were above 40 years of age and from urban and rural areas
DM was the most common underlying risk factor. ROC was the most common presentation.
Rhizopus species was the most common isolate, Apophysomyces and Saksenaea were isolated
from cutaneous lesions. 57.6% of the samples were positive for circulating Mucorales
DNA PCR was positive in 80% of culture negative samples PCR was positive in 42.1%
of samples with no angioinvasion Detection of circulating Mucorales DNA could serve
as an indicator of angioinvasion and help in early diagnosis 36.4% of the patients
recovered Other factors that could influence the PCR results include: use of different
volumes of serum for DNA extraction and commercial extraction kits A larger sample
size and serial sampling during the course of illness is required to critically evaluate
the utility of this test
Jenks
J.
Reed
S.
Mehta
S.
Law
N.
Aslam
S.
Taplitz
R.
Hoenigl
M.
Medicine, University of California San Diego, San Diego, United States of America
P138. Rapid Diagnosis of Invasive Aspergillosis in Bronchoalveolar Lavage: Performance
of the Aspergillus Galactomannan Lateral Flow Assay and the Aspergillus-specific Lateral
Flow Test in a Mixed Patient cohort
Objectives: Early diagnosis and treatment of invasive pulmonary aspergillosis (IPA)
remains the most important factor to reduce mortality and improve outcome. Diagnosis
remains a challenge, however, due to unspecific clinical presentation and radiological
findings. Only very recently rapid tests for IPA have been developed. The objective
of this study was to evaluate the performance of two new CE-marked tests, the Aspergillus
Galactomannan Lateral Flow Assay (LFA; IMMY, Norman, Oklahoma, USA) and the Aspergillus-specific
Lateral Flow Device Test (LFD; OLM, Newcastle, UK) for IPA in a mixed cohort of patients
with and without hematological malignancies.
Methods: The LFA and LFD were retrospectively performed according to the manufacturer’s
instructions in 106 previously frozen bronchoalveolar lavage fluid samples from 106
patients at risk for IPA (23% with underlying hematological malignancies). Samples
were collected between September 2016 and September 2018 at the University of California
San Diego, United States. Performance of the LFA and the LFD were compared to Galactomannan,
and BAL culture. IPA was classified according to revised EORTC/MSG criteria.
Results: Overall, 22 patients met criteria of probable or proven IPA, 9 criteria of
possible IPA, while 75 patients did not fulfill EORTC/MSG criteria of IPA. Sensitivity
of the Apergilllus Galactomanann LFA for probable/proven IPA was 77% (17/22), and
thereby slightly higher than sensitivity for the Aspergillus-specific LFD (59%; 13/22).
Sensitivity was similar to BAL GM (77% with a cutoff of 1.0 ODI), but higher compared
to BAL culture (23%). The LFA resulted negative in 7/9 cases with possible IPA and
47/73 cases without IPA (overall specificity 66%, 54/82). The less than perfect specificity
was driven particularly by non-neutropenic patients (specificity 62%, 43/69), while
specificity was 85% among patients with underlying hematological malignancies. Specificity
was slightly higher for the LFD (70% specificity; 67% among non-neutropenic patients).
Lower specificity among non-neutropenic patients was also observed for the BAL GM
(overall specificity 77%, 72% among non-neutropenic patients), and BAL culture (overall
90%, 88% among non-neutropenic patients).
Conclusion: Our study indicates that both the LFA and LFD may be useful for rapid
diagnosis of IPA in BALF when IPA is clinically suspected. The lower specificity in
non-neutropenic patients without underlying hematological malignancies may be explained
by limited applicability of the EORTC/MSG criteria in non-neutropenic patients. Better
consensus criteria for classifying IPA in those patients are needed.
Kozlova
Y.
1
Frolova
E.
2
Uchevatkina
A.
2
Filippova
L.
2
Borzova
Y.
2
Makhmutova
V.
3
Stepanenko
T.
3
Aak
O.
2
Bogomolova
T.
2
Spiridonova
V.
2
Vasilyeva
N.
4
Klimko
N.
5
1Department of Clinical Mycology, Allergology and Immunology, North-Western State
Medical University named after I.I. Mechnikov, Saint-Petersburg, Russian Federation
2Kashkin Research Institute of Medical Mycology, Saint-Petersburg, Russian Federation
3Multidisciplinary City Hospital №2, St. Petersburg, Russia, Saint-Petersburg, Russian
Federation
4Kaschkin Research Institute of Medical Mycology. Department of Medical Microbiology,
North-Western State Medical University named after I.I. Mechnikov, Saint-Petersburg,
Russian Federation
5Department of Clinical Mycology, Allergology and Immunology, North-Western State
Medical University n.a. I.I. Mechnikov, Saint-Petersburg, Russian Federation
P139. Use of the basophil surface marker CD203c in the diagnosis of fungal sensitization
in patients with cystic fibrosis
Objectives: Allergic bronchopulmonary aspergillosis (ABPA) is the most common form
of aspergillosis in patients with cystic fibrosis (CF). ABPA affects from 2% to 15%
of patients with CF and significantly worsens the course of the underlying disease.
Diagnosis of ABPA with CF is complicated because many diagnostic criteria intersect
with typical manifestations of the underlying disease. Recent studies show that the
sensitization to A.fumigatus associated with a significant reduction in lung function
and an increased incidence of pulmonary exacerbations in CF patients. Thus, early
detection of fungal sensitization very important for the diagnosis of ABPA, and appointment
of adequate therapy which prevents the progression of lung disease. Now there is a
search for new diagnostic tests that will identify fungal sensitization in these patients.To
study possibility of basophil activation test use for identification of fungal sensitization
in patients with cystic fibrosis.
Methods: The study included 190 patients with CF aged 1 to 40 years. All patients
underwent allergological (skin tests with fungal allergens, determination of serum
levels of total IgE (“Polygnostus”, Russia) and specific IgE (sIgE) for fungal allergens(“AlcorBio”,
Russia)) and mycological (microscopy and culture of respiratory substrates) examination.
Computed tomography of the chest was performed according to the indications. Diagnostic
criteria of ABPA 2003 [Stevens et al] were used. Study of the activation of basophils
by flow cytometry was performed using Allergenicity kit (“Beckman Coulter”, USA).
Basophils were identified by CD3-CRTH2+ marker, basophil activation – by elevated
expression of CD203c. For basophil stimulation were used allergens A.fumigatus (“AlcorBio”,
Russia). The basophil activation test with the A. fumigatus allergen was conducted
in 12 CF patients with ABPA and 25 CF patients without ABPA in addition to the standard
allergological examination. The obtained data was processed using the STATISTICA 10
software system.
Results: In patients with cystic fibrosis the frequency of sensitization to A. fumigatus
was 27%, the incidence of allergic bronchopulmonary aspergillosis - 5.7%. The number
of eosinophils, total IgE and specific IgE levels in CF patients with ABPA were significantly
higher than in CF patients without ABPA. In blood of were identified 77.1 (46.3-87.8)
% basophils activated by A.fumigatus allergen, the stimulation index was 19.2 (11.3-30.6).
In the comparison group stimulation index did not exceed the value of 2.5 (p = 0.000).
Direct positive correlation between the level of sIgE to A. fumigatus and the number
of basophils activated by A. fumigatus allergens (r = 0,67; p < 0,05) was established.
In CF patients with ABPA the FVC and body mass index were significantly lower comparing
with patients without fungal sensitization.
Conclusion: The basophil activation test applying, along with traditional methods,
will allow a more differentiated approach to assessing the development of ABPA in
CF patients. Timely identification of the associated with A. fumigatus clinical status
of CF patients will facilitate early and effective assignment of specific therapy.
Lengerova
M.
1
Volfova
P.
1
Langer
J.
1
Pantuckova
D.
1
Pospisilova
J.
1
Bezdicek
M.
1
Kocmanova
I.
2
Mayer
J.
1
Racil
Z.
1
1Department of Internal Medicine - Hematology and Oncology, University Hospital Brno,
Brno, Czech Republic
2Department of Clinical Microbiology, University Hospital Brno, Brno, Czech Republic
P140. rComparison of In-house Real-time Assay and MucorGenius Assay performance for
testing of Clinical Samples from Immunocompromised Patients
Objectives: Diagnostics of infections caused by mucormycetes is still quite complicated
because no serological tests (unlike in aspergillosis) are available. Therefore, PCR
methods are currently the methods of choice. The aim of this study was to compare
MucorGenius assay results (Pathonostics) on samples that previously tested positively
using our in-house PCRs.
Methods: In our laboratory, we use nested real-time PCR assay followed by high-resolution
melting analysis (RT-HRM) for PCR screening of clinical samples from patients at risk
of invasive mucormycosis. This assay enables not only detection but also identification
of detected species. If the result is positive, we confirm the result and determine
the fungal load with species-specific real-time quantitative PCR (RQ-PCR). In this
study, we tested 29 selected clinical samples (10 tissues, 15 bronchoalveolar lavage
(BAL)samples, 3 cerebrospinal fluid samples and 1 endosecret), 25 that had been tested
as positive using our screening assay and 4 mucormycetes negative samples. The results
were compared to tests with the MucorGenius assay.
Results: Out of 25 RT-HRM positive samples, 21 were positive using species-specific
RQ-PCR. A significant fungal load (Ct £ 35) was detected in 10 samples. Using MucorGenius
assay, 17 out of 25 samples (68%) were positive (Ct 18.1 – 37.3). RT-HRM detected
Rhizopus sp. (17 samples), Mucor sp. (3 samples), Lichtheimia corymbifera (2 samples),
Lichtheimia ramosa (2 samples) and Rhizomucor sp. (1 sample). 12 out of 17 samples
with Rhizopus sp. detected revealed low fungal load or were negative. To analyze this
discrepancy, sequencing was performed and in 10 samples and R. stolonifer was identified
(which is not targeted by in-house RQ-PCR). The MucorGenius assay was positive in
7/10 R. stolonifer positive samples (Ct 31.0-37.3).
Conclusion: Using commercial assays for PCR diagnostics is currently preferred because
of standardization; therefore we compared our in-house assay results with a commercial
kit for mucormycetes detection. In this study, we also analyzed samples with discrepant
results and identified R. stolonifer as a frequent finding when testing BAL samples.
As this species is rarely described as a cause of infection, its detection most probably
represents colonization or contamination. Based on these results, we added R. stolonifer
specific RQ-PCR to our diagnostic algorithm (if RT-HRM detects Rhizopus sp. than R.
microsporus, R. oryzae and R. stolonifer RQ-PCRs are used to verify the results).
Due to our experience, the MucorGenius kit is easy to use, as it is a one tube real-time
PCR assay and has very good sensitivity. The main disadvantage is the inability to
identify mucormycetes species. Although this information is not critical to initiating
treatment, it is essential for epidemiological reasons and might help to distinguish
colonization/contamination from a truly ongoing infection. This study was supported
by the Ministry of Health, Czech Republic - conceptual development of research organization
(FNBr, 65269705) and TE02000058.
Mathew
J.
Savio
J.
Mathew
J.
Reddy
M.S.
Microbiology, St. John’s Medical College, Bangalore, India
P141. Pythium insidiosum - sporulation with leaves of grass: Two case reports
Case Report:
Pythium insidiosum is a filamentous, aquatic parasite which was till recently considered
to be a fungus. It is known to cause sight-threatening keratitis. The filaments of
Pythium resemble pauci-septate hyphae of Mucorales. Case history: Both patients presented
with redness, irritation and reduced vision of right eye since one month. There was
no history of trauma or any co-morbid conditions. Patient 1 (27 years old gentleman):
Ocular examination revealed corneal ulcer with feathery margins, stromal infiltrates
and endothelial plaque. Hypopyon and corneal congestion were noted. Fungal keratitis
was the clinical diagnosis. KOH microscopy of corneal scraping revealed broad pauci-septate
fungal hyphae. The patient was started on oral fluconazole and topical voriconazole
and amphotericin-B along with antibiotics. With deterioration in the clinical course,
therapeutic penetrating keratoplasty was performed and the patient was discharged
on topical Natamycin. Progressive loss of vision in the eye was managed with iris
repositioning and tightening of corneal suturing. The patient developed a white corneal
opacity and was later lost to follow-up. Patient 2 (26 years old lady): Ocular examination
revealed a corneal epithelial defect, satellite lesions, corneal scarring with stromal
infiltration and no hypopyon. The impression was healing fungal ulcer/viral keratitis.
Initially, she was started on topical acyclovir. On her follow-up OPD visit she still
complained of pain in the right eye. KOH microscopy of corneal scraping revealed broad,
pauci-septate fungal hyphae. The patient was started on oral ketoconazole and topical
voriconazole. Topical linezolid, doxycycline and azithromycin were also added. On
follow-up visit, the patient was asymptomatic and ocular examination revealed healed
lesions. No surgical management was undertaken. Both samples of corneal scraping on
fungal culture yielded a non-sporulating mould. Colonies were flat, feathery, glabrous
and light-brown coloured. Attempts were made to induce sporulation using the conventional
techniques such as water agar, water culture and using non-nutrient media such as
corn meal agar. With colony morphology resembling Pythium, literature search was made
for specific sporulation techniques. The induction medium required chemicals such
as K2H2PO4, MgCl2. Due to the non-availability of these in our laboratory, we set
out to use the grass on our campus (the common Bermuda grass, Cynodon dactylon) with
yeast extract and sterile distilled water with the hope to induce sporulation. To
our surprise, the isolates produced sporangia and motile zoospores, which were visualized
under light microscope. The isolates were sent to PGIMER, Chandigarh for confirmation
by gene sequencing and the identification was confirmed as Pythium insidiosum. Video
link-zoospore: https://drive.google.com/file/d/1cxHGWR-EwHgx0ucuTQZGy81nwfVagFEj/view
Conclusion: Pythium insidiosum keratitis may present as chronic corneal ulcers. The
simple Grass leaf culture as a technique for induction of sporulation of Pythium insidiosum
can be performed in resource-limited settings. Experimentation can be attempted using
leaves from different species of grass. Increased awareness and high index of suspicion
on direct microscopy (pauci-septate, ribbon-like hyphae) and colony morphology (flat,
feathery colonies) is integral to prevent misdiagnosis as fungal infection. Studies
prove that prognosis of such infections is better when treated by a combination of
antibiotics and antifungals than antifungals alone, which may occur when misdiagnosed
as a fungal infection.
Green
J.
Bruker Microbiology, Glasgow, United Kingdom
P142. An Evaluation to assess the performance of the Fungiplex Aspergillus and the
Fungiplex Aspergillus Azole-R IVD Real-Time PCR Kits following the implementation
of an extraction control
Objectives: This Performance Evaluation was designed to test contrived samples spiked
into serum, plasma and BAL material to assess the impact of a new extraction control
on the limit of detection of the Fungiplex Aspergillus and Fungiplex Aspergillus Azole-R
IVD Real-Time PCR Kits and to ensure sensitivity was unaffected by this change. The
results from this study have been evaluated to determine whether the inclusion of
an extraction control has an impact on the limit of detection defined in previous
studies.
Methods: Simulated samples were prepared and analysed by the clinical evaluators.
Samples prepared in serum, plasma and BAL were spiked with specific concentrations
of Aspergillus fumigatus or Aspergillus terreus genomic DNA prior to extraction. Aspergillus
fumigatus wild type strains were used as well as resistant strains containing DNA
mutations TR34 and TR46 in the Cyp51A gene. Samples were prepared in replicates of
five and negative controls were included for each sample type. Prior to extraction
10 µL of the new extraction control formulation was added to each sample. Extraction
was performed using two commercial extraction platforms; the eMAG® by bioMérieux and
the BioRobot® EZ1™ by Qiagen. The PCR was prepared in duplicate for each sample and
run on the Qiagen Rotor-Gene Q 5plex HRM platform.
Results: When tested with the Fungiplex Aspergillus IVD Real-Time PCR Kit, the limit
of reproducibility for Aspergillus fumigatus and Aspergillus terreus samples was 5
ge (genome equivalents). No difference in sensitivity was observed when the results
from two different extraction platforms were compared, however, on average the resultant
Ct (cycle threshold) values were 1.2 cycles higher when the EZ1™ platform was used.
When tested with the Fungiplex Aspergillus Azole-R IVD Real-Time PCR Kit, the limit
of reproducibility for Aspergillus fumigatus samples containing mutations TR34 and
TR46 was 50 ge. This higher limit of reproducibility was expected as the Cyp51A gene
is single copy. The limit of reproducibility was consistent between all sample types,
where in a previous study, without an extraction control, the limit of reproducibility
had been reported between 50 and 75 ge depending on which sample type was used. The
extraction control was detected in every sample analysed with no significant differences
observed in Ct value reported.
Conclusion: 100% sensitivity was reported for the Fungiplex Aspergillus Kit when analysing
Aspergillus samples at 5 ge and for the Fungiplex Aspergillus Azole-R Kit when analysing
resistant strains at 50 ge. The sensitivity is not dependent on sample type and the
new extraction control implemented in these products did not affect the performance
of either Kit. Data has shown that results from this study are equivalent, and in
some cases, superior to previous studies performed.
Gaajetaan
G.
Kampermann
T.
Tegelen
D. Van
Dingemans
G.
PathoNostics, Maastricht, Netherlands
P143. Evaluation of the clinical usefulness of dermatophyte EQA panels using molecular
tests
Objectives: Determine the relevance of external quality assessment (EQA) panels for
detection of dermatophytes by real-time PCR. For this purpose, the commercially available
DermaGenius® 2.0 Complete real-time multiplex PCR was used and simultaneously it’s
performance was assessed.
Methods: Samples from the Dermatophytosis EQA pilot study (years 2016-2018) from QCMD
(Quality Control for Molecular Diagnostics, Glasgow, UK) and the dermatophytes EQAS
program (years 2017-2018) from INSTAND e.V. (Düsseldorf, Germany) were all tested
with the DermaGenius® 2.0 Complete real-time multiplex PCR after suitable DNA-extraction.
The samples from QCMD were processed with a NucliSENS® easyMag® DNA extraction system
(bioMérieux) and the samples from the EQA from INSTAND e.V. were processed with the
PathoNostics Extraction kit.
Results: The Dermatophytosis EQA pilot study panel from QCMD is provided once a year
and contains 8 samples. All 8 samples in each year were correctly identified with
the DermaGenius® 2.0 Complete real-time PCR. The composition of the EQA panel was
comparable every year and contains 1 negative, three samples with different T. rubrum
concentrations (Ct 26-35) and three samples with different T. interdigitale concentrations
(Ct 27-38). Remarkably, each year the panel contained one sample which was positive
for C. albicans (Ct 34-36), which is not a dermatophyte (and not in the scope of the
panel) but can cause a nail infection. The dermatophytes EQAS panel from INSTAND e.V.
is provided twice a year and contains 4 samples. Most samples were correctly identified
with the DermaGenius® 2.0 PCR, only Nannizzia gypsea was not detected because this
dermatopyte target is not included in the PCR assay. Other dermatophytes included
in the four panels were T. rubrum (4x), T. tonsurans (2x), T. interdigitale (1x),
M. canis (1x), T. violaceum (1x), T. benhamiae (1x) and M. audouinii (1x). Almost
all dermatophytes were detected at very low Ct-values (Ct 19-26) representing a high
DNA load. A negative control was included in each panel and was also identified as
negative by the PCR.
Conclusion: Both dermatophyte EQA panels are intended for performance evaluation of
molecular detection methods. The DermaGenius® 2.0 Complete real-time multiplex PCR
showed good performance on both EQA panels, but the panels from these organisations
have a different scope. The EQA panel from QCMD contains variable DNA concentrations
of common dermatophytes and thereby focuses on sensitivity and clinical prevalence
of dermatophyte species. The EQA panel from INSTAND e.V. panel contains a very high
load of common but also more rare dermatophytes and thereby focussing more on specificity
rather than sensitivity and prevalence/clinical significance. More importantly, the
sample type is very difficult to mimic and therefore quality control samples do not
really reflect the actual situation. Nevertheless, the EQA panel from INSTAND e.V.
supplies a solid sample (lyophilized) and can therefore be processed in a more realistic
way in comparison with the liquid samples from the EQA samples from QCMD. Overall,
it is important to select a quality assessment program which serves your purpose.
Martínez
S.
1
Vallina
E.
1
Costales
I.
1
Milian
L.
2
Llamas
E.
1
Sandoval
M.
1
Fernández
J.
1
Zapico
M.S.
1
Peláez
T.
1
1Servicio De Microbiología Y Parasitología, HUCA, Oviedo, Spain
2Servicio De Microbiología Y Parasitología, CAUSA, Salamanca, Spain
P144. Diagnosis of fungal peritonitis: optimization by evaluating two culture procedures
Objectives: Peritonitis is one of the most serious intra-abdominal infections. Among
them, the most frequent is secondary peritonitis, usually caused by intestinal perforation
and with polymicrobial etiology. Fungal involvement in peritonitis is especially relevant,
as fungal peritonitis is associated to a high mortality rate. Regular culture-based
diagnosis of fungal peritonitis is difficult, due to bacterial growth may mask the
presence of yeasts with slower growth. The use of selective culture media for these
microorganisms means an improvement of the diagnosis. The aim of this study is to
evaluate the improvement of the microbiological diagnosis of fungal peritonitis, comparing
two culture procedures.
Methods: Between 2016 and 2019, a total of 1671 peritoneal fluids were retrospectively
studied. Two time periods were established to evaluate two culture procedures: Period
1 (January 2016 to May 2018) and Period 2 (June 2018 to April 2019). During Period
1, if sample volume was enough, peritoneal fluids were inoculated in blood-culture
bottles (BD BACTEC Plus Aerobic/F culture vials) and incubated in an automatic incubator
(BACTEC FX Instruments, Becton-Dickinson). Otherwise, they were inoculated in growth
media agar plates (blood agar, chocolate agar, McConkey agar and Brucella agar). During
Period 2, all peritoneal fluids were also inoculated at the same time in a fungus
selective culture medium (Gentamycin + Chloramphenicol Sabouraud Agar, Biomèrieux).
Results: A total of 1671 peritoneal fluids were evaluated, 1254 during the first period
and 417 during the second period. of them 147 (8.8%) were positive for fungal growth.
The positivity rates of fungal growth during the first and the second period were,
respectively: 6.7% vs 15.1%. The new culture protocol (Period 2) increased significantly
the number of yeast isolates in peritoneal fluids (p = 0.0001), rising from 7% in
the first period to 19.5% in the second (Table 1). Comparing data by yeast species,
a significant rise in incidence of C. albicans (4.5% vs 10.5%) and C. parapsilo
sis (0.48% vs 3.4%) was observed (Table 2).
Conclusion: Our data show that the incorporation of selective culture medium for fungi
improves significantly the microbiological diagnosis of fungal peritonitis, increasing
the positivity rates and the recovery of fungal isolates. C. albicans was the most
frequent species isolated, followed by C. glabrata and C. parapsilosis. The incidence
of C. albicans and C. parapsilosis increased significantly during the second period
of the study.
Kordalewska
M.
Lee
A.
Zhao
Y.
Perlin
D.S.
Center For Discovery and Innovation, Hackensack Meridian Health, Nutley, United States
of America
P145. Direct detection of Candida auris and its ERG11-associated azole resistance
and FKS1-associated echinocandin resistance in clinical skin swabs
Objectives:
Candida auris is a multidrug resistant yeast, recognized as a cause of invasive infections
and health care associated outbreaks around the world. Major obstacles impacting control
of C. auris spread include common misidentification by diagnostic platforms available
in clinical and public health laboratories; a poor understanding of resistance to
antifungal drugs and disinfectants; and a high propensity to contaminate health care
environments which results in transmission of clonal C. auris isolates. Ultimately,
correct detection and identification of the pathogen and its antifungal susceptibility,
followed by strict adherence to appropriate treatment and infection prevention and
control strategies is crucial for limiting the spread of C. auris. Here, we have developed
and validated platforms for direct detection of C. auris and its ERG11-associated
azole resistance and FKS1-associated echinocandin resistance in clinical skin swabs.
Methods: DNA isolated from 112 de-identified clinical axilla/groin skin swab samples
was tested in the recently developed C. auris-specific SYBR Green qPCR assay (Kordalewska
et al. JCM 2017) with the addition of inhibition control. Enrichment culture (Welsh
et al. JCM 2017) followed by sequencing of rDNA was used as a reference method. 24
samples, determined as C. auris-positive by both qPCR and reference method, were further
screened in the ERG11-associated azole resistance and FKS-associated echinocandin
resistance assays (Hou et al. AAC 2018). Antifungal susceptibility testing with echinocandins
(anidulafungin, micafungin) and azoles (fluconazole, voriconazole) according to CLSI
guidelines and sequencing of ERG11, and FKS1 were used as reference methods.
Results: In the C. auris-specific SYBR Green qPCR assay 45% of swabs were C. auris-negative
(50/112), and 55% were C. auris-positive (62/112). However, only 21% (24/112) of swabs
were culture-positive. The finding that C. auris cells lost their viability in 34%
of swabs, but the DNA was still present (giving a positive real-time PCR result),
was possibly due to the long swabs storage (4-5 months) before processing. Detection
of C. auris was not affected by the presence of other microorganisms present in the
sample. Thus, the assay was found to produce results comparable to the enrichment
culture approach currently implemented at CDC and Antibiotic Resistance Laboratory
Network (ARLN) laboratories. The resistance detection assays identified all C. auris-positive
swabs as FKS1 wild-type, and ERG11 wild-type. The molecular diagnostic results from
the assays were 100% concordant with DNA sequencing results. Moreover, all isolates
exhibited low minimal inhibitory concentration (MIC) values (MIC ranges - anidulafungin:
0.06-0.125 mg/l; micafungin: 0.06-0.125 mg/l; fluconazole: 1-2 mg/l; voriconazole:
≤0.03 mg/l) and were categorized as echinocandin- and azole-susceptible.
Conclusion: Overall, we found the C. auris-specific SYBR Green qPCR assay can be successfully
applied for rapid and accurate detection of C. auris in patient skin swabs, thereby
increasing high-throughput diagnostic options for this emerging pathogen. Moreover,
the ERG11-associated azole resistance and FKS1-associated echinocandin resistance
detection platform has the potential to overcome the deficiencies of existing in vitro
susceptibility-based assays to identify azole and/or echinocandin resistant C. auris,
and thus, it holds promise as a surrogate diagnostic method to direct antifungal therapy
more effectively.
Kampouri
E.
1
Scherz
V.
2
Marquis
P.
3
Pagani
J.-L.
3
Greub
G.
1
2
Lamoth
F.
1
2
1Infectious Diseases Service, University Hospital of Lausanne, Lausanne, Switzerland
2Microbiology Institute, University Hospital of Lausanne, Lausanne, Switzerland
3Intensive Care Department, University Hospital of Lausanne, Lausanne, Switzerland
P146. Role of PCR, mannan antigen and 1,3-beta-d-glucan measurements in cerebrospinal
fluid for the diagnosis and follow-up of Candida meningitis
Objectives: Infections of the central nervous system due to Candida spp. are rare
and usually associated with intracranial devices. Diagnosis is challenging because
of low sensitivity of cultures and lack of specificity of clinical and biological
signs of meningitis. Optimal treatment is not well established and response to therapy
is difficult to assess. We present two cases of Candida albicans meningitis occurring
in the setting of craniofacial surgery without intracranial device for which different
fungal markers (1,3-beta-d-glucan, mannan, PCR) in the cerebrospinal fluid (CSF) were
used for diagnosis and follow-up.
Methods: PlateliaTM Candida Ag plus (Bio-Rad), FungitellTM (Associates of Cape Cod)
and a home-made quantitative C. albicans-specific PCR (targeting 26S rDNA) were used
for detection of mannan antigen (Mn-Ag), 1,3-beta-d-glucan (BDG) and fungal DNA, respectively,
in serum and cerebrospinal fluid (CSF).
Results:
Candida meningitis presented as a complication of neurosurgery, following transsphenoidal
surgery and skull base reconstruction with CSF leakage respectively. Biological analyses
of CSF were consistent with bacterial meningitis, but with negative culture and broad-spectrum
16S rDNA PCR for bacteria. C. albicans was detected by CSF culture in one case and
PCR in CSF in both cases. Antifungal therapy consisted in fluconazole and liposomal
amphotericin B. PCR fungal loads subsequently declined along with clinical and biological
improvement. BDG levels in CSF were very high at diagnosis while negative in serum
and remained elevated during follow-up in both cases. Mn-Ag was measured in one case
with elevated value in CSF (>500 pg/ml vs 20 pg/ml in serum) at diagnosis and significant
decline in follow-up.
Conclusion: These two cases illustrate the potential value of PCR, Mn-Ag and BDG measurements
in CSF for the diagnosis of Candida meningitis. Further analyses are required to define
a threshold of positivity for BDG and Mn-Ag in CSF or the diagnostic value of CSF/serum
indexes. Moreover, PCR and Mn-Ag may be useful for monitoring of response to therapy.
Xess
A.
1
Xess
I.
2
Singh
G.
2
1Microbiology, all india institute of medical sciences, new delhi, India
2Microbiology, All India Institute of Medical Sciences, new delhi, India
P147. Usefulness of Galactomannan antigen test in Serum or Bronchoalveolar lavage
sample in Kidney transplant patients
Objectives: Evaluation of Galactomannan in BAL and Serum samples in Kidney Transplant
Patients
Methods: A total of 127 kidney transplant patients (KTP) suspected of invasive pulmonary
aspergillosis (IPA) were enrolled for the study. Patients were further investigated
radiologically and microbiologically. Galactomannan was done in serum and BAL samples
Receiver Operating Curve (ROC) was generated to determine the optimal cut off value
for Galactomannan in BAL and serum samples.
Results: Out of 127 patients 32(25.2%) were females and 95(74.8%) were males. 76/127(59.8%)
patients presented with fever not responding to antibiotics and 62/127(48.8%) presented
with cough/respiratory distress. 54/127 (42.5%) presented with both fever and cough.
In CT scan, 24/127(18.9%) patients showed consolidation,10/127(7.9%) showed ground
glass appearance, 5/127(3.9%) showed nodules and 3/127(2.4%) patients had only opacity.
Galactomannan antigen was tested in 138 serum and 47 BAL samples of 127 patients.
Forty-four serum samples and 34 BAL samples were positive for galactomannan antigen.
Three patients were positive for fungal elements on KOH-calcofluor stain and two were
only positive for culture. During the course of hospitalization 12/127(9.4%) received
Amphotericin-B, 4/127(3.1%) voriconazole, 3/127(2.4%) caspofungin, 2/127(1.6%) posaconazole
and 1/127(0.8%) itraconazole. Mortality was around 22% (28/128). Receiver operating
characteristic (ROC) curve was plotted for BAL and Serum galactomannan levels. The
value for BAL was found to be 1.0 and for serum it was 0.7.
Conclusion: Corrected BAL and serum galactomannan values for diagnosis of invasive
fungal aspergillosis in kidney transplant patients were found to be 1.0 and 0.7 respectably,
can be useful for diagnosis where the sensitivity of direct demonstration and culture
is very low.
Cruciani
M.
1
White
P.
2
Löffler
J.
3
Morton
C.O.
4
Barnes
R.
5
Donnelly
J.P.
6
Klingspor
L.
7
Buchheidt
D.
8
Maertens
J.
9
Heinz
W.J.
10
Rogers
T. R
11
Warris
A.
12
Mengoli
C.
13
Lockhart
D.
12
Cordonnier
C.
14
1Infectious Diseases, San Bonifacio Hospital, Verona, VERONA, Italy
2Microbiology, Public Health Wales Microbiology, Cardiff, United Kingdom
3Medizinische Klinik Und Poliklinik Ii, Universitätsklinikum Würzburg, Würzburg, Germany
4western Sidney University, Sidney, Australia
5Medical Microbiology and Infectious Diseases, Cardiff University School of Medicine,
Cardiff, United Kingdom
6EAPCRI, Nijmegen, Netherlands
7Laboratory Medicine, Karolinska Insitutet, Stockholm, Sweden
8Mannheim University Hospital, Heidelberg University, Mannheim, Germany
9KU Leuven, Leuven, Belgium
10University hospital, Wuerzburg, Germany
11Department of Clinical Microbiology, St. James’s Hospital, Dublin, Ireland
12Mrc Centre For Medical Mycology, University of Aberdeen, Aberdeen, United Kingdom
13University of Padua, Padua, Italy
14University of Paris, Paris, France
P148. Potential impact of anti-mould prophylaxis on Aspergillus PCR blood testing
for the diagnosis of invasive aspergillosis: a Fungal PCR initiative (FPCRI) systematic
review and meta-analysis.
Objectives: To analyse the impact of anti-mould prophylaxis on the diagnostic accuracy
of Aspergillus PCR for the diagnosis of Invasive Aspergillosis (IA) in immunocompromised
patients.
Methods: Systematic review and meta-analysis of prospective cohort studies evaluating
Aspergillus PCR testing of blood from high-risk neutropenic cancer patients, haematopoietic
stem cell transplant (HSCT) and solid organ transplant recipients. QUADAS-2 was used
to assess methodological quality of included studies. The sensitivity and specificity
of Aspergillus PCR was evaluated by meta-analytical pooling of logit sensitivity and
logit specificity. We also calculated the diagnostic Odd Ratio (DOR). A subgroup analysis
of studies using anti-mould prophylaxis compared to studies not using anti-mould prophylaxis
(or using placebo or fluconazole) was performed
Results: Nine studies used anti-mould prophylaxis (itraconazole, voriconazole, amphotericin
B or caspofungin) in the entire population or in a subset of patients, while 13 studies
did not use any anti-mould prophylaxis. The incidence of IA was significantly lower
in the prophylaxis group compared to the non prophylaxis group (11.43 vs 16.13; p
= 0.0005). The use of anti-mould prophylaxis increased sensitivity (from 0.80 to 0.86;
p = n.s) and decreased specificity (from 0.78 to 0.64; p = 0.059) and DOR (from 17.10
to 11.14; p = n.s.). In sensitivity analysis, removing 3 studies using prophylaxis
with itraconazole has no significant impact on sensitivity, Sensitivity and specificity
of PCR test in studies using or not anti-mould prophylaxis. TP, TN = true positive
and true negative results of PCR test, compared to total cases of IA according to
the reference standard (EORTC/MSG criteria) or no-IA. specificity and DOR.
Conclusion: The cumulative incidence of IA was significantly lower in patients receiving
anti-mould prophylaxis. The use of prophylaxis decreases the specificity of Aspergillus
PCR testing of blood, which is of borderline statistical significance. Although an
increase in sensitivity and decrease in DOR was observed, this did not reach statistical
significance between the 2 groups. Anti-mould prophylaxis reduces the proportion of
proven/probable cases of IA (according to EORTC/MSG criteria) which is associated
with a lower specificity of the Aspergillus PCR testing of blood.
Argy
N.
1
Bonnal
C.
1
Godet
C.
2
Timsit
J.F.
3
Lortat-Jacob
B.
4
Houzé
S.
1
1Laboratoire De Parasitologie Mycologie, Hôpital Bichat-Claude Bernard, 46 rue Henri
Huchard, PARIS, France
2Service De Pneumologie B, Hôpital Bichat-Claude Bernard, 46 rue Henri Huchard, PARIS,
France
3Service De Réanimation Médicale Et Infectieuse, Hôpital Bichat-Claude Bernard, 46
rue Henri Huchard, PARIS, France
4Service De Réanimation Chirurgicale, Hôpital Bichat-Claude Bernard, 46 rue Henri
Huchard, PARIS, France
P149. Comparison of fungal cultures and Aspergillus PCR kits results in SOT patients:
an evaluation of five new commercial kits on respiratory samples.
Objectives: The diagnosis of invasive aspergillosis (IA) is difficult in patients
without hematological malignancies. For diagnosis, direct microscopy, histopathology
and culture are strongly recommended and, in solid organ transplant patients (SOT),
broncho-alveolar lavage (BAL) galactomannan measures are recommended as well. Recent
recommendations suggest that BAL-PCR should be considered in conjunction with other
diagnostic tests in lung transplanted patients particularly1. Commercially Aspergillus
fumigatus and Aspergillus spp PCR kits are now available but their evaluation in clinical
practice remains to be performed. The aim of our study was to compare the results
obtained with some of those Aspergillus PCR kits and cultures performed on respiratory
samples in non-hematological patients.
Methods: Five kits (Ademtech MycoGENIE® Aspergillus fumigatus and MycoGENIE® Aspergillus
species, AsperGenius® Species PathoNostics (A fumigatus, A terreus, Aspergillus spp),
Fungiplex® IVD PCR (Aspergillus spp, A terreus) Bruker and Aspergillus ELITe MGB®
ElitechGroup (Aspergillus spp.)) were evaluated on respiratory samples which were
routinely analyzed for patients (including SOT) in our hospital. The PCR results were
considered as not acceptable if the matching between fungal cultures and PCR results
represents less than 70% of the samples tested (in bold in results).
Results: Forty respiratory samples (BAL, bronchial aspirates) from 36 patients were
included. For 30 samples, 8 A fumigatus, 14 Aspergillus spp (4 A flavus, 4 A niger,
2 A hiratsukae, 1 A terreus, 1 A nidulans, 1 A calidoustus and 1 Aspergillus spp.)
and 8 Penicillium spp. were isolated. Twelve were negative. For MycoGENIE®
A fumigatus, MycoGENIE®
Aspergillus species, AsperGenius®
A fumigatus, AsperGenius®
Aspergillus spp, Fungiplex® and ELITe MGB® respectively, the results were: - PCR positive
results/number of samples tested and A fumigatus positive cultures: 7/8, 7/8, 5/8,
6/8, 5/7, 3/8, - PCR positive results/number of samples tested and Aspergillus spp
positive cultures: 0/12, 6/12, 1/10, 6/10, 6/12, 2/12, - PCR negative results/number
of samples tested and Penicillium spp. positive cultures: 6/7, 6/8, 8/8, 3/8, 4/7,
7/7 - PCR negative results/number of samples tested and negative cultures: 7/8, 10/12,
7/9, 5/9, 8/12, 11/12. MycoGENIE® Aspergillus fumigatus shows a high concordance for
A fumigatus positive cultures without false positive results for Aspergillus spp or
Penicillium spp. AsperGenius® A fumigatus does not give satisfactory results for positive
cultures. Neither of the Aspergillus spp. PCR kits have a good correlation with the
culture results.
Conclusion: MycoGENIE® Aspergillus fumigatus seems to be suitable for the diagnosis
of AI due to A fumigatus and must be prospectively studied in non-hematological patients.
For Aspergillus spp. PCR kits, all except ELITe MGB® gave unsatisfactory results for
negative results, mainly because of the cross reactivity with other hyalohyphomycetes.
This is a real problem for lung transplanted patients who are often colonized with
filamentous fungi and may have positive PCR results without AI. On the other hand,
it is difficult to conclude about their correlation with positive cultures because
it may reflect colonization without infection. The cohort was too small for a clinical
evaluation therefore prospective evaluation of these PCRs in patients without hematological
malignancies are needed. Ullmann AJ et al. Clin Microbiol Infect. 2018
Vergidis
P.
1
Moore
C.B.
2
Novak-Frazer
L.
2
3
Rautemaa-Richardson
R.
3
4
Walker
A.
3
Denning
D.
3
4
Richardson
M.D.
2
3
1Infectious Diseases, Mayo Clinic, Rochester, United States of America
2Mycology Reference Centre, Excellence Center For Medical Mycology (ecmm), Wythenshawe
Hospital, Manchester University NHS Foundation Trust, Manchester, United Kingdom
3Division of Infection, Immunity and Respiratory Medicine, University of Manchester,
Manchester, United Kingdom
4National Aspergillosis Centre, Manchester University NHS Foundation Trust, Manchester,
United Kingdom
P150. rHigh-Volume Culture and Quantitative Real-Time PCR for the Detection of Aspergillus
in Sputum
Objectives: Sputum culture is an insensitive method for the diagnosis of pulmonary
aspergillosis. Growth of the organism allows identification of the causative species
and susceptibility testing, both of which can inform treatment choices. The current
practice is to culture an aliquot of diluted sputum. We assessed the value of culturing
large volumes of unprocessed sputum, a method that we have termed high-volume culture
(HVC).
Methods: Specimens were processed by conventional culture (using an aliquot of homogenized,
diluted sputum on Sabouraud agar at 37°C and 45°C for up to 5 days) and HVC (using
undiluted sputum on Sabouraud agar at 30°C for up to 14 days). A separate specimen
was tested by quantitative real-time PCR (qPCR). Aspergillus MycAssay (Myconostica)
was used from January 2015 until February 2016. Aspergillus FUMI (Progenie) was used
from February until October 2016. Aspergillus Elite MGB (ELITechGroup) was used from
October 2016 through February 2017. Antifungal susceptibility testing was performed
by the EUCAST standard.
Results: We obtained 290 sputum specimens from 231 patients with the following conditions:
Chronic pulmonary aspergillosis (67.1%), allergic bronchopulmonary aspergillosis/severe
asthma with fungal sensitization (25.1%) and Aspergillus bronchitis (7.8%). The sensitivity
of conventional culture was 16.2% (95% CI: 12.4-20.9%) and that of HVC was 52.4% (95%
CI: 46.7-58.1%) (p<0.001). qPCR had an overall sensitivity of 49.0% (95% CI: 41.2%-56.9%),
comparable to that of HVC (p = 0.46). Rates of growth by HVC in correlation with transformed
Ct values (TCt) for MycAssay and FUMI are shown in Fig. 1. There is a correlation
between higher TCt values and the percentage of growth by HVC. By combining HVC and
qPCR, the sensitivity increased to 66.0% (Fig. 2. Solid bars patients not receiving
antifungal treatment. Dotted bars represent specimens of patients receiving treatment).
A. fumigatus was the most commonly isolated species (82.9%) followed by A. niger (9.2%),
A. montevidensis (3.3%) and A. flavus (2.6%). Among A. fumigatus isolates, pan-azole
resistance was detected in 17.2%. At the time of sputum collection, 52.1% of patients
were receiving antifungal treatment. Among patients with CPA, the sensitivity of HVC
was higher compared to conventional culture for those treated with itraconazole (48.0%
vs 12.0%, p<0.001) as well as those not receiving antifungal drug treatment (60.5%
vs 26.7%, p<0.001). This trend is replicated in patients treated with other antifungals
for all three conditions. HVC allowed for detection of azole-resistant isolates that
would have been missed by conventional culture.
Conclusion: The recovery rate of Aspergillus spp. is significantly higher for HVC
compared to conventional culture. HVC can be performed in any microbiology laboratory
without the need for additional equipment or technical skills. The sensitivity of
HVC is comparable to that of qPCR, although susceptibility testing is not possible
by the latter technique. Combination of the two methods can lead to further increase
in the recovery of Aspergillus from sputum specimens. Use of HVC of expectorated sputum
for the detection of fungi can have a significant impact in the diagnosis and management
of patients with pulmonary mycoses.
Springer
J.
1
Teschner
D.
2
Kessel
J.
3
Cornely
O.A.
4
Liebregts
T.
5
Krause
S.W.
6
Schwartz
S.
7
Kiehl
M.G.
8
Heinz
W.J.
1
Willinger
B.
9
Einsele
H.
1
Rickerts
V.
10
Löffler
J.
1
1University hospital, Wuerzburg, Germany
2University Medical Center of the Johannes Gutenberg University, Mainz, Germany
3University Hospital Frankfurt, Frankfurt, Germany
4German Centre For Infection Research, Partner Site Bonn-cologne, Cologne, Germany
and Clinical Trials Centre Cologne (zks Köln), University of Cologne, Cologne, Germany
5University Hospital Essen, Essen, Germany
6University Hospital Erlangen, Erlangen, Germany
7Charité Berlin, Berlin, Germany
8Klinikum Frankfurt/Oder, Frankfurt/Oder, Germany
9Medical University of Vienna, Vienna, Austria
10Robert Koch Institut Berlin, Berlin, Germany
P151. Aspergillus, Mucorales, Fusarium: Detectable in BAL by real-time PCR?
Objectives: Invasive fungal infections (IFI) remain a major complication in patients
with haematological malignancies (HM) and post allogeneic haematopoietic stem cell
transplantation (HSCT). In these patients, invasive aspergillosis (IA) is the most
common cause of mortality due to infection. Early detection of fungal infections has
the potential to facilitate a more effective management of invasive disease. Bronchoalveolar
lavage samples (BAL) directly obtained from the focus of infection are often used
as diagnostic tool. In this study, BAL samples were tested using different real-time
PCR assays and correlated to EORTC/MSG classification for IFI (DePauw 2008).
Methods: In a multi-centre approach, nine hospitals collected BAL samples from HM/HSCT
patients comprising a CT scan suspicious for IFI. DNA was isolated from supernatant
and BAL pellet and analysed for the presence of fungal DNA. Analysis was performed
in Wuerzburg only by using 3 specific real-time PCR assays and one panfungal assay
(Khot 2009, 28S 10f-12r). All samples were analysed for the presence of Aspergillus
and Mucorales, additionally some for Fusarium and fungal DNA.
Results: In total, 294 samples were collected. 69/294 showed Aspergillus DNA, 20/294
Mucorales, 2/141 Fusarium and 62/69 panfungal DNA (Table 1). Sequencing of panfungal
amplicons revealed mostly DNA of yeasts (mainly Malassezia sp. and Candida sp.) which
were classified as non-invasive/contaminations. Only in 7 cases (7.9%; 6 Aspergilli,
1 Mucorales) the result of the specific assay could be confirmed. Therefore no further
PCR analysis using the panfungal assay was performed. In correlation to EORTC/MSG
criteria (6 proven, 85 probable, 130 possible IFD and 26 undetermined cases; 47 without
data) Aspergillus DNA was detected in 42 probable/proven cases, Mucorales in 11, Fusarium
in 1 and panfungal DNA in 24 (only 7 if excluding contaminations) (Table 2). DNA of
Aspergillus and Mucorales was detected in just one or none control samples (undetermined
cases), 10/69 samples (14.5%) showing DNA of Aspergillus were also positive for Mucorales
(n = 9) or Fusarium (n = 1). Changing the perspective revealed that almost half of
the Mucorales positive samples were double-infected with Aspergillus (9/20; 45%).
Conclusion: qPCR was able to detect DNA of specific fungi in BAL samples. Specific
assays for Aspergillus, Mucorales and Fusarium proved to be helpful to detect fungal
pathogen DNA, whereas the panfungal approach mainly detected non-invasive contaminations.
The number of double-infections seems to be higher than expected (14.5% to 45%).
Domingo
M.P.
1
Nabal
S.
2
Redrado
S.
1
Lopez
C.
2
Rodriguez-Vigil
C.
3
Muñoz
A.
3
Calvo
C.
3
Rezusta
A.
2
Pardo
J.
4
Galvez
E.
1
1Instituto de Carboquimica (ICB-CSIC), Zaragoza, Spain
2Microbiology, Hospital Miguel Servet, Zaragoza, Spain
3Oncology Pediatric, Hospital Miguel Servet, Zaragoza, Spain
4Centro De Investigaciones Biomedicas, Universidad De Zaragoza, ARAID, Zaragoza, Spain
P152. Analysis of the efficacy of bis(methyl)gliotoxin for early invasive aspergillosis
detection and to guide antifungal treatment in neutropenic pediatric oncology patients.
Objectives: Invasive pulmonary aspergillosis (IPA) is a leading cause of morbidity
and mortality in immunocompromised patients. This is due in part to the absence of
optimal diagnostic modalities, which hamper early specific disease detection. Systemic
detection of serum biomarkers is a standard noninvasive practice used to early diagnose
and treat IPA in high risk population. During the last years, doubts have been raised
regarding the use of galactomannan (GM) in onco-hematological population mainly due
to the large number of patients with false positive test as well as the low efficacy
in patients under antifungal profylaxis. In a previous study, we demonstrated the
high diagnostic accuracy of bis(methyl)gliotoxin (bmGT) compared to GM for IPA diagnosis
in adult oncohematological patients. However, the performance of bmGT as a diagnosis
biomarker in pediatric patients is unknown. The objective of this study is to compare
the ability of bmGT and GMN detection for IPA diagnosis in oncopediatric patients
and to analyse the efficacy of bmGT to serve as guide for voriconazol treatment.
Methods: The presence of bmGT and voriconazole levels in sequential series of serum
from 7 oncopediatric patients at risk of IA (prospective samples) is simultaneously
quantified by High Performance Thin Layer Chromatography (HPTLC) as well as LC/MS/MS.
Results: We have established a fast and sensitive technique for simultaneous separation,
detection and quantification of bmGT and voriconazole in serum and BAL of pediatric
patients. bmGT and GM were negative in all possible IA cases (5 patients). Two patients,
presenting prolonged neutropenia after chemotherapy treatment, fever, hypoxemia and
pulmonary infiltration compatible with Aspergillosis in high resolution CT, were categorised
as probable IA. GM and bmGT was monitored in serum and bronchoalveolar lavage. In
one patient, GM and bmGT were detected in serum and with the voriconazole treatment
GM was negativized and the bmGT levels decreased. bmGT was positive earlier than GM
allowing early initiation of treatment. In the second patient, GM was negative and
bmGT positive in both serum and BAL, so treatment with voriconazole was started. Treatment
decreased bmGT values coinciding with the patient clinical improvement.
Conclusion: BmGT has allowed us the early diagnosis and successful treatment of pediatric
IA, being a promising tool for early detection of IA in neutropenic pediatric patients.
Choi
J.-K.
1
2
Cho
S.-Y.
1
2
3
Park
C.
2
Lee
D.-G.
1
2
3
1Division of Infectious Diseases, Department of Internal Medicine, College of Medicine,
The Catholic University of Korea, Seoul, Republic of Korea
2Vaccine Bio Research Institute, Seoul, Republic of Korea
3Catholic Hematology Hospital, Seoul, Republic of Korea
P153. Identification for major Aspergillus species in paraffin-embedded tissue: newly
developed multiplex real-time PCR method using hydrolysis probes
Objectives: Species identification is crucial for determining appropriate antifungal
agents in treatment of fungal infections. However, current culture-based methods are
insensitive due to low positive rate and slow process. Microscopic examination of
tissue specimens has limitations in distinguishing fungal organisms. Detection of
fungal DNA by polymerase chain reaction (PCR) method in tissue samples is expected
to be useful for the diagnosis of fungal infections. Herein, we describe the performance
and clinical impact of a multiplex fungal PCR assay of formalin-fixed paraffin-embedded
(FFPE) sample for the diagnosis of Aspergillus infections.
Methods: FFPE samples previously collected from patients diagnosed as fungal infections
by histopathologic finding between June 2009 and June 2012 were used for PCR analysis,
and retrospective chart review was also performed for correlation with clinical diagnosis.
Real-time PCR primers and multiplex hydrolysis probes were newly designed for four
major Aspergillus species (A. fumigatus, A. niger, A. terreus, and A. flavus) based
on molecular analysis data of β-tubulin gene (benA) in Aspergillus isolates. The method
of real-time PCR was qualified and quantified using the reference genomic DNA (gDNA)
of Aspergillus species. DNA was extracted from FFPE sample using TaKaRa DEXPAT kit
(TaKaRa Biomedicals, Shiga, Japan). The amplified benA amplicon was analyzed by comparing
with the standard curve of the reference gDNA. Multiplex PCR was performed on 72 FFPE
samples. The sample quality was validated by human globulin detection, and 61 qualified
samples were analyzed.
Results: Twenty-seven of 35 cases clinically suspected as aspergillosis presented
positive results in this real-time PCR method (sensitivity: 77.1%). The positive rate
was higher than that of the fungal culture (12/35, 34.3%) or galactomannan test (20/33,
60.6%). Among the 23 cases with culture-negative proven/probable invasive aspergillosis,
17 cases were confirmed as positive by real-time PCR. Aspergillus gene was identified
in 5 of 9 IFI cases that causative fungal organisms were not confirmed in clinical
and pathological examination. In cases with non-Aspergillus infection, Aspergillus
gene was detected in 2 of 13 cases. By real-time PCR for FFPE specimen, additional
informations, not only detection of benA gene, but also species-level identification,
were obtained in 51.7% (32 of 61) cases included in this study.
Conclusion: This study revealed that this newly developed real-time PCR method for
FFPE tissue could improve diagnostic accuracy of aspergillosis. Further study is needed
to understand mixed mold infections or false positive cases with additional assays
including PCR for non-aspergillus mold.
Kutsevalova
O.
1
Antonets
A.
2
Kit
O.
3
Panova
N.
1
Lysenko
I.
4
Dmitrieva
V.
3
Pak
E.
3
Kozyuk
O.
3
Marykov
E.
3
Klyasova
G.
5
1Laboratory of Clinical Microbiology, Rostov Research Institute of Oncology, Rostov-on-Don,
Russian Federation
2Laboratory of Molecular Oncology, Rostov Research Institute of Oncology, Rostov-on-Don,
Russian Federation
3Rostov Research Institute of Oncology, Rostov-on-Don, Russian Federation
4Oncohematology Department, Rostov Research Institute of Oncology, Rostov-on-Don,
Russian Federation
5National Research Center for Hematology, Moscow, Russian Federation
P154. New approaches to the verification of bacterial and candidal bloodstream infection.
Objectives: To evaluate the prognostic significance of procalcitonin and mannan antigen
of Candida spp (MA) for early and accessible verification of the causative agent of
bloodstream infection (BSI).
Methods: We examined 349 patients with suspected BSI from 16 intensive care, oncology
and oncohematology units of 9 hospitals in the Southern Federal District of Russia.
The diagnostic algorithm included sterility blood testing and a study of biochemical
markers with immunoenzymatic assays. Sterility blood testing was performed using ‘BacT/ALERT3D
analyzer. Identification of strains and detection of their antimicrobial drugs sensitivity
was made with Vitek-2 automatic analyzer (BioMerieux, France). Simultaneously we measured
the levels of procalcitonin and MA with the use of Procalcitonin kit ELISA-BEST (Russia)
and Platelia™ Candida Ag Plus kit (France) correspondingly. Only high values of procalcitonin
(10 and above ng/ml) were considered as expedient in the diagnosis of bacterial sepsis.
The measurement of MA made it possible to judge about the involvement of Candida spp.
in the infectious process. The result of MA testing was regarded as positive with
the concentration ≥125 pg/ml.
Results: Positive blood cultures were obtained in 84 (24.1%) patients. Bacteria accounted
for 77.4% (65 strains), Candida spp. - 22.6% (19 isolates). In 3 cases (3.6%) bacterial-candidal
associations were detected which worsened the condition of the patients. In a parallel
study of biochemical markers, an increased level of one of them was in 205 (58.7%)
patients. High values of procalcitonin accounted for 68 (33.2%) that was in favor
of a severe bacterial infection. Positive results for MA were in 118 (57.6%) patients.
Positive results of MA testing in the presence of clinical symptoms and risk factors
for the development of invasive candidiasis suggested a candidal BSI. In 19 (9.2%)
patients two biochemical markers were elevated indicating a possible mixed infection
(picture 1,2). For 144 (41.3%) patients with initial negative blood culture testing
and normal biomarker levels additional studies were needed to exclude or confirm BSI.
As a result, in 26 patients a negative results were regarded as a cancer relapse.
118 patients needed dynamic blood testing. 70 patients received a positive results.
14 of them had high procalcitonin and 56 had a positive MA. 18 patients also had a
positive blood culture (4 patients had bacteria isolated, 14 - candida). 52 patients
were observed with only positive biomarkers (10 with procalcitonin, 42 with MA). The
rest 48 patients were with fever of unknown origin.
Conclusion: A comprehensive approach to the diagnosis of BSI has increased the percentage
of pathogen verification to 58.7%. It should be taken into consideration that both
blood culture and biomarkers testing of bacterial infection revealed almost the same
diagnostic significance. However, MA testing improved dramatically the early diagnosis
of a candidal BSI despite negative blood culture testing which is probably due to
the prolonged cultivation of Candida spp. in the blood. The inclusion of biochemical
markers testing in the diagnostic algorithm for suspected BSI allowed to identify
the causative infectious agent as early as possible and to start adequate antibacterial
or antifungal therapy.
Jabeen
K.
1
Ghanchi
N.
2
Farooqi
J.
2
Masood
K. Iqbal
2
Siddiqui
O. Ali
2
Humayun
A.
2
Hasan
Z.
2
1Pathology and Laboratory Medicine, Aga Khan University, Karachi, Pakistan, Pakistan
2Pathology and Laboratory Medicine, Aga Khan University, Karachi, Pakistan
P155. Validation and ongoing evaluation of a real time PCR assay for Pneumocystis
jirovecii detection in bronchoalveolar fluid specimens; data from Pakistan
Objectives: Pneumocystis pneumonia (PCP) is a frequent opportunistic infection in
HIV-positive and immunocompromised individuals. Healthy individuals have been shown
to harbor the organism without clinical signs of infection and may play a role in
the transmission of PCP. PCR based assays for detection of P. jirovecii are reported
to be more sensitive compared to conventional immunofluorescence assays (IFA). We
report experience of a tertiary care hospital laboratory in Pakistan which recently
shifted from IFA based to PCR based diagnosis for PCP pneumonia.
Methods: The study was conducted at the clinical laboratory of Aga Khan University
Hospital, Karachi Pakistan. In the first phase (January-May 2018), a real time PCR
assay was validated against twenty archived PCP positive or negative bronchoalveolar
lavage (BAL) specimens. Once validation was completed satisfactorily, PCR based assay
was introduced and offered to clinicians in June 2018. All BAL specimens submitted
for detection for PCP from June 2018-May 2019 after the introduction of test were
included and evaluated. Clinical data collected as routine part of reporting was assessed.
Positivity rate for Pneumocystis detection was evaluated for PCR during this period.
Additionally PCP data using IFA was retrieved for the year 2016-2017 to compare the
positivity rate of IFA with PCR over these two time periods.
Results: Initially twenty samples from patients clinically suspected of PCP were compared
using both IAF and PCR. PCR confirmed five (25%) positive cases compared to IAF which
confirmed 3(12%). After introduction of PCR in the laboratory, a total of 145 suspected
patient BAL samples were tested for P. jirovecii of which 25 (17%) were PCR positive.
Majority of the PCR confirmed patients were male (72%). Clinical data available on
18/25 (72%) positive patients revealed 4 (16%) patients positive for human immunodeficiency
virus; 3 (12%) with underlying autoimmune disease; 6 (20%) with malignancy, 2 (4%)
on corticosteroid therapy and 1 patient on immunosuppressive therapy post renal transplant.
PCP detection increased from 4.5% during 2016-2017 using IFA based assay to 17% using
PCR based assay during June 2018-May 2019.
Conclusion: A high positivity rate of 17% was seen in clinically suspected PCP patients
using PCR based diagnosis in this study. PCP cases were reported in both HIV positive
and negative patients. There was an improvement in case detection using PCR compared
to IFA. However, high cost of PCR compared to IFA limits wider availability of PCR
based PCP diagnosis in resource limited settings.
Lim
W.
1
Smit
S.
2
Fahal
A.H.
3
Sande
W. Van De
4
1Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, Netherlands
2Department of Plant Science - Bioinformatics, Wageningen University & Research, Wageningen,
Netherlands
3Mycetoma Research Center, University of Khartoum, Khartoum, Sudan
4Department of Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam,
Netherlands
P156. A comparative genome approach to identify specific DNA sequence for PCR diagnosis
of Madurella mycetomatis
Objectives: The current gold standard of identifying eumycetoma causative agents is
to culture the isolate and assess the morphological characteristics. This method of
identification is time consuming and can often lead to misidentifications. Precise
and early detection of the causative agent is crucial for clinical management. Currently
precise species identification can only be achieved by sequencing the internally transcribed
spacer (ITS) regions or by using species-specific PCRs. Only for M. mycetomatis a
species specific PCR was developed, but recently it was demonstrated that this PCR
did cross-react with other Madurella species. We therefore looked into the sequenced
M. mycetomatis genome in search for unique and specific protein coding sequences which
could be transformed into a species specific diagnostic PCR.
Methods: We used a comparative genome approach to identify unique sequences for M.
mycetomatis based on its predicted protein coding sequences. The homology of these
sequences were identified using blastclust, localization of the proteins was identified
using WolfPSORT and SignalP was used to predict the secretion signals in these predicted
proteins sequences. Based on these sequences, PCR primers were developed and validated
in a collection of 61 M. mycetomatis, 4 Madurella tropicana, 3 Madurella pseudomycetomatis,
3 Madurella fahalii, 2 Falciformispora senegalensis, 3 Trematosphaeria subthermophilia,
3 Thielavia terrestris, 3 Trematosphaeria grisea, 2 Chaetomium globosum, 1 Aspergillus
fumigatus and 1 Aspergillus terrus isolates using conventional PCR.
Results: By using a comparative genomic approach, we were able to identify at least
32 M. mycetomatis unique protein sequences. For the top 16 most promising candidate
sequences, PCR primers were developed. of these primer pairs, only 12 were able to
detect all M. mycetomatis isolates tested. of these 12, only 1 did not react with
any other fungal species tested.
Conclusion: Based on this comparative genomics approach, we identified a novel M.
mycetomatis specific PCR primer pair to detect M. mycetomatis.
Lamoth
F.
1
Nguyen
M.H.
2
Tissot
F.
1
Squires
K.
2
Eggimann
P.
1
Flückiger
U.
3
Siegemund
M.
3
Zimmerli
S.
4
Calandra
T.
1
Marchetti
O.
1
Clancy
C.J.
2
Bochud
P.-Y.
1
1Infectious Diseases Service, Lausanne University Hospital, Lausanne, Switzerland
2University of Pittsburgh Medical Center, Pittsburgh, United States of America
3University Hospital of Basel, Basel, Switzerland
4Department of Infectious Diseases, University Hospital of Bern, Bern, Switzerland
P157. Performance of T2Candida Panel for the diagnosis of intra-abdominal candidiasis
Objectives: Intra-abdominal candidiasis (IAC) is a frequent and life-threatening infection
in critically ill patients following complicated abdominal surgery. Diagnosis of IAC
is difficult because of the lack of sensitivity of blood cultures and the limited
specificity of indirect markers, such as the beta-D-glucan (BDG) test. The T2Candida
Panel (T2C, T2Biosystems) is a magnetic resonance-based molecular diagnostic test
that identifies the five most common pathogenic Candida spp, (C. albicans and C. tropicalis
[CA/CT], C. glabrata and C. krusei [CG/CK], and C. parapsilosis) directly in whole
blood specimens within 7 hours. T2C demonstrated excellent performance for the diagnosis
of candidemia, but data for non-candidemic invasive candidiasis are lacking. We tested
T2C’s efficiency in detecting IAC.
Methods: We used the T2C panel to test frozen whole blood samples from critically-ill
patients previously enrolled in a prospective cohort study of the Fungal Infection
Network of Switzerland (FUNGINOS), who were at high risk for IAC due to at least one
of the following conditions: i) recurrent gastro-intestinal tract perforation, ii)
abdominal surgery and Candida colonization, iii) necrotizing pancreatitis or iv) burns
(>25% body surface area). IAC was defined as growth of Candida spp. in a per-operative
culture sample from an intra-abdominal abscess or peritoneal fluid. The samples were
collected within 72h of IAC diagnosis or between days 7-10 from study inclusion for
patients without IAC. Results of concomitant serum BDG (Fungitell) determined previously
were included in the analysis.
Results: Whole blood samples from 46 patients were available for testing. Most patients
had Candida colonization of ≥1 non-sterile site (98%) and Candida score ≥3 (89%).
Eighteen (33%) of them had IAC: 13 were due to CA, 2 to CT, 1 to mixed CA/CG and 2
to Candida spp. not included in the T2C Panel (C. kefyr and C. lusitaniae). Antifungal
therapy was ongoing at time of IAC diagnosis and blood sampling in 2 (11%) cases.
Table 1 shows the comparative diagnostic performance of blood cultures, T2C and BDG.
Blood cultures were positive in 2/18 (11%) IAC cases. T2C was positive in 6/18 (33%)
IAC cases (including the 2 candidemic patients) with Candida spp. matching those isolated
in cultures. Analysis limited to IAC cases due to Candida spp. of the T2C panel yielded
a sensitivity of 37.5%. A positive T2C result was found in 2 of the 28 patients who
did not fulfill IAC criteria (specificity 93%). While discordant results for T2C and
BDG were observed in 21 (46%) cases, concordant positive results were associated with
IAC in 100% of cases and concordant negative results with absence of IAC in 90% of
cases.
Conclusion: T2C has higher sensitivity than blood cultures and higher specificity
than BDG for IAC diagnosis. T2C and BDG are anticipated to be most useful in guiding
management of patients at risk for IAC, in particular when they provide concordant
results to confirm or exclude IAC in cases for which intra-abdominal cultures are
not feasible or delayed.
Nawel
A.-A.
1
Foulet
F.
1
Angebault
C.
1
Suyyagh
S.
1
Maubon
D.
2
Botterel
F.
1
1Parasitologie-mycologie, CHU Henri MONDOR, Créteil, France
2Univ. Grenoble Alpes, CNRS, CHU Grenoble Alpes, Grenoble INP*, TIMC-IMAG, 38000 Grenoble,
France * Institute of Engineering Univ. Grenoble Alpes, GRENOBLE Cedex, France
P158. Performances of the GenMark’s ePlex® blood culture identification fungal pathogen
(BCID-FP) panel: a prospective French evaluation
Objectives: Fungemia is associated with high rate of morbidity increasing length of
hospital stay and mortality. Delay of effective antifungal treatment is associated
with unfavorable outcome and rapid species identification contributes to adapt antifungal
therapy. ePlex® Blood Culture Identification fungal pathogen panel (ePlex® BCID-FP)
developed by GenMark® Diagnostics, Inc. (Carlsbad, CA, USA) is a fully automated easy-to-use
cartridge designed to detect 15 fungal pathogens from positive blood culture (BC)
including the emerging pathogen Candida auris. The objective of this study was to
evaluate prospectively the performance of ePlex® BCID-FP test using clinical BC samples.
Methods: The evaluation was performed prospectively on consecutive fungal BC over
2 years-period (from April 2017 to Mars 2019) in Henri Mondor University Hospital
in France. BCs taken from patients were incubated in BacT/ALERT® 3D automated system
(Biomérieux®). Once detected positive for fungi, the BC underwent classical subcultures.
Then, species identification was performed on MALDI-TOF MS Andromas® system (identification
within 24h). In parallel to this conventional procedure, ePlex® BCID-FP was tested
(hands-on-time < 5 min and time-to-result: 1.5h).
Results: A total of 58 BC, corresponding to 52 patients, were tested on ePlex® using
the BCID-FP. Invalid results due to technical issues were obtained in 10/58 tests
(17.2%). Among the 48 valid tests, results were fully concordant with classical identification
process in 45/48 BC isolated species (94%) including one fungemia caused by two species
with C. tropicalis and C. lusitaniae. Identifications were available the day of BC
positivity in less than two hours. This technique saved at least 24 hours compared
to conventional methods. The non-concordant results consisted on one C. parapsilosis
missed by ePlex and two species absent from the BCID-FP panel (1 C. metapsilosis and
1 Saccharomyces cerevisiae). Species distribution on valid tests was: 19 C. albicans
(41%), 12 C. parapsilosis (26%), 8 C. glabrata (18%) and 7 others (C. krusei (n =
3), C. tropicalis (n = 2), C. dublininiensis (n = 1) and C. lusitaniae (n = 1) (15%).
Thus, fluconazole could be introduced in first intention in 76% of cases, saving the
use of caspofungin.
Conclusion: To our knowledge, this is the first large prospective evaluation of the
ePlex® BCID-FP on more than 50 fungal BC. ePlex® BCID-FP accurately identified 78%
of the fungal species from positive BC. Focusing on targets which are currently present
in the panel, 100% of correct identification was achieved. A prospective clinical
study evaluating the time-to-result benefit on antifungal stewardship and on length
of hospital stay is needed.
Zoran
T.
1
2
Seelbinder
B.
1
Sieber
P.
1
Schaeuble
S.
1
Loeffler
J.
2
1Pidomics, Leibniz Institute for Natural Product Research and Infection Biology -
Hans Knoell Institute, Jena, Germany
2Department of Internal Medicine Ii, WÜ4i, University Hospital Wuerzburg, Wuerzburg,
Germany
P159. Integrating miRNA and mRNA expression profiles for improving diagnosis of invasive
aspergillosis
Objectives: Invasive aspergillosis (IA) is a life-threatening disease affecting immunocompromised
patients. Due to unspecific symptoms of this disease and limitation of current methods
based on fungal biomarkers, diagnosis of IA remains challenging. MicroRNAs (miRNAs)
are small non-coding RNAs involved in the post-transcriptional regulation of several
biological processes including various disease pathways and immune response to numerous
pathogens. Due to their involvement in gene regulation, their stability and ubiquitous
presence in biofluids they are considered as good biomarkers for different diseases.
The aim of our in vitro study was to investigate miRNA profiles in immune cells following
fungal and bacterial exposure and identify potential miRNAs and their target genes
that may be useful as diagnostic and prognostic human biomarkers for improving IA
diagnosis.
Methods: We used next generation sequencing (NGS) to investigate miRNA profiles in
monocyte-derived dendritic cells (moDCs) generated from healthy volunteers. Immune
cells were stimulated for 12 hours with inactivated Aspergillus fumigatus germ tubes
and a clinical isolate of Escherichia coli. Next, we evaluated transcriptome by RNA-sequencing
of moDCs infected with E. coli and A. fumigatus and investigated in silico interaction
between differentially expressed miRNAs (DEMs) and their targets. After correlation
analysis and prediction of interactions with mRNA, selected miRNA and their immunological
relevant target genes were further experimentally investigated.
Results: Our data showed 32 significantly DEMs in moDCs stimulated with fungal and
bacterial pathogens in comparison to normal expression in unstimulated moDCs. 28 miRNAs
were differentially regulated after infection with A. fumigatus and 8 miRNAs after
infection with E. coli. We identified 24 miRNAs significantly differentially regulated
in moDCs after fungal but not bacterial infection. Among all significantly DEMs in
stimulated moDCs, the following four miRNAs were observed to be differentially expressed
after infection with A. fumigatus and E. coli: hsa-miR-9-5p, hsa-miR-155-5p, hsa-miR-155-3p
and hsa-miR-1303. Based on two DEMs specific to A. fumigatus exposure and one DEM
identified in moDCs infected with A. fumigatus as well as E. coli, we further investigated
potential targets that had been predicted by investigating transcriptome data. Integration
of hsa-miR-9-5p and gene expression showed 45 common differentially expressed (DE)
target genes in both types of infection. Furthermore, our data showed 26 unique DE
target genes in moDCs after exposure to A. fumigatus and 13 in moDCs infected with
E. coli (n = 13). We validated the expression of three selected DEMs and their selected
immunological relevant target genes in additional donors with Real-Time PCR, where
we confirmed our observations from NGS data.
Conclusion: Our data showed distinctive miRNA profiles between uninfected moDCs as
well as moDCs after exposure to A. fumigatus and E. coli, suggesting miRNAs profiles
can be used to detect and distinguish bacterial from fungal infection. Furthermore,
characteristic miRNA and mRNA expression profiles may be used as a diagnostic and
prognostic biomarker of IA. Further experimental validation is necessary to confirm
predicted miRNA-mRNA interactions and investigate the potential use of miRNA and mRNA
expression profiles for IA diagnosis.
Gits-Muselli
M.
1
2
White
P.
3
Mengoli
C.
4
Chen
S.
5
Crowley
B.
6
Dingemans
G.
7
Frealle
E.
8
Gorton
R.
9
Guiver
M.
10
Hagen
F.
11
Halliday
C.
12
Johnson
G.
13
Lagrou
K.
14
Lengerova
M.
15
Melchers
W.
16
Novak-Frazer
L.
17
Rautemaa-Richardson
R.
18
19
Scherer
E.
20
Steinmann
J.
21
22
Cruciani
M.
23
Barnes
R.
24
Donnelly
J.P.
25
Loeffler
J.
26
Bretagne
S.
27
Alanio
A.
27
1Molecular Mycology, Institut Pasteur, PARIS, France
2Parasitology-mycology Laboratory, Lariboisière Saint-Louis Fernand Widal Hospital,
Assistance Publique-Hôpitaux de Paris (AP-HP), PARIS, France
3Microbiology, Public Health Wales Microbiology, Cardiff, United Kingdom
4Molecular Medicine, University of Padova, Padova, Italy
5Medicine, The University of Sydney, Camperdown, New South Wales, Australia
6Department of Virology, St James’s Hospital, Dublin, Ireland, Dublin, Ireland
7PathoNostics, Maastricht, Netherlands
8Parasitology-mycology, CHU Lille, Lille, France
9Infection Sciences, Health Services Laboratories, London, United Kingdom
10Public Health Laboratory, National Infection Service Public Health England, Manchester
University NHS Foundation Trust, Manchester, UK, Manchester, United Kingdom
11Medical Mycology, Westerdijk Fungal Biodiversity Institute, Utrecht, Netherlands
12Clinical Mycology Reference Laboratory, Centre for Infectious Diseases and Microbiology
Laboratory Services, Institute of Clinical Pathology and Medical Research, New South
Wales Health Pathology, Westmead Hospital, and the University of Sydney, Australia,
Sydney, Australia
13Scientific Development, OLM diagnostics, Newcastle, United Kingdom
14Laboratory of Clinical Bacteriology and Mycology, Department of Microbiology and
Immunology, Excellence Center For Medical Mycology (ecmm), KU Leuven, Leuven, Belgium
15Department of Internal Medicine - Hematology and Oncology, University Hospital Brno,
Brno, Czech Republic
16Medical Microbiology, RadboudUMC, Nijmegen, Netherlands
17Mycology Reference Centre, Excellence Center For Medical Mycology (ecmm), Wythenshawe
Hospital, Manchester University NHS Foundation Trust, Manchester, United Kingdom
18Division of Infection, Immunity and Respiratory Medicine, University of Manchester,
Manchester, United Kingdom
19Manchester Academic Health Science Centre, Manchester, United Kingdom
20Parasitology - Mycology - Umr6249 Cnrs Chrono-environnement, University Hospital
Besançon, Besançon, France
21Institute of Clinical Hygiene, Medical Microbiology and Clinical Infectiology, Klinikum
Nuernberg, Nuremberg, Germany
22Institute of Medical Microbiology, University Hospital Essen, Essen, Germany
23Infectious Diseases, San Bonifacio Hospital, Verona, VERONA, Italy
24Medical Microbiology and Infectious Diseases, Cardiff University School of Medicine,
Cardiff, United Kingdom
25EAPCRI, Nijmegen, Netherlands
26Department of Internal Medicine Ii, WÜ4i, University Hospital Wuerzburg, Wuerzburg,
Germany
27Paris-diderot, Sorbonne Paris Cité University, Institut Pasteur, Molecular Mycology
Unit, Cnrs Cmr2000, Parasitology-Mycology Laboratory, Lariboisière Saint-Louis Fernand
Widal Hospitals, Assistance Publique-Hôpitaux de Paris, Paris, France
P160. rThe Fungal PCR Initiative’s evaluation of in-house and commercial Pneumocystis
jirovecii qPCR assays: towards a standard for a diagnostics assay
Objectives: Quantitative real-time PCR (qPCR) is increasingly used to detect Pneumocystis
jirovecii for the diagnosis of pneumocystis pneumonia (PCP). PCP is more severe and
mortality rates are significantly higher in non-HIV patients than in HIV-infected/AIDS
people, despite a lower fungal load during infection. Currently, definite thresholds
for Pneumocystis pneumonia (PCP) in non-HIV patients have not been established to
be consensual and universally used in different diagnostic laboratories. Therefore,
diagnosis of PCP remains a challenge because of the importance of quantification of
the low fungal loads. An evaluation of the performance of different qPCR assays is
needed to assist laboratory standardization of quantification.
Methods: Through the Fungal PCR Initiative, a working group of the International Society
for Human and Animal Mycology, one inter- and one intra-laboratory comparison of qPCR
assays was performed. For the interlaboratory study, sixteen reference laboratories
in eight countries running 19 assays analysed a panel consisting of two negative and
three positive specimens from a pool of three different P. jirovecii positive bronchoalveolar
lavage (BAL) fluids at three different dilutions (pure 1:1, 1:100 and 1:1000). Participants
were asked to test the panel specimens as part of their routine molecular biology
workflow. The results were collected using a dedicated Google form that included requests
for the qualitative result (positive/negative), the quantification cycle (Cq) values
for each replicate related to each specimen and specific information regarding the
methods and workflows applied. The intra-laboratory study compared 10 different assays
with the same person and qPCR thermocycler, by testing 10 previously confirmed P.
jirovecii qPCR-negative and 9 previously confirmed P. jirovecii qPCR-positive BALs.
Those 10 assays consisted of 5 in-house assays and 5 commercial kits and included
3 RT-qPCR (1 commercial and 2 in-house) and 7 qPCR assays (4 commercial and 3 in-house)
Results: from 20 qPCR assays (16 qPCR amplifying DNA only and 4 RT-qPCR assays amplifying
Whole nucleic acids (WNA, i.e DNA+RNA) were obtained from 16 laboratories across eight
countries (including seven European countries and one Australian). The different targets
evaluated were mtSSU (n = 2), mtLSU (n = 16), MSG (n = 1) and beta-tubulin (Tub, n
= 1). The inter-laboratory analytical sensitivity was 100% at 1:1 genomic load, 95%
at 1:100, and 82.5% at 1:1000. Analytical specificity was 100% for all assays but
yielded a false-positive test (95% specificity). For both evaluations and for all
genomic loads, testing whole nucleic acid (RNA plus DNA) using reverse-transcriptase
RT-qPCR was superior to qPCR (p≤0.001) testing DNA only. (Figure 1 and Figure 2).
The target gene mitochondrial small sub-unit (mtSSU) was significantly more sensitive
than the mitochondrial large subunit, the major surface glycoprotein, or the beta-tubulin
(Figure 1 and Figure 2).
Conclusion: Thus, RT-qPCR targeting the mtSSU gene had the best sensitivity and could
serve as a basis for standardizing the P. jirovecii load, which is essential if qPCR
is to be incorporated into clinical care pathways.
Brown
L.
1
Rautemaa-Richardson
R.
1
2
3
Novak-Frazer
L.
1
3
Hill
S.
1
3
Hassan
D.
3
Richardson
M.D.
1
3
1Division of Infection, Immunity and Respiratory Medicine, University of Manchester,
Manchester, United Kingdom
2National Aspergillosis Centre, Manchester University NHS Foundation Trust, Manchester,
United Kingdom
3Mycology Reference Centre, Excellence Center For Medical Mycology (ecmm), Wythenshawe
Hospital, Manchester University NHS Foundation Trust, Manchester, United Kingdom
P161. Identification of Cyp51A-mediated triazole resistance in Aspergillus fumigatus
using pyrosequencing: an audit of UK National Aspergillosis Centre cases
Objectives: Globally, triazole resistance rates are rising. Azole-naïve patients acquiring
an azole-resistant isolate from the environment, or because of long courses of azole
treatment is a growing concern. TR34/L98H within the cyp51A gene of A. fumigatus leads
to pan-triazole resistance and is the most common mutation found in environmental
isolates. Other mutations in this gene have also been associated with various patterns
of azole resistance. Failure of microbiological testing to detect resistance delays
the initiation of appropriate antifungal therapy and negatively impacts the clinical
outcomes of patients with aspergillosis. Therefore, empiric combination therapies
are increasingly used which has significant stewardship implications. We compared
the performance of high-volume culture (HVC) and Aspergillus fumigatus PCR combined
with pyrosequencing (AfPCR-Pyro) for the detection of triazole resistance in aspergillosis
patients.
Methods: We performed a retrospective audit over a 27-month period (between January
2017 and March 2019) at the Mycology Reference Centre Manchester (MRCM) and the UK
National Aspergillosis Centre (NAC), UK. Respiratory samples collected from patients
with chronic pulmonary aspergillosis (CPA) or allergic bronchopulmonary aspergillosis
(ABPA) and analysed by both HVC and AfPCR-Pyro were included in the study. Hospital
and laboratory databases were searched for patient history, laboratory findings and
if the pyrosequencing result triggered a change in antifungal treatment.
Results: Three hundred and forty-nine samples from 162 patients were analysed for
susceptibility using AfPCR-Pyro. of these 28% (96/349) were positive for A. fumigatus
by HVC while 68% (236/349) were reported with no growth after 14 days. The remaining
3% (9/349) of samples were positive for other Aspergillus spp, but excluded from further
analysis as pyrosequencing is limited to A. fumigatus. Resistance was found by pyrosequencing
in 29% (99/340) of samples compared to 15% (52/340) by HVC. Resistance was found by
both culture and pyrosequencing in 9% (30/340) of cases, with agreement in susceptibility
profiles between the two methods in 66% (63/96) of cases. Resistance was identified
by pyrosequencing in 23% (54/236) of culture negative samples. If pyrosequencing had
not been used in these patients, 46 resistant cases would have been missed. Pyrosequencing
identified the environmental TR34/L98H mutation in 20% (69/340) of samples. Importantly,
resistance was recognised by pyrosequencing before HVC results in 17% (57/340) of
cases. A change in antifungal therapy was initiated in 19% of cases (58/299) due to
pyrosequencing results.
Conclusion: Compared to HVC, AfPCR-Pyro has a higher sensitivity for detecting triazole
resistance. The technology allows for prompt recognition of resistant organisms and
the selection of appropriate antifungal treatment. Increased availability of pyrosequencing
would likely lead to improved patient outcomes and better antifungal stewardship.
Kristensen
L.
1
Gertsen
J.B.
1
Petersen
T.M.
1
Handberg
K.
1
Normand
A.C.
2
Hendrickx
M.
3
Nørskov-Lauritsen
N.
1
1Department of Clinical Microbiology, Aarhus University Hospital, Aarhus N, Denmark
2Ap-hp, Groupe Hospitalier Pitié-salpêtrière, Service de Parasitologie Mycologie,
Paris, France
3Service of Mycology and Aerobiology, Bccm/ihem Fungal Collection, Sciensano, Brussels,
Belgium
P162. Evaluation of two mass spectrometry platforms, the Bruker MALDI Biotyper and
the MSI online application, for the identification of clinically important moulds
Objectives: The matrix-assisted laser desorption ionisation time-of-flight mass spectrometry
(MALDI-TOF MS) method has emerged as a rapid identification method of filamentous
fungi. The aim of this study was to evaluate the performance of the Bruker MALDI Biotyper
and the recently introduced MSI (mass spectrometry identification) platform to identify
a collection of moulds, followed by an evaluation in the clinical setting.
Methods: Thirty-four well-characterised mould isolates (18 species from 10 genera)
were selected from our collection of fungal strains. For the subsequent evaluation,
75 clinical isolates were prospectively collected. Aspergillus fumigatus and Aspergillus
flavus with typical morphology were excluded. The isolates were cultured 2-3 days
on Sabouraud glucose agar (Oxoid). Prior to MS analysis, a simple ethanol-formic acid-acetonitrile
inactivation/extraction procedure was used. Acquisition of mass spectra was performed
with the Bruker MALDI Biotyper, software v. 3.1. Data analysis was performed using
the general Bruker library (MBT 7311 MSP) combined with the Bruker Fungi Library 1.0,
and with the MSI online library after uploading of spectra. In the initial assessment,
identification thresholds of 2.0 and 1.7 for species and genus, respectively, were
used. The data were compared to a species identification threshold of 1.7. Based on
these results the 1.7 threshold for species identification was applied in the subsequent
evaluation.
For MSI online identification, a species identification threshold of 20 was used.
Identification by sequencing of internal transcribed spacer (ITS2) was used as gold
standard.
Results: Initial evaluation
ITS sequence identified 25 isolates to the species level and nine isolates to the
species complex level (insufficient separation of closely related species).
Bruker (species threshold 2.0): 22 of 34 isolates (65%) were correctly identified
to the species or species complex level, six isolates (18%) were correctly identified
to the genus level, six isolates (18%) were not identified.
Bruker (species threshold 1.7): 28 of 34 isolates (82%) were correctly identified
to the species or species complex level while six isolates (18%) were not identified.
MSI: 31 of 34 isolates (91%) were correctly identified to the species or species complex
level. One Fusarium oxysporum was misidentified as Fusarium acutatum, two isolates
(6%) were not identified. Clinical evaluation
The clinical isolates comprised 19 species from 11 genera.
ITS sequence identified 55 isolates to the species level and 20 isolates to the species
complex level.
Bruker: 60 of 75 isolates (80%) were correctly identified to the species or species
complex level. Fifteen isolates (20%) were not identified.
MSI: 70/75 isolates (93%) were correctly identified to the species or species complex
level. Five isolates (7%) were not identified.
Conclusion: Both the Bruker library (species identification threshold 1.7) and the
MSI platform were accurate and reliable in the initial evaluation. The results were
confirmed in the clinical setting, however, compared to Bruker the MSI library obtained
the highest degree of identification to the species or species complex level (93%)
with a considerably lower number of unidentified isolates.
Seeth
C. Gronfors
Axelov
K. Gieng
Ullberg
M.
Özenci
V.
Clinical Microbiology, Karolinska University Hospital, karolinska Institutet, Stockholm,
Sweden
P163. Rapid identification of a rare fungi: A skillful mycologist against the state
of the art methods
Case Report: Modern diagnostics methods in clinical mycology have become widely available.
However, it is important to acknowledge that these methods cannot replace the conventional
methods requiring theoretical knowledge and hands on experience. The patient was 52
y old, female with a history of cystic fibrosis. Previous sputum samples showed growth
of Staphylococcus aureus and Pseudomonas aeruginosa. On 16 April 2019 the patient
contacted cystic fibrosis clinic and presented decreased lung capacity. The Forced
expiratory volume in one second (FEV1) was 53% expected value. Sputum sample was taken
for both bacterial and fungal culture. Bacterial cultures showed growth of Pseudomonas
aeruginosa on 17 April 2019. Growth of mold was observed after 6 days on Sabouraud
agar at 30 C. Microscopy from agar plates showed hyphi without septa. In combination
with the presence of abundant and relatively rapid growth the preliminary laboratory
diagnosis was reported as Zygomycetes spp. on 23 April 2019. One day later new microscopy
from the plates showed the presence of spherical-, multi-spored- sporangia without
columellae. The laboratory technician who had never seen a similar morphology reported
the isolate preliminary as Mortierella spp. The report was solely based on her theoretical
knowledge in combination with clinical experience. The isolate could not be diagnosed
by repetitive MALDI-TOF MS tests and was sent to internal transcribed spacer (ITS)
sequencing. The results from the ITS sequencing showed that the isolate was Umbelopsis
isabellina (99%) on 2 May 2019. The literature search showed that Umbelopsis isabellina
is used as synonym for Mortierella spp. Mortierella spp. is a mold belonging to the
order Mortierellales according to the most recent taxonomy and is considered a pathogen
solely of animals. To our knowledge, there has been only one other case report describing
an invasive disease in an immunocompromised patient with Mortierella wolfii, isolated
from the liver biopsy and blood culture (Layios et al., 2014). In that case the isolate
was identified by ITS sequencing. Therefore the present case is the first describing
the preliminary identification of Mortierella spp. with a conventional method. It
is important to note that the conventional method could identify Mortierella spp.
seven days earlier than ITS sequencing. It is still not clear if the present case
with Mortierella spp. is colonization or an infection. The patient is currently being
followed up clinically and further lower respiratory samples will be taken up. In
the laboratory it feels comfortable to have access to both conventional and modem
diagnostic methods. The present study describes the importance of hands on experience
in diagnosis of rare fungal patogens.
Rocchi
S.
1
Mengoli
C.
2
White
P.
3
Barnes
R.
4
Donnelly
J.P.
5
Loeffler
J.
6
Millon
L.
1
1Parasitology - Mycology - Umr6249 Cnrs Chrono-environnement, University Hospital
Besançon, Besançon, France
2Molecular Medicine, University of Padova, Padova, Italy
3Microbiology, Public Health Wales Microbiology, Cardiff, United Kingdom
4Medical Microbiology and Infectious Diseases, Cardiff University School of Medicine,
Cardiff, United Kingdom
5Division of Infectious Diseases, San Antonio Center for Medical Mycology, San Antonio,
United States of America
6Department of Internal Medicine Ii, WÜ4i, University Hospital Wuerzburg, Wuerzburg,
Germany
P164. An inter-laboratory multi-centre evaluation of the performance of Mucorales
PCR assays when testing serum specimens: A study by the ISHAM Fungal PCR Initiative
and the ModiMucor study group - FPCRI Mucor Laboratory Working group (A. Alanio, M.
Cogliati, S. Fuchs, F. Hagen, C. Halliday, R. Hare, C. Klaassen, M. Lackner M. Lengerova,
W. Posch, B. Sendid, J. Springer, B. Willinger) - ModiMucor study group (F. Botterel,
M. E. Bougnoux, S. Bretagne, C. Damiani, F. Dalle, J. Denis, M. Gitts-Muselli, X.
Iriart, F. Morio, P. Poirier, E. Scherer)
Objectives: Real-time qPCR detection of Mucorales DNA in serum, plasma and BAL fluid
has been shown to be sensitive and early tool for diagnosing mucormycosis. Several
qPCR assays have been developed, but little comparison and no standardization is available.
In 2016, the Mucorales Laboratory Working Group of the ISHAM Fungal PCR Initiative
(FPCRI) organised inter-laboratory evaluations of Mucorales PCR assays currently in
use, with the objectives of: 1) determining the uniformity of qualitative detection
(positive/negative) and 2) assessing qPCR performance. Participants were members of
the FPCRI, laboratories involved in the ModiMucor* study and three other French laboratories
which have already implemented this tool to diagnose mucormycosis.
Methods: For the 1st panel (A), four sera (2ml/sample) were inoculated with genomic
DNA (between 27 to 116 pg of DNA/mL of serum) from four different mucorales species
(Rhizomucor pusillus, Lichtheimia corymbifera, Cunninghamella bertholetiae, Rhizopus
oryzae). For the 2nd panel (B), six sera (2 ml/sample) were inoculated with three
concentrations of R. pusillus and L. corymbifera equivalent to one, 10 and 100 genomes/mL
of serum. For each panel, a negative control serum was also sent. Twenty laboratories
analysed the panel A (10 FPCRI, two Modimucor/FPCRI, seven Modimucor, three others
French laboratories) and 22 laboratories analysed the panel B (two more FPCRI laboratories).
All participants were requested to use their own DNA extraction methodology. The 12
French participants used the same qPCR technique (combination of three qPCR assays
targeting the four most frequent genera: Lichtheimia, Mucor, Rhizopus, Rhizomucor)
(1). For panel B, only quantitative PCR results were analysed (two techniques that
used conventional PCR were excluded). Twenty-two laboratories returned multiple datasets,
leading to 26 different datasets for analysis: - 16 datasets using a single technique
(1) - 10 datasets using other techniques (Four Pathonostics MucorGenius®, Six "in-house"
assays, (2-5), three of which were not previously described).
Results: For panel A, all datasets were negative when testing the negative control
serum. Conversely, 85-90% of the sera inoculated with “common” Mucorales (R. pusillus,
R. or yzae, L. corymbifera) were positive. Detection of sera containing C. bertholetiae
DNA was lower (12%). For panel B, the probability of qPCR positivity increased predictably
with DNA quantity, generating a mean PCR efficiency of 0.85. Detection of Lichtheimia
DNA was optimal, irrespective of the technique. Regarding the qPCR systems, Millon
et al. and MucorGenius® techniques generated greater positivity rates, with earlier
Cq values.
Conclusion: Despite the diversity of techniques, Mucorales DNA detection in sera was
very reproducible with little inter-laboratory variability providing support for including
Mucorales PCR in the EORTC/MSG definitions of invasive fungal disease and for its
use in the clinical diagnosis of mucormycosis ModiMucor*: French prospective multicenter
study for evaluation of Mucorales PCR (PHRC (Projet Hospitalier de Recherche Clinique)
national-Modimucor 2014-A00580-47.)
References 1- Millon et al. CID 2013 2- Springer et al. J. Med. Microbiol. 2016 3-
Lengerova et al. J Clin Microbiol 2014 4- Machouart et al. J Clin Microbiol 2006 5-
Hrncirova K., J Clin Microbiol 2010
Gaajetaan
G.
Kampermann
T.
Tegelen
D. Van
Dingemans
G.
PathoNostics, Maastricht, Netherlands
P165. Comparison of a point-of-care (PreventID®) with the DermaGenius® Nail real-time
PCR kit for the detection of dermatophytes species in nail samples
Objectives: Compare the performance of a point-of-care test for the detection of dermatophyte
fungi (PreventID®) with the DermaGenius® 2.0 Nail real-time multiplex PCR assay on
a set of clinical nails. Both methods are compared to microscopy as the gold standard
method.
Methods: Left over nail samples were split into different parts. The culture and microscopy
of all nail samples was performed by an external clinical microbiology laboratory.
One part of the nail sample was used for DNA-extraction with the PathoNostics Extraction
kit and identification of the pathogen was enabled by using the DermaGenius® 2.0 Nail
real-time multiplex PCR. Finally, an immunochromatographic poin-of-care test (PreventID®
Dermatophyte) was applied to determine the presence of dermatophyte-derived antigens
in the nails.
Results: Microscopy of the nails was considered as the gold standard. The DermaGenius®
2.0 Nail real-time multiplex PCR was able to detect all positive samples, resulting
in a sensitivity of 100%. Specificity was however lower (83%) due to detection of
dermatophyte DNA in microscopy-negative samples. Interestingly, these samples were
culture-positive. Culture resulted both in a reduced specificity (67%) and sensitivity
(57%). Both the sensitivity (79%) and specificity (83%) for the PreventID® Dermatophyte
test were promising. Although the PCR assay is sensitive, the clinical relevance is
still a matter of debate. High Ct-values indicate limited amounts of DNA and do not
always represent a clear infection. Interestingly, the Ct-values for samples which
were negative with the PreventID® test were between 26-33 with the DermaGenius® real-time
PCR. In addition, readability of the test strips was limited in some samples while
Ct-values of 22-28 with the DermaGenius® PCR was obtained, indicating a high fungal
load. These samples were also positive with microscopy.
Conclusion: Microscopy and the DermaGenius® 2.0 Nail real-time PCR showed a good correlation
and are useful for rapid identification of dermatophyte infection. For specific pathogen
identification, the DermaGenius® PCR can be used. Culture can result in false negatives
and in combination with a long turnaround time, it has limited value in routine diagnostics.
The use of an immunochromatographic point-of-care test is uncommon in dermatophyte
diagnostics but was included in this study to determine its suitability. Although
the PreventID® Dermatophyte test results in a sensitivity of 79%, some dermatophyte
infections with low Ct-values were missed. The difference in sensitivity could be
explained by the fact that the PreventID® test is using a protein target while the
DermaGenius® PCR detects DNA, which is normally not influenced by treatment. But more
important is that the interpretation of the PreventID® test is based on visual inspection
which makes the test less reliable. The PreventID® should be compared in a larger
sample set to determine suitability for routine dermatophyte diagnostics. Currently,
microscopy and the DermaGenius® 2.0 Nail real-time PCR seem to be the most reliable
and suitable methods for diagnosis of dermatophyte infections in nails.
Brown
L.
1
Rautemaa-Richardson
R.
1
2
Rowbotham
E.
1
Chen
S.
3
Jones
B.
4
4
1Division of Infection, Immunity and Respiratory Medicine, University of Manchester,
Manchester, United Kingdom
2Mycology Reference Centre, Excellence Center For Medical Mycology (ecmm), Wythenshawe
Hospital, Manchester University NHS Foundation Trust, Manchester, United Kingdom
3Medicine, The University of Sydney, Camperdown, New South Wales, Australia
4NHS Greater Glasgow and Clyde Health Board, Glasgow, United Kingdom
5Institute of Infection Immunity & Inflammation, University of Glasgow, Glasgow, United
Kingdom
P166. Polymerase chain reaction on respiratory tract specimens of immunocompromised
patients to diagnose Pneumocystosis - A systematic review
Objectives:
Pneumocystis jirovecii pneumonia (PCP) is a severe, life-threatening infection affecting
immunocompromised patients. The highest rates are seen in those with HIV infection,
but other groups are increasingly affected such as solid organ recipients and those
receiving long-term corticosteroid therapy. Polymerase Chain Reaction (PCR) is highly
sensitive for the detection of P. jirovecii when compared to microscopy, but may not
differentiate between infection and colonisation. The Fungal PCR Initiative group
(FPCRI) working group of the international Society for Human and Animal Mycology (ISHAM)
aims to develop international standards for P. jirovecii PCR. A systematic review
to determine the diagnostic accuracy of PCR testing in respiratory tract specimens
for PCP was undertaken.
Methods: Following protocol registration with PROSPERO, a systematic review of the
literature was performed using PubMed, Scopus, Embase and Cochrane without applying
any language or date limits. Studies where PCR techniques were used on respiratory
specimens for the diagnosis of PCP in humans were included if they (i) compared the
PCR test results with the diagnosis made using standard laboratory methods and clinical
presentation; (ii) sufficient information was provided to assess the strength of diagnosis;
(iii) results were reported as false- positive, true-positive, false-negative and
true-negative; and (iv) evaluation of the test(s) was performed in prospective cohorts
of patient populations, defined as groups of individuals at high risk for PCP. Where
relevant, the diagnostic performance of PCR techniques to the beta-d-glucan (BDG)
assay, was compared. Breakpoint analyses for differentiating colonisation from infection
will also be investigated.
Results: A total of 2843 unique records were identified. Abstracts of these records
were sent out to six pairs of reviewers to be assessed against the inclusion and exclusion
criteria. The reviewers were in agreement on 2371 abstracts; 472 abstracts with reviewer
discrepancies were sent out for a third review. After screening, 2509 records were
excluded and 334 full-text articles are now being assessed for eligibility by pairs
of reviewers. Papers meeting the inclusion criteria after reading in full will be
included in the meta-analysis to be carried out by the group. Risk of bias will be
assessed using the QUADAS-2 tool.
Conclusion: This systematic review will provide essential information about the diagnostic
accuracy of PCR tests in respiratory tract specimens for PCP required for the development
of a standardised method for Pneumocystis PCR which will be later validated in clinical
trials.
Hunter
E.
1
Richardson
M.D.
1
2
Denning
D.
1
3
1Division of Infection, Immunity, and Respiratory Medicine, University of Manchester,
Manchester, United Kingdom
2Mycology Reference Centre, Excellence Center For Medical Mycology (ecmm), Wythenshawe
Hospital, Manchester University NHS Foundation Trust, Manchester, United Kingdom
3National Aspergillosis Centre, Manchester University NHS Foundation Trust, Manchester,
United Kingdom
P167. Evaluation of the LDBio Aspergillus ICT lateral flow assay as a point-of-care
test for serological antibody detection in allergic bronchopulmonary aspergillosis
Objectives: Allergic bronchopulmonary aspergillosis (ABPA) is an immunological pulmonary
disorder complicating asthma, as a result of hypersensitization to Aspergillus species
(predominantly A. fumigatus). It is estimated to affect 2.5% of adults with asthma,
which accounts for approximately 4.8 million patients worldwide. Early recognition
and diagnosis of ABPA is critical to improve patient symptoms and prevent or delay
progression of bronchiectasis and development of chronic pulmonary aspergillosis (CPA).
It is especially important to make the distinction between asthma and Aspergillus
sensitization. LDBio Diagnostics has recently commercialized a lateral flow assay
(Aspergillus ICT) that detects Aspergillus antibodies less than 30 minutes, requiring
minimal laboratory equipment. Herein, we evaluate this assay for specific diagnosis
of ABPA as compared to asthma controls.
Methods: A retrospective, ongoing study was performed using 94 patient sera collected
at the National Aspergillosis Centre (NAC, Manchester). Patient sera were selected
based on a clinical diagnosis of ABPA and classified as ‘proven’ (n = 70) or ‘probable’
(n = 24). Control sera (n = 93) were obtained from the Manchester Allergy, Respiratory
and Thoracic Surgery (ManARTS) biobank. LDBio Aspergillus ICT was performed as per
manufacturer’s instruction. Results were read at intervals, and determined after 20
to 30 minutes as recommended (any line at the “T” marker considered positive), both
manually and using the Qiagen ESEQuant LR3 lateral flow reader. Serological Aspergillus-specific
IgG and IgE, and total IgE titres were measured by ImmunoCAP.
Results: We found the LDBIO Aspergillus ICT to have a sensitivity of 88.6% across
for 70 proven ABPA cases, and 89.4% for all cases (‘proven’ and ‘probable’) combined.
Specificity was 91.4% for 93 control sera (Table 1). A comparison of ICT result with
Aspergillus-specific IgG and IgE titres showed no evident immunoglobulin isotype bias
(Figure 1). Using digital measurements obtained using the Qiagen LR3, we found no
correlation between ImmunoCAP Aspergillus-specific IgE level and Aspergillus ICT test
line intensity. Finally, assessment of 3 assay lots using 30 samples showed perfect
agreement between lots when tests were read visually.
Conclusion: The LDBio Aspergillus ICT lateral flow assay exhibits good sensitivity
for serological screening for ABPA. The assay is easy to perform and interpret, using
minimal equipment and resources. As such, it provides a valuable point-of-care screening
resource to rapidly distinguish between asthma and Aspergillus sensitization.
Hunter
E.
1
Harris
C.
2
Richardson
M.D.
1
3
Denning
D.
1
2
1Division of Infection, Immunity, and Respiratory Medicine, University of Manchester,
Manchester, United Kingdom
2National Aspergillosis Centre, Manchester University NHS Foundation Trust, Manchester,
United Kingdom
3Mycology Reference Centre, Excellence Center For Medical Mycology (ecmm), Wythenshawe
Hospital, Manchester University NHS Foundation Trust, Manchester, United Kingdom
P168. Effect of patient immunodeficiencies on the diagnostic performance of serological
assays to detect Aspergillus-specific antibodies in chronic pulmonary aspergillosis
Objectives: Prevalence of chronic pulmonary aspergillosis (CPA) is estimated at approximately
3 million cases worldwide, and serological detection of Aspergillus-specific antibody
is a critical component in the CPA diagnostic pathway. Some patients with CPA, however,
have subtle immune deficits, including low CD4 or CD19 (B cell) counts, mannose binding
lectin deficiency and/or low gamma IFN or IL12 production. It is possible these deficits
contribute to poor Aspergillus antibody production to one or more ordinarily immunodominant
antigens, and may lead to false negative results by diagnostic assays used to detect
Aspergillus-specific antibodies. Herein, we examine the potential effect of patient
immunodeficiencies on the accuracy and performance of CPA serological diagnostics.
Methods: We analyzed patient data, as available, from 167 cases of clinically confirmed
CPA that were previously evaluated by ImmunoCAP Aspergillus-specific IgG EIA and LDBio
Aspergillus ICT lateral flow assay, to determine the presence of deficiencies in the
following: mannose binding lectin (MBL), IgG, IgA, IgM, IL12 production, IL17 production,
and/or cell markers (CD4, CD19, CD56). We compared patient data with test results
(ImmunoCAP, ICT) as well as for ImmunoCAP ‘seronegative’ patients -- defined as cases
for which ImmunoCAP Aspergillus IgG was consistently and repeatedly positive for up
to 3 years of patient history. Seronegative cases accounted for 12.6% of all cases
evaluated (n = 21). ‘Seropositive’ was defined as all other CPA cases (n = 146).
Results: We found the rate of false negatives by ImmunoCAP Aspergillus IgG EIA to
be more prevalent in patients with low levels of IgG, IgA, or IgM. The performance
LDBio Aspergillus ICT appears to relatively unaffected by these (Figure 1). Similar
findings were observed for IFN gamma production. Additionally, though not significant,
we observed a trend in lower levels of CD4, CD19, or CD56 cell markers amongst seronegative
CPA patients as compared to seropositive CPA patients, according to ImmunoCAP results
(Figure 2).
Conclusion: In select cases of CPA, ImmunoCAP EIA yields a false negative result,
making serological diagnosis difficult and requiring increased diagnostic sensitivity.
Our previous findings demonstrated that LDBio Aspergillus ICT show greatly improved
sensitivity and ability to detect Aspergillus-specific antibody in patient sera, especially
for ImmunoCAP IgG-negative (‘seronegative’) cases. Our analysis of patient immunodeficiencies
reveals that ImmunoCAP false negatives are more prevalent in patients with certain
immunodeficiencies, and that diagnostic performance of ImmunoCAP Aspergillus-specific
IgG EIA may be more adversely affected by these patient conditions, as compared to
LDBio Aspergillus ICT.
Hare
R.
1
Abou-Chakra
N.
1
Datcu
R.
1
Arendrup
M.C.
1
2
3
1Unit of Mycology, Statens Serum Institut, Copenhagen, Denmark
2University of Copenhagen, Dept. of Clinical Medicine, Copenhagen, Denmark
3Department Og Clinical Microbiology, Rigshospitalet, Copenhagen, Denmark
P169. The diagnostic value of a molecular assay for Fusarium infections recently introduced
in the Danish national reference laboratory
Objectives:
Fusarium keratitis and other infections remain difficult to diagnose even in the modern
mycology laboratory. Molecular methods have enabled genus and species specific detection
independent of culture and have improved diagnostic speed, sensitivity and accuracy
of fungal infections. A specific diagnostic assay for Fusarium infections has been
introduced in the Danish mycology reference laboratory and this study sought to evaluate
the clinical performance.
Methods: Clinical samples from patients suspected for fusariosis were subjected to
Fusarium PCR (detecting Fusarium solani, Fusarium dimerum and Fusarium oxysporum)
during the period October-2017 to April-2019. The real-time multiplex Fusarium PCR
assay using Taqman probes was slightly modified from that described by Salehi et al.
2016 (Pubmed ID 27605714). Patient samples were DNA extracted using the Qiacube (Qiagen,
Denmark) standard protocol for blood and tissue samples, with up to 200 µL input volume
and elution in 100 µL. Bronchoalveolar lavage samples were processed on the NucliSENS
easyMAG (Biomérieux, Denmark) DNA extraction platform with up to 1 mL input volume
and elution in 60 µL. Specimens were subjected to PCR in combination with culture
and Blankophor microscopy, except for one patient (PCR only) due to insufficient amount
of material. PCR was performed on the Applied Biosystems 7500 or the Quantstudio 5
thermocyclers (Thermo Fisher Scientific, Denmark). Positive and negative template
controls were applied but not PCR inhibition or DNA extraction controls. PCR results
were compared for each patient to our other laboratory results and to results from
the Danish Microbiological Database (MiBa), which captures clinical microbiological
results from every laboratory in the entire country.
Results: In total, 71 samples from 34 patients, among which 50 samples were from 29
patients with suspected keratitis, were included. Five patients (14.7%) accounted
for 19/33 (57.6%) samples positive for Fusarium spp. DNA (Table). Four PCR positive
patients provided 12/32 (37.5%) samples microscopy positive (septate hyphae) and 10/32
(31.3%) culture positive with Fusarium species (Table). The fifth PCR positive patient,
was culture negative, but clinically diagnosed with a fungal keratitis and responded
to targeted antifungal treatment. The remaining 29 patients provided 38 PCR negative
samples. The majority of those patients (18/29) had culture positive samples for other
potential culprits (clarification pending) and none were culture and microscopy positive
for Fusarium.
Conclusion: The diagnostic platform for Fusarium infections has proven accurate to
the species level and was applied successfully in three incidents of Fusarium keratitis,
one invasive fusariosis and one cutaneous infection. These data indicate a superior
sensitivity of PCR compared to microscopy and culture. of note, no clinically false
positive findings were identified. Still, the specificity needs to be confirmed including
a larger sample set. PCR performance may be further improved upon ongoing optimisation
of the extraction procedures and quality control as well as introduction of a PCR
inhibition control. In conclusion, the Fusarium PCR assay appears to be a significant
improvement of the overall diagnostic algorithm at this reference laboratory both
in speed and accuracy and thus important for those suffering from this uncommon but
sight or potentially life-threatening disease.
Hare
R.
1
Abou-Chakra
N.
1
Datcu
R.
1
Arendrup
M.C.
1
2
3
1Unit of Mycology, Statens Serum Institut, Copenhagen, Denmark
2University of Copenhagen, Dept. of Clinical Medicine, Copenhagen, Denmark
3Department Og Clinical Microbiology, Rigshospitalet, Copenhagen, Denmark
P170. rMucorales PCR: a diagnostic improvement at the Danish national reference laboratory
Objectives: Invasive Mucorales infections are associated with a poor prognosis for
affected patients due to challenging therapeutic management strategies and often inadequate
diagnostic tools. Molecular methods are increasingly complementing conventional culturing
and microscopy to improve the overall diagnostic performance for invasive Mucorales
infections. In the Danish mycology reference laboratory, a molecular assay detecting
Mucorales DNA has been introduced which in a recent EQA panel managed by the Mucorales
subgroup of the Fungal PCR Initiative scored 100% (7/7 correct). This study sought
to evaluate the clinical performance.
Methods: Clinical samples from patients suspected for invasive Mucorales infections
were subjected to two Mucorales PCR tests (1: Rhizopus microsporus, Rhizopus arrhizus
and Mucor spp. and 2: Lichtheimia spp. and Rhizomucor spp.) during the study period
October-2017 to April-2019. Both real-time multiplex Mucorales PCRs (Taqman probes)
were slightly modified from those described by Salehi et al. 2016. Patient samples
were DNA extracted using the Qiacube (Qiagen, Denmark) standard protocol for blood
and tissue samples, with up to 200 µL input volume and elution in 100 µL. Formalin
fixed paraffin-embedded tissue samples were processed using Neo-clear (Sigma-Aldrich,
Denmark) followed by the Tissue protocol as described above. BALs were processed on
the NucliSENS easyMAG (Biomérieux, Denmark) DNA extraction platform with up to 1 mL
input volume and elution in 60 µL. PCR was performed on the Applied Biosystems 7500
and since mid-2018 the Quantstudio 5 thermocyclers (Thermo Fisher Scientific, Denmark).
Positive and negative template controls were applied but not PCR inhibition or DNA
extraction controls. PCR results were compared to culture and Blankophor microscopy
whenever possible and to results from the Danish Microbiological Database (MiBa),
which captures clinical microbiological results from every laboratory in the entire
country.
Results: In total, 116 samples from 18 patients were included. Four patients diagnosed
with invasive mucormycosis contributed with 91 samples of which 38 were Mucorales
PCR-positive (41.8%, range 25-100% per patient) (Table). Among those four patients,
microscopy revealed pauci-septate hyphae in 31/85 (36.5%) and Mucorales species was
cultured from 10/81 (12.3%) samples (Table). All patients received targeted treatment
and surgical interventions guided by PCR results and all survived. The remaining 14
patients had 25 PCR negative samples, of which 20/21 and 19/19 were also negative
by microscopy and culture, respectively. One patient, was microscopy positive with
pauci-septate hyphae and by pan-fungal ITS DNA sequencing, Cunninghamella spp. DNA
was detected, which is not included in the current Mucorales tests.
Conclusion: Both diagnostic tests for Mucorales proved accurate to the genus or species
level and with a sensitivity comparable to that for microscopy but significantly superior
to that for culture (p<0.001). No clinically false positive findings were reported
suggesting a high specificity. Yet, further evaluation including a larger sample set
is warranted. PCR performance may be further improved upon ongoing optimisation of
extraction procedures and PCR parameters as well as introduction of a PCR inhibition
control. In conclusion, the Mucorales PCR assay appears to improve the diagnostic
armamentarium and may play a pivotal role in managing patients suffering from Mucorales
infections.
Gorton
R.
1
White
P.
2
Loeffler
J.
3
Barnes
R.
4
Donnelly
J.P.
5
Cruciani
M.
6
Hare
R.
7
Linton
C.
7
8
Johnson
E.
8
Novak-Frazer
L.
9
Cogliati
M.
10
Mengoli
C.
11
Willinger
B.
12
Posch
W.
13
Clancy
C.J.
14
Melchers
W.
15
Lackner
M.
13
Hamal
P.
16
Paholcsek
M.
17
Sendid
B.
18
Nguyen
M.H.
14
Arastehfar
A.
19
Ugoji
U.
20
Vanderbosch
B.
15
Selitsch
B.
12
Fuchs
S.
13
Vyzantidis
T.
21
1Infection Sciences, Health Services Laboratories, London, United Kingdom
2Microbiology, Public Health Wales Microbiology, Cardiff, United Kingdom
3Department of Internal Medicine Ii, WÜ4i, University Hospital Wuerzburg, Wuerzburg,
Germany
4Medical Microbiology and Infectious Diseases, Cardiff University School of Medicine,
Cardiff, United Kingdom
5Division of Infectious Diseases, San Antonio Center for Medical Mycology, San Antonio,
United States of America
6Infectious Diseases, San Bonifacio Hospital, Verona, VERONA, Italy
7Unit of Mycology, Statens Serum Institut, Copenhagen, Denmark
8Public Health England UK National Mycology Reference Laboratory, Bristol, United
Kingdom
9Mycology Reference Centre Manchester, Manchester University NHS Foundation Trust,
Manchester, United Kingdom
10Laboratorio di Micologia Medica, Dipartimento di Scienze Biomediche per la Salute,
Universita degli Studi di Milano, Milano, Italy
11Molecular Medicine, University of Padova, Padova, Italy
12Medical University of Vienna, Vienna, Austria
13Division of Hygiene and Medical Microbiology, Medical University of Innsbruck, Innsbruck,
Austria
14University of Pittsburgh Medical Center, Pittsburgh, United States of America
15Medical Microbiology, RadboudUMC, Nijmegen, Netherlands
16Department of Microbiology, Palacký University, Faculty of Medicine and Dentistry
and University Hospital Olomouc, Olomouc, Czech Republic
17Faculty of Medicine, Department of Human Genetics, University of Debrecen, Debrecen,
Hungary
18Laboratory of Mycology and Parasitology, University Hospital of Lille, Lille Cedex,
France
19Westerdijk Fungal Biodiversity Institute, Utrecht, Netherlands
20The Bourne Laboratory, Royal Holloway University of London, London, United Kingdom
21Medical Biopathology-microbiology, Aristotle University of Thessaloniki, Thessaloniki,
Greece
P171. An evaluation of the analytical specificity of PCR assays for the detection
of Candida species – A study by the ISHAM working group, the Fungal PCR initiative
Objectives: The use of PCR to detect Candida in clinical samples has been described
for two decades, yet standardization is limited, hampering its widespread application.
Following the successful efforts of the Fungal PCR initiative (FPCRI) in standardizing
Aspergillus PCR the FPCRI has expanded it efforts to standardising the molecular detection
of other fungi, including Candida. This research describes the efforts of the FPCRI
Candida Laboratory Working Party to determining the analytical specificity (detection
range and cross-reactivity) of Candida PCR assays currently used in clinical mycology
centres.
Methods: A panel of 12 samples containing genomic DNA extracted from nine yeast species
(Candida albicans (2 concentrations), Candida glabrata (Nakaseomyces glabrata) (2
concentrations), Candida krusei (Pichia krusei), Candida parapsilosis, Candida tropicalis,
Candida auris, Clavispora lusitaniae, Meyerozyma guilliermondii and Dipodascus geotrichum)
and commercially bought human genomic DNA was distributed to 11 centres. The recipients
were requested to perform Candida PCR following local protocols. Qualitative results
(positive/negative), together with associated C
T
values (for real-time PCR) were reported for each sample, along with the assay’s technical
details.. True positivity rates (detection of the correct species) and false positivity
rates (detection of species not included in the panel, or misidentification as another
species in the panel) were calculated for the overall panel and for individual samples.
Regression analysis has been performed to identify any technical variables associated
with improved analytical specificity.
Results: Across 11 datasets ten different PCR protocols were utilized. At the high
DNA concentration detection of the five main causes of invasive candidiasis (C. albicans,
C. glabrata, C. krusei, C. parapsilosis, C. tropicalis) was 92.7%, ranging from 100%
for C. parapsilosis to 90.9% for the other species. Detection of less common yeast
pathogens (C. lusitaniae, M. guilliermondii) was 36.3%. At the lower DNA concentration
the detection of C. albicans and C. glabrata was 72.7% and 54.5%, respectively. For
C. auris, 63.6% of datasets were negative, but 36.4% generated an incorrect identification.
Similarly for Dipodascus geotrichum, 45.5% were correctly negative but 45.5% provided
an incorrect identification; only one assay identified Dipodascus geotrichum correctly.
One assay generated a false positive result when testing human DNA. Nine centers used
real-time PCR. Analysis of C
T
values for the five main Candida species indicated variance in the sensitivity of
the assays with a mean Sd of 5.7 (range 4.3 – 6.9) with higher variance in C
T
values observed for C. parapsilosis, C. tropicalis or lower concentrations of C. albicans
and C. glabrata, shown in Figure 1 and Table 1.
Conclusion: The detection of the main Candida pathogens that account for >95% of invasive
disease is excellent, albeit at lower concentrations detection may be reduced. The
detection of less common species is poor. In particular, only one of the 11 assays
evaluated was designed to detect and identify C. auris but failed to do so which,
given the susceptibility profile and infection control concerns associated with C.
auris, highlights the design limitations of current Candida PCR assays. Preliminary
analysis of C
T
values for the main Candida pathogens indicates differing sensitivity levels across
real-time Candida assays.
Gorton
R.
1
Wey
E.
2
Hussein
M.
1
Patabendige
K.
1
Johnson
G.
3
1Infection Sciences, Health Services Laboratories, London, United Kingdom
2Microbiology, Royal Free Hospital Foundation Trust, London, United Kingdom
3Scientific Development, OLM diagnostics, Newcastle, United Kingdom
P172. An evaluation of human β-globin gene detection to assess the quality of broncho-alveolar
lavage and sputum specimens submitted for the diagnosis of Pneumocystis Pneumonia
(PcP).
Objectives: The use of PCR to diagnose Pneumocystis Pneumonia (PcP) from clinical
samples is recommended in recent guidelines for the diagnosis of PcP. One component
of the diagnostic pathway, specifically sampling of broncho-alveolar lavage and sputum,
is poorly controlled and often the laboratory receives specimen that is not standardised
in volume or portion. This research aims to evaluate the use of a human β-globin gene
(HgG) PCR from respiratory samples as a method for quality control of specimens, with
the hypothesis that adequate levels of HgG present in a specimen is an indicator of
a good quality sample,
Methods: DNA was extracted from 47 BAL and 18 sputua using the Diasorin Arrow with
DNA extraction cartridges. 500μL of BAL concentrate was centrifuged at 8000g for 10
minutes. The supernatant discarded; 50μL of glass beads were added and ribolysed for
45 seconds. The lysate was suspended in 240 μL Diasorin buffer 2 and 10μl proteinase
K and incubated at 56°C for 10 minutes. Extraction was performed as per manufacturer’s
instructions on the Arrow platform. PCR was performed using the OLM PneumID assay
with 6µL template in a total reaction volume of 20µL, following manufacturer’s instructions.
PCR was performed on the RotorGeneQ platform. Outliers were determined using the 1.5
x IQR rule.
Results: HgG C
T
characteristics are presented in Table 1 and Figure 1a) and b).
The mean HgG C
T
value from BAL was 19.49 (IQR 21.73, 23.49) and the lower threshold was determined
to be 26.13. 10.6% (5/47) of the BAL samples had HgG amplification below the threshold.
One sample was negative for P. jirovecii, two samples were sub-clinical (internal
threshold 1E+5) positive for P. jirovecii measuring 2.3E+1 and 8.5 E+4 copies per
mL. The remaining two samples were positive for P. jirovecii measuring 2.1 E+7 and
8.3 E+9 copies per mL. The mean HgG C
T
value from sputum was 18.04 (IQR 19.16, 20.26) and the lower threshold was determined
to be 21.91. 16.7% (3/18) of the sputum samples had HgG amplification below the threshold.
One sample was negative and two samples were positive for P. jirovecii at 9 E+5 and
1.9 E+6 copies per mL. From the total dataset of 65 specimens 47.7% (31/65) were positive
for P. jirovecii. No significant difference (p = 0.38) was observed between the measured
copies per mL using the OLM PneumID assay and the FTD Pneumocystis assay indicating
that inclusion of the HgG PCR did not competitively inhibit the detection of P. jirovecii.
Conclusion: This study has demonstrated HgG detection enables the laboratory to quality
control respiratory specimens by determining if the specimen contains sufficient host
material, which can be used as an indicator of good quality specimen. In this study
almost 90% of samples had measured HgG above the established threshold, therefore
negative and positive P. jirovecii PCR results could be reported with confidence.
Where the HgG is below the acceptable threshold, negative or low positive samples
should be interpreted with caution, as results may be derived from poor quality samples,
seen for >10% of samples in this study.
Salah
H.
1
2
Kolecka
A.
2
Houbraken
J.
2
Taj-Aldeen
S.J.
1
Boekhout
T.
2
3
1Department of Laboratory Medicine and Pathology, Division of Microbiology, Hamad
medical corporation, Doha, Qatar
2Westerdijk fungal biodiversity Institute, Utrecht, Netherlands
3Institute of Biodiversity and Ecosystem Dynamics, University of Amsterdam, Amsterdam,
Netherlands
P173. Optimization of Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight Mass
Spectrometry for identification of filamentous fungi
Objectives: Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight Mass Spectrometry
(MALDI-TOF MS) has been widely used in clinical laboratories for routine identification
of bacteria and yeast. However, there remain difficulties when applied to filamentous
fungi. The standard liquid cultivation method recommended for identification is labour
intensive and time-consuming. The aim of this study was to develop a quicker and less
laborious method for cultivating filamentous fungi which leads to a reliable identification
using Bruker MALDI-TOF MS.
Methods: Reference strains of Aspergillus section Fumigati (n = 55) and section Flavi
(n = 104), which were previously identified by molecular methods, were cultivated
on top of and in between polycarbonate filters placed on Sabouraud’s dextrose agar
(SDA) plate, and by the Bruker standard liquid cultivation method. After 24 hours
of incubation at 35°C, mycelia were harvested for ethanol/formic acid protein extraction.
Main Spectra Profiles (MSPs) were created and databases were constructed for each
cultivation method. These databases were validated by identification of 50 reference
strains and 100 clinical strains of Aspergillus and Penicillium species which were
previously characterized by DNA sequencing.
Results: Growth: On top of a polycarbonate filter: The strains grew as a monolayer
of mycelia with some spores in the middle. In between two polycarbonate filters: The
strains grew as a mycelial monolayer without spores. Bruker method: Mycelial growth
without sporulation MSP creation: 55/55 MSPs of Aspergillus section Fumigati strains
were created by cultivation in between 2 polycarbonate filters, 52/55 by cultivation
on top of a polycarbonate filter and 47/55 by Bruker standard method. Out of 104 Aspergillus
section Flavi strains, 94, 92 and 76 MSPs were created by cultivation on top of and
in between polycarbonate filters, and Bruker standard method, respectively. Validation:
Identification of 50 reference strains of Aspergillus and Penicillium species against
in-house databases resulted in 95%, 90% and 62% correct identifications for the strains
cultivated on top of a polycarbonate filter, in between 2 polycarbonate filters and
by Bruker method, respectively. Identification against Bruker database (BDAL) resulted
in 79%, 64% and 61% correct identifications by 1 polycarbonate filter, 2 polycarbonate
filters, and Bruker method, respectively. Identification against in-house databases
and Bruker database together yielded 87%, 82% and 61% correct identifications by 1
polycarbonate filter, 2 polycarbonate filters, and Bruker method, respectively (Figure
1).
Identification of clinical strains: Out of 100 clinical Aspergillus and Penicillium
strains cultivated on top of a polycarbonate filter, 81% were correctly identified
against in-house databases of strains grown on top of a polycarbonate filter, 78%
with 2 polycarbonate filters and 59% by Bruker commercial database (BDAL) (Figure
2).
Savings: For 50 strains, the cultivation method using polycarbonate filter was 270
minutes quicker and 81 Euros cheaper than Bruker standard method.
Conclusion: Cultivation of filamentous fungi on top of a polycarbonate filter showed
significantly improved results compared to the Bruker standard liquid cultivation
method in terms of correct identification, MSP creation, time consumption, cost, and
labour intensity. This can reliably be applied for identification of clinically significant
filamentous fungi.
Guegan
H.
1
Iriart
X.
2
Robert-Gangneux
F.
1
Berry
A.
2
Bougnoux
M.-E.
3
Gangneux
J.P.
1
1Laboratoire De Parasitologie-mycologie, CHU de Rennes, Rennes, France
2Laboratoire De Parasitologie-mycologie, CHU de Toulouse, Toulouse, France
3Laboratoire De Parasitologie Mycologie, APHP Hôpital Necker-Enfants-Malades, Paris,
France
P174. Interest of Mucorales PCRs in pulmonary samples and comparative evaluation of
MucorGenius® assay and in-house Mucorales PCRs
Objectives: Mucormycosis is a life-threatening fungal infection whose diagnosis remains
challenging due to a lack of reliable diagnostic tests. Clinical and radiological
signs are often unspecific. The contribution of histopathological and cultural procedures
for early diagnosis is limited. In this context, Mucorales in-house real-time PCRs
have been previously shown to be of high interest for a reliable and early diagnosis
both in serum and respiratory specimens. A multiplex real-time PCR assay (MucorGenius®,
Pathonostics, the Netherlands) has been newly marketed to detect the main genera involved
in human mucormycosis. The aim of this study was i) to assess the diagnostic performances
of MucorGenius® assay in pulmonary samples compared to in-house Mucorales PCRs, and
ii) to evaluate the occurence of Mucorales detection among invasive aspergillosis
(IA) patients.
Methods: We retrospectively included 320 pulmonary samples between January 2012 and
May 2019: bronchoalveolar lavage fluids (n = 293), tracheal aspirations (n = 13),
sputum samples (n = 5), pleural fluids (n = 3), and lung biopsies (n = 6) collected
from 320 patients with respiratory symptoms at Rennes, Toulouse and Paris-Necker teaching
Hospitals (France). Among the 320 patients, 30 patients were classified as a proven
mucormycosis based on a positive direct examination and/or a positive culture in the
respiratory sample and/or a positive serum mucorales PCR. Fifty-eight patients were
proven/probable IA cases according to EORTC-MSG criteria. The 232 remaining patients
were considered as having no IA and no mucormycosis (negative group). In-house real-time
PCRs consisted in 3 assays targeting respectively Mucor-Rhizopus spp, Lichteimia spp,
and Rhizomucor spp. MucorGenius® targeted the same genera, including Cunninghamella
spp. Amplification was performed according to manufacturer’s instructions using a
LC480 (Roche) device.
Results: The global concordance of both tests was high, of 97.8% (kappa coefficient
= 0.877). Discordant results were mainly related to a higher sensitivity of in-house
PCRs compared to MucorGenius® assay (100% (30/30) versus 90.0% (27/30). The 3 cases
missed by MucorGenius® PCR were related to low fungal burden (mean Cq of in-house
PCRs of 39.5±1.4) and were positive for Mucor-rhizopus (n = 2) and Rhizomucor (n =
1) with in-house PCRs. The specificity of both tests was similar (98.6% (286/290)
versus 99.3% (288/290). No Mucorales DNA was detected in IA patients using in-house
PCRs whereas one was found positive with MucorGenius® assay, without other biological
evidence for mucormycosis. of the 4 positive-PCR samples in the negative group, one
was probably a true positive result, from a real mucormycosis episod missed at the
time of diagnosis (hematological malignancy, imaging compatible with IA during aplasia,
early death under voriconazole treatment). The 3 other could be associated with fungal
colonization.
Conclusion: The commercial MucorGenius® assay provides the possibility to detect in
one well the main mucorales responsible for human mucormycosis, and then could reduce
the diagnostic delay and improve the outcome. MucorGenius® assay is an easy to use
marketed kit including internal quality control, that shows good diagnostic performances
with 97.8% of concordance compared to in-house PCRs, missing 3 low fungal burden cases.
Ceballos-Garzon
A.
1
2
Giraldo
C.M. Parra
2
Amado
D.
2
Velez
N.
2
Rodríguez
C.
3
1Department of Parasitology and Medical Mycology of The University of Nantes., Universite
de Nantes, Nantes, France
2Microbiology, Pontificia Universidad Javeriana, Bogotá, Colombia
3Bruker Mexicana, Ciudad de México, Mexico
P176. Development and Validation of an In-House Candida auris database in MALDI TOF
MS to improve the yeast identification.
Objectives: to construct an In-house Colombian database to improve Candida auris identification
by MALDI-TOF MS.
Methods: 30 clinical isolates of C. auris were used to construct an in-house data
base. For each isolation, protein extraction was performed using the formic acid/ethanol
method according to the Bruker Daltonics protocol. Minimum 20 mass spectra of each
one was obtained. The library was named “Candida auris Colombia”. For its validation
300 Colombian isolates of C. auris were used, comparing the results between the BDAL
(Bruker) and Candida auris Colombia (in house) libraries. Finally, Cohen’s kappa index
was used to compare the results of the classification with both libraries.
Results: From 30 clinical isolates, 770 mass spectra were obtained for the construction
of the database. The validation was carried out with 300 isolates to compare the identification
of results in the BDAL and Candida auris Colombia libraries (Figure 1). Our library
allowed us to obtain an identification result for 100% of the evaluated isolates and
improve significantly the scores obtained, which shows a better performance compared
to the library present in the Bruker spectrometer.
Figure 1 BDAL and Candida auris Colombia libraries identification results. A Total
results and B Matrix of concordance identification from BDAL and Colombia databases.
The MALDI-TOF MS results according to the manufacturer’s technical specifications
as follows: Correct genus and species identification (>2,300), correct genus and probably
species identification (≥2.0), correct genus identification and (1.7–2.0) no reliable
identification (<1.7).
Conclusion: The current bruker database has only 3 C. auris isolates from Korea and
Japan, what it entails with identifications problems and low punctuation. Our database
had a remarkable improvement in the identification of the 300 isolates, evidencing
that the strengthening of the database is a great opportunity to improve the punctuation
and C. auris identification.
Wilopo
B.
1
Hunter
E.
1
Page
I.
1
Moore
C.
1
2
Richardson
M.D.
1
2
Denning
D.
1
2
1Division of Infection, Immunity and Respiratory Medicine, The University of Manchester,
Manchester, United Kingdom
2Mycology Reference Centre, Excellence Center For Medical Mycology (ecmm), Wythenshawe
Hospital, Manchester University NHS Foundation Trust, Manchester, United Kingdom
3National Aspergillosis Centre, Manchester University NHS Foundation Trust, Manchester,
United Kingdom
P177. Aspergillus-specific IgG assay optimum cut-off for the diagnosis of chronic
pulmonary aspergillosis
Objectives: Chronic pulmonary aspergillosis (CPA) is a fungal disease characterised
by cough, haemoptysis and weight loss present for at least for three months in non-immunocompromised
people. It is caused by Aspergillus spp. especially Aspergillus fumigatus that can
reach the respiratory tract by airborne transmission. The diagnosis of CPA can be
made by a variety of clinical symptoms, radiological and laboratory evidence of Aspergillus.
Most patients with CPA have positive Aspergillus precipitating antibodies or an abnormally
high level of Aspergillus-specific IgG (Aspf IgG). The Bordier Aspergillus fumigatus
ELISA kit is one assay that can detect Aspf IgG and the result is deemed positive
when the optical density (OD) index is higher than 1.0. We have re-evaluated this
cut-off in patients with CPA in the UK.
Methods: Aspf IgG levels in sera from 114 CPA patients and 150 British healthy controls
were measured by the Bordier Aspergillus fumigatus ELISA kit. The results obtained
were organised in MS Excel spreadsheets and analysed were performed using SPSS. Sensitivity
and specificity at a number of points on the receiver operating characteristics (ROC)
curve were described. The optimum diagnostic cut-off for the Bordier assay was calculated
using Youden’s J statistic (sensitivity + specificity - 1).
Results: Based on statistical analyses, we determined that the ROC area under the
curve (AUC) of the Bordier Aspergillus fumigatus ELISA kit was 0.928. This assay has
77.2% sensitivity and 99.3% specificity using the manufacturer’s cut-off (1.0). The
sensitivity and specificity of this assay at various points on the ROC curve improves
to 83.3% sensitivity and 97.3% specificity if the OD index cut-off is lowered to 0.9.
At this cut-off, the diagnostic odds ratio and diagnostic accuracy are 145 and 90.9%.
Conclusion: We have performed a ROC AUC analysis comparing Aspf IgG levels in a large
group of patients with CPA compared to healthy British controls. Diagnostic performance
improves if the OD index cut-off falls from 1.0 to 0.9.
Liu
W.-D.
1
Shih
M.-C.
2
3
Wang
Y.-W.
4
Lin
S.-C.
4
Lee
Y.-F.
5
Lin
S.-W.
4
6
Chou
W.-C.
5
7
Wu
U.-I.
1
8
Chen
Y.-C.
1
9
1Department of Internal Medicine, Division of Infectious Disease, National Taiwan
University Hospital, Taipei City, Taiwan
2Institute of Epidemiology and Preventive Medicine, College of Public Health, National
Taiwan University, Taipei City, Taiwan
3Department of Medical Education, National Taiwan University Hospital, Taipei City,
Taiwan
4Department of Pharmacy, National Taiwan University Hospital, Taipei City, Taiwan
5Department of Laboratory Medicine, National Taiwan University Hospital, Taipei city,
Taiwan
6Graduate Institute of Clinical Pharmacy, National Taiwan University, Taipei City,
Taiwan
7Department of Internal Medicine, Division of Hematology, National Taiwan University
Hospital, Taipei City, Taiwan
8Department of Internal Medicine, National Taiwan University Cancer Center, Taipei
City, Taiwan
9Center of Infection Control, National Taiwan University Hospital, Taipei City, Taiwan
P178. Influence of Intravenous Human Immunoglobulins on the Accuracy of Aspergillus
Galactomannan Antigen Assays
Objectives: Galactomannan (GM) enzyme immunoassay (EIA), a diagnostic tool that detects
the cell wall component secreted by Aspergillus species, provide a non-invasive method
for early diagnosis of invasive aspergillosis (IA). However, its performance in diagnosing
IA can be limited by potential false positivity caused by cross-reaction with other
fungal species, food consumption and administration of certain antibiotics and blood
products. False positive results were also demonstrated in a few patients having received
intravenous immunoglobulins (IVIG). Some cases were attributed to cross-reactivity
between the GM kits and saccharose contained in some IVIG products. Recently, we observed
a growing number of patients prescribed with maltose-stabilized IVIG had false positive
GM results despite absence of factors reported in the literature. We thus sought to
determine if the false positivity was due to the processing procedures and presence
of other unrecognized cross-reactive antigens contained in IVIG.
Methods: Two new, unopened bottles of human IVIG (TBSF, CSL Behring, Australia) was
randomly obtained from our hospital pharmacy. Each vial contained 3g (6%) human IVIG
and 14.6 mmol maltose in 50ml solution. From each bottle, solution were aspirated
for dilution using 0.9% saline to the concentration of 1% and 0.1%, respectively in
order to simulate the peak (15.8 g/L) and trough (7.4 g/L) of serum IVIG level in
an adult having received IVIG infusion at a concentration of 0.2-0.6 g/Kg. A total
of three different concentrations (6%, 1% and 0.1%) of IVIG together with negative
controls (0.9% saline) were prepared in duplicates for GM detection using the Platelia
Aspergillus EIA Kits (Bio-Rad) according to the manufacturer’s instructions. In order
to explore if heat treatment and addition of EDTA required in standard sample processing
procedures were associated with false positivity, samples were prepared with and without
the investigated pretreatment, respectively for comparisons. A GM test result was
considered positive if the optical density index (ODI) reached 0.5 or above. In addition,
conventional bacteria and fungus cultures, pan-fungal polymerase chain reaction (PCR)
were performed using undiluted samples from the bottles of IVIG to assess the presence
of contaminants. All procedures were performed under strict sterile conditions.
Results: Using standard procedures with heat pretreatment and addition of EDTA, all
IVIG samples tested positive for GM regardless of the concentrations used for the
experiments (Figure 1), while all 0.9% saline controls yielded negative results. Pre-treatment
of samples with boiling water-bath resulted in considerable elevation of GM ODI in
both presence and absence of EDTA compared to samples without heat treatment. No fungus
or other microorganisms were isolated from our IVIG samples using PCR or conventional
culture methods.
Figure 1. GM ODI of IVIG samples prepared with different concentrations and pretreatment
methods.
Conclusion: IVIG treatment can cause false GM positivity owing to the reaction between
the contents in the IVIG products and standard pretreatment procedures of the assays.
Interpretation for a positive GM assay result should be cautious in patients having
received human IVIG infusion.
Magallon
A.
1
Basmaciyan
L.
1
Valot
S.
1
Curraize
C. Goulard De
2
Sautour
M.
3
Bador
J.
2
Dalle
F.
4
1Mycology, University Hospital of Dijon, Dijon, France
2Bacteriology, University Hospital of Dijon, Dijon, France
3Laboratoire Parasitology Mycology, CHU Dijon, Dijon, France
4Parasitology-mycology, University Hospital Dijon, Dijon, France
P179. Evaluation of the relevance of use of the BD BACTECTM mycosis IC/F, BD BACTECTM
Plus Aerobic/F, BD BACTECTM Lytic/10 anaerobic/F et BD BACTECTM Peds Plus/F culture
bottles system for fongemia detection: A 4-year retrospective study at the University
Hospital of Dijon, France.
Objectives: Fongemia are severe invasive fungal diseases that combine rapid evolution
with high mortality. The early diagnosis of these pathologies is therefore fundamental
in the care of patients and is a vital emergency. Currently, only the BD BACTECTM
Automated Blood Culture System (Becton Dickinson, Franklin Lakes, New Jersey, USA)
has a bottle specifically dedicated to the detection of fungal agents, the BD BACTECTM
Mycosis IC/F bottle. The objective of this study was to (i) evaluate the fongemia
detection performance of the BD BACTECTM Mycosis IC/F culture bottles in comparison
to the so-called "bacteriological" culture bottles (i.e. BD BACTECTM Plus Aerobic/F
bottles, BD BACTECTM Lytic/10Anaerobic/F bottles, and BD BACTECTM Peds Plus/F) in
different clinical situations and so (ii) the relevance of their use.
Methods: This retrospective study was conducted over a period of 4 years (i.e. from
January 2013 to December 2016) at the University Hospital of Dijon. 331 pairs of blood
cultures (i.e. each pair combining a BD BACTECTM Mycosis IC/F culture bottle with
at least one of the bacteriological culture bottle). Univariate analysis by the MacNemar
chi² test was carried out globally as well as in the subgroups corresponding to the
various clinical situations encountered.
Results: of the 331 pairs of positive for fungi blood cultures included in the study,
the BD BACTECTM Mycosis IC/F culture bottles showed a diagnostic advantage over bacteriological
culture bottles in 57.4% of cases (p <0.01). In addition, for 96 pairs of blood cultures,
only the BD BACTECTM Mycosis IC/F culture bottles was positive for fungi versus 66
pairs in which only the bacteriological culture bottles was positive for fungi. On
the other hand, a fungus / bacterium co-infection was observed in 46 of the 331 pairs
of blood cultures included in the study. In this "co-infection" subgroup, the overall
"diagnostic advantage" variable was significantly in favor of the BD BACTECTM Mycosis
IC/F culture bottles (n = 30, p<0.05). Multivariate analysis revealed an association
between diagnostic performance and (i) the existence of yeast-bacteria co-infections,
(ii) the mode of sampling, (iii) the type of fungus and (iv) diagnostic indication
or follow-up of the patient, showing the relevance of the use of the BD BACTECTM Mycosis
IC/F culture bottles in addition to those of bacteriology.
Conclusion: This study showed the necessity of the of the BD BACTECTM Mycosis IC/F
culture bottles in the diagnosis of candidemias, in particular in case of bacterial
co-infections.
Lopez
A. Gomez
Hernández
C. Rueda
Pozo
R. Pando
Reference Laboratory On Mycology, CNM-ISCIII, MAJADAHONDA-MADRID, Spain
P180. rGliotoxin (GT) and bis-methyl-Gliotoxin (bmGT) characterization during Aspergilllus
fumigatus infection.
Objectives: To develop a new method based on liquid-liquid extraction and chromatographic
separation to specifically and simultaneously characterize both mycotoxins. We also
describe the primary bmGT and GT’s role as defence molecule during Aspergillus progression
in two cell lines infection model of IA that mimic human disease by measurement of
GT and bmGT amount and their cytotoxic effect.
Methods: An UHPLC/PDA method was first developed and validated to specifically characterize
both compounds. Chromatographic separation was achieved using an ACQUITY UPLC-H Class
apparatus. The analytical conditions were selected after testing different parameters
such as extraction procedures, column type, mobile phase composition, etc. We applied
the developed method to define temporal kinetics of GT and bmGT production by using
a fungal infection in two different cell line models, A549 (ATCC CCL-185, alveolar
basal epithelial cell of human adenocarcinoma), and RAW 264.7 (ATCC TIB-71, murine
macrophages) and an Asperillus fumigatus clinical isolate, GT producer. After the
establishment of a specific infection model (ratio1:1, inoculum size 5x10t5 spores),
we harvested each supernatant from cell line cultures at fixed time points, for assessment
of GT/bmGT production. Galactomannan (GM) antigen was also quantified in order to
establish the fungal burden at each time. In addition, a microscopic evaluation was
performed to prove spores progression in each cell line. Specific stains (Sytox, calcofluor)
were used to better define the characteristics of infection
Results: The best overall selectivity and peak shape were achieved by using a silica
column (ACQUITY UPLC® HSS, C18, 2,1 x 75 mm 1,8μm particle size) combined with a mobile
phase consisting of a gradient of water and acetonitrile and a flow rate of 0.1 mL/min.
The developed method was applied to analyze the extracted supernatant (using chloroform)
obtained from each condition. In the laboratory condition established, GT was firstly
detected after 30 h of incubation. The kinetic profile showed a maximum production
after 48 h and then a continuous decrease. Enrichment of GT in infected cell lines
did not correlate with GM. Additionally, the level of bmGT, the inactive derivate
of GT, was detected later. Contrary to GT, the levels of bmGT showed a continuous
increase after 48 h of incubation. No great differences were found in GM, GT and bmGT
amounts by cell lines studied. In both models, GT showed a concentration-dependent
cytotoxic effect. of note, bmGT did not show any toxic effect and appear to be more
stable than GT.
Conclusion: 1) GT and bmGT may be excreted to extracellular media during the course
of Aspergillus fumigatus infection, although these components showed different kinetics
during the Aspergillus fumigatus growth. 2) The chromatographic method described was
successfully applied to characterize these secondary metabolites, having potential
as a diagnostic technique for aspergillosis.
Giannakopoulou
D.-A.
Zachrou
E.
Zarkada
E.
Vyzantiadis
T.-A.
First Department of Microbiology, Medical School, Aristotle University of Thessaloniki,
Thessaloniki, Greece
P181. In vitro comparison between two commercial kits for the detection of Aspergillus
galactomannan. Preliminary results.
Objectives: Galactomannan is an important component of fungal cell wall, mainly measured
for the early diagnosis and monitoring of invasive Aspergillosis. Several commercial
kits have been released and the aim of this study was to compare the recently available
kit of Dynamiker Aspergillus Galactomannan Assay (Dynamiker Biotechnology Co, Ltd,
China) to the internationally used and broadly evaluated PlateliaTM Aspergillus Ag
(Bio-Rad, France).
Methods: Both kits are immunoenzymatic assays, based on the same principle with slight
technical differences. An advantage of the Dynamiker method is the use of breakable
strips, while both produce results after the calculation of an index value. The interpretation
of positivity is based on the cut-off index of 0.5 for serum and 1.0 for BAL, defined
after years of use and evaluation of results produced by the Bio-Rad kit. Forty four
(39 serums, 4 BALs, 1 pleural fluid) specimens were tested by both methods (18 positive
by the Bio-Rad kit). The positive and negative controls of the Bio-Rad kit were also
tested by the Dynamiker one. Further on, in order to test the precision of the Dynamiker
kit, twelve specimens were tested for their inter-day variation between two or three
sequential measurements and their intra-day variation concerning double measurements.
Alongside, two positive specimens (one serum and one BAL) were further diluted in
three sequential two-fold sub dilutions (1:2, 1:4 and 1:8) in order to test the detection
ability of the kit.
Results: The values (mean±SEM) were 1.12±0.24 by Bio-Rad and 0.68±0.12 by Dynamiker.
Although, the strict numeric values were quite different, they didn’t differ statistically
(p = 0.35) and they were positively correlated (r = 0.65, p = 0.000001). The precision
of the Dynamiker kit was good with an intra-run CV (coefficient of variation) of 13.01%
and an inter-run CV of 13.25%. However, while all negative (26 specimens) by Bio-Rad
were found again negative by Dynamiker, this was not the case with the positive specimens,
where there was a disagreement in six out of eighteen (33.3%, positive by Bio-Rad
and negative by Dynamiker) and the strict numeric values differed statistically (p
= 0.016). Both Bio-Rad positive and negative controls were found at the same level
with the Dynamiker and all dilutions of the tested two positive samples provided positive
results.
Conclusion: If the long used Bio-Rad kit is going to be considered the reference kit
for the comparison of other recently available commercial kits, then it is probable
that the here-tested Dynamiker kit is quite efficient and could probably offer a reliable
alternative. However, these preliminary results show that there is a possible issue
of sensitivity by not being able to detect a percentage of positive samples, while
the kit is highly specific. Further in vitro studies with larger number of samples
in combination to clinical information and outcome would provide a more documented
and evaluated use of the kit.
Alobaid
K.
1
Chadha
A.
2
Alansari
A.
3
1Mycology Reference Lab, Mubarak Alkabeer hospital, Jabryia, Kuwait
2Microbiology Unit, Mubarak Alkabeer hospital, Jabryia, Kuwait
3Medical Department, Mubarak Alkabeer hospital, Jabryia, Kuwait
P182. External Validation of a bedside prediction rule for candidemic patients
Objectives: The aim of this study is to examine Ostrosky-Zeichner’s rule (CPR) on
predicting cases of candidemia among hospitalized patients in ICU and non ICU settings.
Methods: This is a prospective study on cases of candidemia from March 2017 till May
2018 in a secondary hospital. All candiemic cases were analyzed and tested by applying
Ostrosky-Zeichner’s rule (CPR). Another group of patients who developed bacteremia
were also tested as a control. The inclusion criteria for cases and controls are (1)
positive blood culture in a patient with signs of infection, (2) hospital acquired
infections. CPR was modified to include ICU and non ICU patients. The rule included
presence of central line or broad spectrum antibiotic plus two of the following: steroids,
immune suppressive agents, pancreatitis, major surgery, TPN, and major surgery.
Results: In the period from March 2017 till May 2018, 50 cases of candidemia were
found. Two cases were excluded, as they represented contamination. Control group involved
60 patients. Majority of cases were hospitalized in medical wards (27), seven patients
were surgical, 12 patients were hospitalized in ICU, and two patients were pediatric
cases. The sensitivity, specificity, PPV, and NPV of modified Ostrosky-Zeichner’s
rule were 40.4%, 98.3%, 67%, and 95% respectively. Area under the curve (AUC ROC)
is 0.69 and 95 % CI is (0.62-0.76). Although 29 cases (59.6%) were not predicted by
the rule, 6 patients (21%) had femoral catheters.
Conclusion: Clinical prediction rules are helpful adjunctive tool to rationalize the
use of antifungals. Modified Ostrosky-Zeichner’s rule, in particular, appears to have
good specificity for candidemic patients. The results also suggest that around 40%
of patients can be treated empirically before culture results and this may improve
survival. The possibility of femoral catheters as a risk factor for candidemia needs
further evaluation. As shown in other studies, the high negative predictor value helps
avoiding or stopping unnecessary use of antifungals.
Ignatyeva
S.
1
Spiridonova
V.
1
Bogomolova
T.
2
Shadrivova
O.
3
Borzova
Y.
1
Desyatik
E.
2
Vasilyeva
N.
2
Klimko
N.
4
1Kaschkin Research Institute of Medical Mycology, North-Western State Medical University
named after I.I. Mechnikov, Saint-Petersburg, Russian Federation
2Kaschkin Research Institute of Medical Mycology. Department of Medical Microbiology,
North-Western State Medical University named after I.I. Mechnikov, Saint-Petersburg,
Russian Federation
3Department of Clinical Mycology, Allergy and Immunology, North-Western State Medical
University named after I.I. Mechnikov, Saint-Petersburg, Russian Federation
4Department of Clinical Mycology, Allergology and Immunology, North-Western State
Medical University named after I.I. Mechnikov, Saint-Petersburg, Russian Federation
P183. Real Time PCR and galactomannan test for detection of Aspergillus spp. in bronchoalveolar
lavage fluid of patients with aspergillosis
Objectives: The success of treatment of fungal infection depends on early etiological
diagnostics. The aim of the study was to test a real time PCR with High Resolution
Melt analysis (mHRM-RT-PCR) and galactomannan (GM) for detection of Aspergillus spp.
in bronchoalveolar lavage fluid (BAL) of patients with aspergillosis.
Methods: We tested 33 BAL from 33 patients with aspergillosis in Saint-Petersburg
between 2015 and 2018 yy. As controls, 50 BAL were collected from patients without
mycoses. BAL was examined by direct microscopy with calcofluor white and culture.
Fungal DNA was extracted from clinical samples by a chloroform-isoamyl extraction
method. DNA amplification was performed using aspergillus - specific primers and EvaGreen
based mHRM-RT-PCR on Rotor-Gene 6000 cycler. Detection of GM was performed using “Platelia
Aspergillus Ag” (Bio-Rad). The test was considered as positive with a cut-off of ≥0,5.
We calculated the sensitivity and specificity for the PCR assay and GM with the level
of statistical significance <0,05.
Results: Direct microscopic examination of BAL from patients with aspergillosis was
positive in 42% cases, Aspergillus spp. were isolated in 91% cases. Cultures of 33
BAL samples revealed various Aspergillus spp.: A. fumigatus (63%), A. niger (19%),
A. flavus (6%), A.versicolor (3%). From 3 patients (9%) two different species were
isolated: A.fumigatus + A.niger (6%) and A.fumigatus + A.flavus (3%). GM test was
positive in 23 BAL samples from patients with confirmed aspergillosis. Sensitivity
of PCR assay in BAL was higher, than sensitivity of GM test (84% vs. 70%, p = 0,013).
At the same time, specificity of GM test was higher than of PCR assay in patients
without aspergillosis (87% vs. 71%, p = 0,018). The positive results of PCR assay
in patients with aspergillosis correlated with positive results of microscopy and
cultural investigations from BAL in 75% and 78%, positive GM test - in 55% and 95%,
respectively. Complex investigation of biological materials by immunological and molecular
methods increased the diagnostic effectiveness to 91 - 92%.
Conclusion: This study indicated that the mHRM-RT-PCR may be a very useful tool for
detection of Aspergillus spp. in bronchoalveolar lavage fluid of patients with aspergillosis.
Real Time PCR assay together with GM test increase the diagnostic effectiveness of
biological materials investigations.
Ignatyeva
S.
1
Spiridonova
V.
1
Bogomolova
T.
2
Avdeenko
Y.
1
Shadrivova
O.
3
Zyuzgin
I.
4
Chudinovskikh
Y.
5
Popova
M.
6
Uspenskaya
O.
7
Vasilyeva
N.
2
Klimko
N.
8
1Kaschkin Research Institute of Medical Mycology, North-Western State Medical University
named after I.I. Mechnikov, Saint-Petersburg, Russian Federation
2Kaschkin Research Institute of Medical Mycology. Department of Medical Microbiology,
North-Western State Medical University named after I.I. Mechnikov, Saint-Petersburg,
Russian Federation
3Department of Clinical Mycology, Allergy and Immunology, North-Western State Medical
University named after I.I. Mechnikov, Saint-Petersburg, Russian Federation
4N.N. Petrov National Medical Research Centre of Oncology, Saint Petersburg, Russian
Federation
5N.N. Petrov National Medical Research Centre of Oncology, Ministry of Health of Russian
Federation, St. Petersburg, Russian Federation
6I.Pavlov First Saint Petersburg State Medical University, St. Petersburg, Russian
Federation
7Leningrad Regional Clinical Hospital, St. Petersburg, Russian Federation
8Department of Clinical Mycology, Allergology and Immunology, North-Western State
Medical University n.a. I.I. Mechnikov, Saint-Petersburg, Russian Federation
P184. Detection and identification of mucormycosis etiologic agents in biological
samples of oncohematological patients using multiplex real time PCR
Objectives: Mucormycosis is an opportunistic infection characterized by rapid progression,
high morbidity and mortality. The success of treatment of fungal infection depends
on early etiological diagnostics. The aim of the study was to test a multiplex real
time PCR with High Resolution Melt analysis (mHRM-RT-PCR) on clinical samples for
simultaneous detection and identification of Mucormycetes in biological samples.
Methods: We tested 12 clinical samples from 10 oncohematological patients with mucormycosis
in Saint-Petersburg between 2014 and 2018 yy. As controls, 21 tissue samples and 20
BAL were collected from patients without mycoses. Fungal DNA was extracted from clinical
samples by a chloroform-isoamyl extraction method. DNA amplification was performed
using mucormycetes - specific primers and EvaGreen based mHRM-RT-PCR on Rotor-Gene
6000 cycler.
Results: The mHRM-RT-PCR allows to identify In clinical samples from patients with
aspergillosis and mucormycosis the representatives of Aspergillus to the genus and
Mucormycetes to the species level: Rhizopus arrhizus, Mucor racemosus, Rhizomucor
pusillus, Lichtheimia corymbifera. We tested 3 native tissue, 7 formalin-fixed paraffin-embedded
tissue samples and 2 BAL of patients with mucormycosis. In 1 of 3 native tissue and
2 BAL with positive direct microscopic examinations Lichtheimia corymbifera was isolated.
In 7 formalin-fixed paraffin-embedded tissue non-septate mycelium of mucormycetes
was detected. mHRM-RT-PCR was positive in native, formalin-fixed paraffin-embedded
tissue samples and BAL of all patients with mucormycosis. PCR assay allowed to identify
the representatives of Mucormycetes: Lichtheimia corymbifera in 10 and Rhizomucor
pusillus in 2 from 12 samples. In biological specimens of 2 patients the PCR assay
detected a mixed infection by Aspergillus and Mucormycetes spp. The results of mHRM-RT-PCR
in patients with mucormycosis correlated with traditional methods. In 21 controls
tissue samples and 20 BAL PCR test was negative.
Conclusion: The multiplex RT-PCR has high sensitivity and specificity in patients
with mucormycosis. This study indicated that the mHRM-RT-PCR may be a very useful
tool for detection of etiologic agents of mycoses, particularly in the case of a mixed
infection caused by Aspergillus and Mucormycetes spp.
Tolnai
E.
Nwozor
K. Okechukwu
Fidler
G.
Szasz
R.
Rejto
L.
Biro
S.
Paholcsek
M.
Faculty of Medicine, Department of Human Genetics, University of Debrecen, Debrecen,
Hungary
P185. Clinical application of free circulating microRNAs in patients with hematologic
malignancies as promising biomarkers in monitoring invasive aspergillosis
Objectives: MicroRNAs (miRNAs) are small 19- to 23- nucleotide long, noncoding single
stranded RNA molecules. They play an important role in regulating various biological
processes through complementary base pairing with 3’- untranslated regions (3’ UTR)
of the corresponding target mRNAs. MiRNAs are key regulators of various physiological
and pathophysiological processes like cell proliferation, differentiation, apoptosis,
inflammation and cancer. Altered expression levels of miRNAs have been associated
with many diseases. Increasing number of evidences demonstrate that specific miRNAs
can be a great promise as novel biomarkers for clinical diagnosis of many types of
diseases due to their stability in various body fluids. However, little is known about
the potential use of circulating miRNAs as biomarkers in fungal infections. Invasive
aspergillosis (IA) is a life-threatening infection caused by Aspergillus especially
in immunocompromised patients. With this pilot study our aim was to verify the different
expression profiles of 21 pre-selected target miRNAs in hematologic malignant patients
with (proven, probable IA) and without (possible IA and healthy controls) IA. We assessed
the association of IA disease characteristics with the abundance of the free circulating
microRNAs in the liquid biopsy samples of these patients to identify potential diagnostic
markers for further studies.
Methods: We obtained whole blood (WB) samples from 40 patients at the onset of fevered
neutropenia (FN) and from 5 healthy volunteers. Total RNA was isolated from the WB
samples using the MagMaxTM mirVanaTM Total RNA Isolation Kit (Thermo Fisher Scientific).
MiRNAs were reverse transcribed to cDNA with TaqMan® Advanced miRNA cDNA Synthesis
Kit (Thermo Fisher Scientific). TaqMan® quantitative real-time PCR (miRNA assay) was
used for detecting miRNA expression profiles. Relative miRNA expressions were calculated
with the ΔΔCt method.
Results: We found that 7 out of the 21 miRNAs were strongly associated with IA by
being significantly (p<0.05; p<0,01) upregulated in our patient group compared to
the control group. Increase in miRNA expressions were also observed in the case of
further 7 miRNAs which expression differences did not prove to be statistically significant.
Conclusion: Based on the result of this pilot study, we detected significant expression
differences in the case of seven miRNAs. We think that these may be promising and
effective biomarker targets to support the diagnosis of IA. We also believe that the
diagnostic strategy relying on the combination of multiple circulating miRNAs promises
better positive predictive values in monitoring of IA.
Kozlov
G.
1
Vasilyeva
N.
2
Riabinin
I.
2
1Department of Technology For Microbiological Synthesis, Saint-Petersburg State Institute
of Technology, Saint-Petersburg, Russian Federation
2Kaschkin Research Institute of Medical Mycology. Department of Medical Microbiology,
North-Western State Medical University named after I.I. Mechnikov, Saint-Petersburg,
Russian Federation
P190. rIndication of opportunistic micromycetes in mycobiota of municipal solid waste
compost by metagenomic approach
Objectives: Objective of the study is to perform the genotaxonomic analysis of mycobiota
of thermal-treated compost from municipal solid waste (CMSW) to identify the microfungi
of medical interest.
Methods: The metagenomic study of samples using an internal transcribed spacer (ITS2)
as a tacosomic marker was performed on the Illumina MiSeq instrument (Illumina, USA)
according to the Manufacturer’s protocol. DNA-extraction, post-PCR purification of
apmplicons and DNA concentration measurement were made by utilization PowerSoil DNA
Isolation (Qiagen, Germany), AMpure XP magnetic beads (Beckman Coulter, USA) commercial
kits and Qubit 4 Fluorometer (ThermoFisher Scientific, USA) respectively. Bioinformatic
data processing was performed using QIIME 1.9.1 software, Silva and Unite databases.
Classification algorithm of open-reference operational taxonomic units was utilized.
Results: Among a variety of microfungi from CMSW-mycobiota representatives of 20 taxa
to varying degrees associated with pathologies and the indigenous human microbiota
were revealed. They can be divided into three groups. Group A consist of micromycetes,
which do not colonize the human body normaly (or occasional contaminants of the body’s
open loci) but can cause opportunistic mycoses: Aspergillus niger, Cryptococcus uniguttulatus,
Rhodotorula glutinis. Group B including micromycetes, sometimes occuring in biomaterials
of human body, as well as colonizing environmental objects: Candida zeylanoides#,
C. inconspicua*, C. membranifaciens#, Pichia norvegensis*, Meyerozyma guilliermondii#,
Kluyveromyces marxianus, Lodderomyces elongisporus, Issathenkia orientalis*, Trichosporon
asahii and Cutaneotrichosporon cutaneum. Group C: micromycetes that live mainly in
the human body (sometimes in animals) and are not common inhabitants of abiotic objects:
C. albicans, C. parapsilosis#, C. metapsilosis, C. glabrata*, C. tropicalis#, Malassezia
sp.
Conclusion: CMSW is expected to be a habitat for microorganisms that are interesting
in two aspects: (1) cause of their capability of carrying out uniqe metabolic pathways
associated with the biodegradation of plant polymers, synthetic plastics and metallic
materials and, on the other hand, (2) cause of representation by them the «microbiome
imprint» of human population of a settlement area. Some of the detected yeast organisms
are known to be resistant to antimycotics: the lists of groups B and C contain species
with intrinsic resistance to fluconazole (*) as well as containing strains with secondary
resistance mechanisms (#). The most unusual representative among the identified microbiota
was Malassezia sp. Despite the deep adaptation of malasseziae to the humans and animals
skin and numerous unsuccessful attempts to find their free-living related yeast, the
application of molecular genetics technologies in the 21st century made it possible
to identify the genetic material of these fungy in the most unexpected habitats (deep-sea
sediments, invertebrates, some freshwater fishes, soil of arctic zones etc.). Ways
to supply the high nutritional needs of malasseziae in the environment are not yet
clear, theoretically, Malassezia spp. in CMSW can borrow the polyunsaturated fatty
acids necessary for them directly from the remnants of dietary fats, or through symbiotic
interaction with other members of the microbial community. Cunducted research made
it possible to consider the CMSW-mycobiota as a potential niche associated with the
circulation of opportunistic micromycetes in the anthropogenic ecosystem of the city.
Drakulovski
P.
1
Gouveia
T.
1
Pierru
J.
2
Krasteva
D.
1
Pottier
C.
1
Arianiello
E.
2
Bellet
V.
1
Roger
F.
1
Bertout
S.
1
1Laboratoire De Parasitologie Et Mycologie Médicale, University of Montpellier, Montpellier,
France
2Centre De Sauvegarde De La Faune Sauvage, LPO, Villeveyrac, France
P191. Hunt for Cryptococcus: two years survey of the wild avian fauna of Southern
France
Objectives:
Cryptococcus sp. are environmental yeast whose natural biotope is still poorly understood.
Indeed, C. neoformans has been isolated mostly from pigeon faeces but also from vegetable
surfaces and several trees. C. gattii has been mostly isolated from decaying wood
of various tree species, like Eucalyptus, Pinus, Olea, and Ceratonia trees but also
from animals. C. laurentii, C. albidus have been isolated from soil, water but also
from animal faeces. A few other species like C. terrestris or C. aerius are considered
environmental only. In order to clarify the biotope of these yeasts, we are conducting
a two year survey of the wild avian fauna in Southern France.
Methods: Six hundred and four birds from 70 genera and 86 species, randomly selected
from the animals arriving for care at the Center for Protection of the Wild Fauna
of Herault were sampled. Sampling was performed during care protocols either by direct
cloacal swab or, when impossible, by swabbing of faecal drops present in cages. The
samples were treated as per the standardized protocol used by the ISHAM Cryptococcal
Working Group i.e transferred onto Niger Agar seed medium and grown at 28°C and 37°C
during 7 days minimum. Each suspected Cryptococcus colony was checked for the presence
of a capsule by India ink staining, for its urease activity and identified by API
ID32C and ITS sequencing.
Results: Identification allowed to find 39 C. albidus, 30 C. magnus, 13 C. laurentii,
2 Cutaneotrichosporon curvatus, 2 C. oeirensis, 2 C. diffluens, 2 C. carnescens, 2
Filobasidiella elegans, 1 C. wiriengae, 1 C. terrestris and 1 C. aerius coming from
33 different bird species. No C. neoformans nor C. gatti were found so far. Global
cryptococcal prevalence was of 15.7%.
Conclusion: Several Cryptococcus were found from bird wherein they were not described
until now. As such, this study brings new data on the avian species potentially carrying
various Cryptococcus sp yeast.
Bonadio
F.
1
Caetano
M.
2
Siqueira
J.P.
3
Almeida
M.
1
1Infectious Disease, Medical School, sao jose do rio preto, Brazil
2UNESP, São José do Rio Preto, Brazil
3Infeciosas Disease, FAMERP, São José do Rio Preto, Brazil
P192. Microbial monitoring and environmental influence before and after hospital architectural
design
Objectives: To assess the microbial contamination and the environmental impact of
reform in a hemodynamic service hospital ward, in a Hospital of the Interior of the
State of São Paulo, Brazil.
Methods: Environmental samples were collected from 10 surfaces (floor, walls, sinks,
and counters), in two different moment, in rooms of medical procedures. RODAC® plates
were imprinted in the different surfaces. Considering fungi and bacteria, plates were
incubated at 35°C for up to hours to allow all organisms to grow. Colonies were isolates
in agar BHI (DIFCO®) with identification of bacterial species conducted by automated
system VITEK®, and fungi by slide culture in Potato Agar (DIFCO®) and biochemical
characteristic. After first sampling, the rooms of the Hemodynamics Service in the
hospital ward was reformed, using all principles and materials commercially available
to control microorganisms. Innovative material and procedures of civil construction
were adopted, including paint, mortar, and coating following normative criteria according
to Brazilian legislation. Then, 10 new surface samples were obtained from the same
points of the previous collection, at the same incubation, isolation, and identification
criteria.
Results: In the first sampling, it was detected contaminated surfaces with high microbial
load: 100% for bacteria, and 80% for fungi, ranging from 1 to 400 CFU, according to
the collection point. Considering the microbial diversity, there was prevalence for
Gram positive bacteria from Staphylococcus spp, and filamentous fungi, especially
Aspergillus spp., Acremonium spp., and Scopulariopsis spp. After the reform, microbiological
analysis showed a significant reduction of microbial counts, as well as reduction
in the diversity of bacterial and fungal species. It was noted that 80% of the cultures
were negative. No fungi were observed and the bacterial isolates are still being identified.
Conclusion: The hospital environment should be constantly monitored for its competence
in non-proliferation of infectious agents. The presence of bacteria and fungi on surfaces
may constitute a risk factor for dissemination and occurrence of infectious diseases,
with great impact on Public Health. Old constructions may favor the dissemination
of these organisms. This study confirmed the high microbial contamination in the hospital
environment before reform and showed that a well-designed architectural design, with
appropriate material and adoption of normative procedures, can minimize the risks
of contamination and dissemination of potentially pathogenic microorganisms.
Cartier
N.
1
Sénéchal
R. Le
1
Becerra
M.
1
Bailly
E.
1
Cateau
E.
1
Chandenier
J.
2
3
Mulot
B.
4
Leclerc
A.
4
Desoubeaux
G.
2
3
1Parasitology - Mycology - Tropical Medicine, Hôpital Bretonneau, Tours, France
2Parasitology - Mycology - Tropical Medicine, Hôpital Bretonneau, TOURS, France
3Cepr Inserm U1100, Université de Tours, Tours, France
4Clinique Vétérinaire, ZooParc de Beauval & Beauval Nature, Beauval, France
P193. Study of fungal environmental contamination in nests of the penguin enclosure
of a large French animal zoo park.
Objectives:
Aspergillus fumigatus is an environmental mold with opportunistic pathogenicity. It
is responsible for aspergillosis, a disease particularly common in penguins that are
kept in a confined or insufficiently ventilated environment. At ZooParc de Beauval
(Saint-Aignan, France), the incidence of aspergillosis was estimated by autopsy at
4% in Humboldt penguins (Spheniscus humboldti) during the period [April 2018 - April
2019]. Antifungal treatment is based on the administration of azole drugs. This study
assessed the environmental contamination in the penguin enclosure and searched for
azole resistances in A. fumigatus isolates.
Methods: Two sets of environmental samples were carried out in the spring and fall
2018, by swabbing the artificial nests of the penguin enclosure in ZooParc de Beauval.
The collected specimens were inoculated onto malt extract agar at 35°C. In case of
fungal growth, a count of the colony number, as well as a phenotypic and molecular
identification were achieved. Each DNA of all the A. fumigatus isolates was systematically
sequenced to look for azole resistance mutations in CYP51 gene.
Results: Overall in spring and fall sampling, 0.8 and 2.6 mean fungal CFU per sample
of penguin nest were counted, i.e. 83% and 100% nests were contaminated with at least
one fungal species, respectively. Moreover, Aspergillus fumigati section was isolated
in 67% and 75% of them. Thus, the concordance rate was 67% between the two sets of
samling. Through to DNA sequencing, all but one isolate (Aspergillus nishimurae) were
eventually identified as A. fumigatus stricto sensu. To date, only one resistance
mutation for azole drugs was detected once in the CYP51 gene (G138A).
Conclusion: Despite the geographical location of ZooParc de Beauval in a rural environment,
intensive adjacent agricultural practices do not exhibit any impact on the selection
of resistant strains. However, the very high contamination rates herein encourage
great caution and regular checks of the fungal populations.
Haghani
I.
1
Shams-Ghahfarokhi
M.
2
Shokohi
T.
3
Hedayati
M.T.
3
Asl
A. Dalimi
4
Fathi
M.
4
1Medical Mycology, Mazandaran university of Medical sciences, Sari, Iran
2Department of Mycology, Faculty of Medical Sciences, Tarbiat Modares University,
Tehran, Iran
3Department of Medical Mycology, Invasive Fungi Research Center, School of Medicine,
Mazandaran University of Medical Sciences, Sari, Iran, Sari, Iran
4Department of Parasitology, Faculty of Medical Sciences, Tarbiat Modares University,
Tehran, Iran
P200. rSpecific Identification and Antifungal Susceptibility Pattern of Fungal Species
Isolated from Patients with Onychomycosis
Objectives: Onychomycosis is a common nail problem, accounting for up to 50% of all
nail diseases. The aim of the present study was to determine the species distribution
based on the restriction fragment length polymorphism and susceptibility patterns
of the causative agents of onychomycosis.
Methods: This cross-sectional study was conducted on nail samples collected from 257
patients suspected of onychomycosis within a year. Fungal isolates was identified
by polymerase chain reaction-restriction fragment length polymorphisms (PCR-RFLP)
with the enzymes Msp I, Mva I, Alw I and sequencing.
Results: According to the results, Out of the 257 patients participating in the study,
onychomycosis was diagnosed in 180 (70.03 %.) cases, among which 51.1% were caused
by non-dermatophyte molds (NDMs), 34.4% by yeasts, and 10.6% by dermatophytes. Numerous
cryptic species recovered from onychomycosis for the first time. In the majority of
cases, novel triazoles and imidazoles (i.e., efinaconazole, luliconazole, and lanoconazole)
showed potent activity in comparison to other antifungal agents. The minimum inhibitory
concentration (MIC) of luliconazole and lanoconazole ranged within 0.001 to >1μg/ml
and their geometric mean MICs were 0.0154 and 0.0309 μg/ml against all isolates, respectively.
Conclusion: It seems that obtained data will be useful to improve the knowledge of
researchers, clinicians, and dermatologists about onychomycosis distribution, species
diversity and adoption of appropriate treatment.
Barat
S.
Angulo
D.
SCYNEXIS, Inc., Jersey City, United States of America
P201. Ibrexafungerp (formerly SCY-078) Demonstrates Activity Against Candida auris:
In Vitro, In Vivo and Clinical Case Studies of Candidemia
Objectives:
Candida auris is a growing global threat; a pathogen associated with high mortality
(up to 60%), multi-drug resistance, the ability to spread from person-to-person and
surface-to-person, presenting high risk for outbreaks in healthcare facilities. Ibrexafungerp
is a novel IV/oral glucan synthase inhibitor (triterpenoid) antifungal with activity
against Candida, Aspergillus and Pneumocystis spp, in Phase 3 development. Given the
potent activity of ibrexafungerp against Candida spp., Scynexis has embarked on a
development program to understand the activity and effectiveness against Candida auris.
We will present the current preclinical and clinical data sets of ibrexafungerp against
Candida auris.
Methods:
In vitro studies tested ibrexafungerp against >100 clinical isolates of C. auris.
Other in vitro studies evaluated the effects of ibrexafungerp against C. auris biofilms.
In vivo activity against C.auris was evaluated using a disseminated murine model and
a cutaneous infection guinea pig model. In humans, an ongoing open-label trial of
ibrexafungerp for treatment of patients with infections caused by C. auris (the CARES
study) has been initiated in the USA and India.
Results:
In vitro and in vivo studies demonstrated that ibrexafungerp is active against C.
auris, including MDR strains. The MIC mode for ibrexafungerp was 1ug/ml and the MIC50
and MIC90 were 0.5 and 1 ug/ml, respectively. Many echinocandin resistant C. auris
isolates have shown susceptibility to ibrexafungerp. Further, ibrexafungerp has been
shown to reduce biofilm thickness. In animal models of C. auris infection, treatment
with ibrexafungerp resulted in improved survival and reduced fungal burden in both
the murine model of disseminated infection and the guinea pig model of cutaneous infection
as compared to untreated controls. In humans, two patients with difficult to treat
C. auris candidemias were enrolled in the CARES study and responded positively to
oral ibrexafungerp with eradication of the infection.
Conclusion: This data demonstrate that ibrexafungerp possess potent in vitro and in
vivo activity as well as promising clinical activity. Therefore, continued clinical
evaluation of ibrexafungerp as an option to treat C. auris infections is warranted.
Uhrlass
S.
1
Sitaru
C.
2
Scholz
C.
2
Gebhardt
M.
3
Baunacke
A.
4
Friedrichs
C.
5
Ranke-Greve
I.
6
Cleffmann
U.
6
Koch
D.
1
Muetze
H.
1
Krueger
C.
1
Rahmig
N.
7
Hipler
U.-C.
7
Nenoff
P.
1
1Partnership Dr. C. Krueger & Prof. P. Nenoff, Laboratory of medical microbiology,
Roetha OT Moelbis, Germany
2Klinik Für Dermatologie Und Venerologie- Mykologisches Labor, Universitätsklinikum,
Freiburg, Germany
3Hautarztpraxis, Zwickau, Germany
4Hautarztpraxis, Radeberg, Germany
5Medizinisches Labor Ostsachsen, Görlitz, Germany
6Dr. Stephan Bartels, Hautarztpraxis, Göttingen, Germany
7Klinik Für Hautkrankheiten, Universitätsklinikum, Jena, Germany
P202. Microsporum ferrugineum in Germany – an anthropophilic dermatophyte on the rise
Objectives: In Germany, the anthropophilic dermatophyte Microsporum (M.) ferrugineum
was nearly never isolated during the last 50 years. Currently, starting with the year
2016, single strains were detected, and small outbreaks of infections due to this
difficult to differentiate fungus have been observed in Germany. From July 2016 until
April 2019, altogether 19 M. ferrugineum strains were isolated. The dermatophytes
originated from patients all over Germany.
Methods: Cultural investigation revealed slowly growing colonies with white thallus
and peripheral yellow brownish submerged hyphae bundles. The reverse side of the furrowed
colonies showed cream coloured to yellow staining. Microscopically, large spherical
and oval double-walled intercalary localized chlamydospores, typical ‘bamboo‘ hyphae,
and acute-angled branched hyphae developed. Fungal culture material from all isolates
was identified by PCR and genomic Sanger sequencing of the internal transcribed spacer
(ITS) region and/or the translation elongation factor (TEF)-1α gene. Reference strain
for comparative identification was M. ferrugineum CBS 497.48 (Centraalbureau voor
Schimmelcultures CBS, Utrecht, The Netherlands, www.westerdijkinstitute.nl). Patients
were children and adolescents under 18 years, mainly males. Suggested source of infection
was martial sports, e.g. wrestling, judo, and boxing. Surprisingly, a significant
part of affected patients counted to Russian Germans. A migrant 3 years old boy from
Afghanistan suffering from tinea capitis was also among the patients. Another strain
was isolated from a 10 years old wrestler with suspicion of tinea corporis. There
was no background of migration or contact to foreigners, the boy did not stay abroad.
The mycological challenge is the cultural identification of M. ferrugineum. Confusion
with the morphological similar species M. canis, but also M. audouinii, and Trichophyton
verrucosum is possible.
Results: The comparison of the found ITS-2 sequence with known sequences deposited
at the database of the National Center for Biotechnology Information (NCBI), Bethesda,
Maryland, U.S., confirmed the species identification M. ferrugineum. The phylogenetic
analysis of the dermatophytes – the dendrogram of fungal strains – demonstrated the
genetic differences between M. ferrugineum strains and M. audouinii or M. canis. The
three species could clear distinguished from each other. In particular, sequencing
of the TEF-1α gene allowed discrimination between M. ferrugineum and M. audouinii
or M. canis better than sequencing the ITS-2-region. Meanwhile, the M. ferrugineum
strain 208361/2016 is deposited at the „Deutsche Sammlung von Mikroorganismen und
Zellkulturen” (DSMZ, German Collection of Micro organisms and Cell cultures) in Braunschweig,
Germany (DSM no. 103785). The DNA sequences of the M. ferrugineum strains are currently
deposited as MF173061- ITS - and as MF173060 - TEF-1α gene - at the NCBI database.
Conclusion: In Germany, in the last decades, M. ferrugineum was not isolated and described
so far. Currently, with this forgotten dermatophyte must be expected in particular
in migrants and asylum seekers. The species identification of M. ferrugineum represents
a challenge for the dermatologist. If there are morphological and microscopic features
suspicious for such a rare Microsporum-species, molecular identification of the causative
pathogen should be performed. For oral therapy of tinea capitis due to M. ferrugineum
griseofulvin should be given, alternatively itraconazole or fluconazole.
Uhrlass
S.
1
Rimek
D.
2
Hubka
V.
3
Schroedl
W.
4
Reuschel
M.
5
Brasch
J.
6
Muetze
H.
1
Koch
D.
1
Krueger
C.
1
Graeser
Y.
7
Nenoff
P.
1
1Partnership Dr. C. Krueger & Prof. P. Nenoff, Laboratory of medical microbiology,
Roetha OT Moelbis, Germany
2Gesundheitsschutz, Thueringer Landesamt für Verbraucherschutz, Bad Langensalza, Germany
3Laboratory of Fungal Genetics and Metabolism, Institute of Microbiology ASCR, Prague,
Czech Republic
4Ag Mykologie, Institut für Bakteriologie und Mykologie, Leipzig, Germany
5Klinik Für Heimtiere, Reptilien, Zier- Und Wildvögel, Stiftung Tierärztliche Hochschule,
Hannover, Germany
6Klinik Für Dermatologie, Venerologie Und Allergologie, Universitätsklinikum Schleswig-Holstein,
Kiel, Germany
7Institut Für Mikrobiologie Und Hygiene, Universitätsmedizin – Charité, Berlin, Germany
P203. Arthroderma chiloniense - a new geophilic dermatophyte – isolated from human
and hedgehog
Objectives: Due tot he new taxonomy of the dermatophytes, geophilic fungal species
are summarized within the genus Arthroderma (A.). Until now known Arthroderma species
are e.g. A. insingulare, A. gertleri, A. uncinatum, A. thuringiensis, besides, there
are the three relatively new species A. amazonicum, A. crocatum, and A. vespertilii.
Altogether four strains of an until now not known geophilic fungus - suggested as
mould or dermatophyte - were isolated between 2011 and 2017 from different patient´s
materials. Underlying dermatoses were a hand eczema or suspected tinea manus, nail
changes (nail plate pigmentation) to exclude an onychomycosis, and skin scrapings
vom the lower leg and body trunk to exclude a tinea corporis. An additional strain
of A. chiloniense was found in clinical material originationg from the skin and stings
of a hedgehog with alopecia diffusa and scaly skin.
Methods: Conventional and molecular mycological diagnostic was performed. Kind of
growth of the fungal colonies on common fungal media was assessed. Fungal culture
material from all five isolates was identified by PCR and genomic Sanger sequencing
of the internal transcribed spacer (ITS: 18S rRNA, ITS1, 5.8S rRNA, ITS2, and 28S
rRNA) region, the translation elongation factor (TEF)-1α gene, and β-tubulin. Human
strains 216228/16- DSM106573, 211495/17- DSM106575, 211496/17- DSM106574 and 215488/17-
DSM108904 are deposited at the „Deutsche Sammlung von Mikroorganismen und Zellkulturen”
(DSMZ, German Collection of Micro organisms and Cell cultures) in Braunschweig, Germany.
The from hedgehog isolated strain with laboratory number 250173/2018 has already been
submitted to the DSMZ, too.
Results: The white, flat, fast growing colonies presented with yellow reverse sides.
Microconidia are small, elongated, sessile lateral directly at the hyphae or the conidiophores,
also in dense aggregates. Later arthroconidia and multiple chlamydospores have developed,
however, no macroconidia. Urea splitting of all isolates was positive. By sequencing
of the gene regions ITS-1 and ITS-2, TEF-1α and β-tubulin showed, that the new species
showed the highest genetic similarity to the species A. crocatum and A. amazonicum.
Ultimately, the sequences of the here isolated unknown fungi could be assigned to
the new described geophilic dermatophyte Arthroderma chiloniense sp. nov.
Conclusion:
A. chiloniense presents a new geophilic dermatophyte, first described in Kiel, Germany,
in 2018, and published 2019. This species is obviously widely prevalent in Germany.
Isolated from human beings under the suspicion of a dermatophytosis of the skin or
nails, seems to indicate that A. chiloniense possesses pathogeic potential. Here,
A. chiloniense, for the first time was isolated from an animal, an European hedgehog.
Chayakulkeeree
M.
1
Ungulkraiwit
P.
2
Chongtrakool
P.
3
Ngamskulrungroj
P.
3
Groot
T. De
4
Meis
J.
5
1Medicine, Division of Infectious Diseases and Tropical Medicine, Faculty of Medicine
Siriraj Hospital, Mahidol University, Bangkok, Thailand
2Department of Medicine, Vejthani Hospital, Bangkok, Thailand
3Microbiology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok,
Thailand
4Medical Microbiology and Infectious Diseases, Canisius-Wilhelmina Hospital, Nijmegen,
Netherlands
5Department of Medical Microbiology and Infectious Diseases, Excellence Center For
Medical Mycology (ecmm), Canisius Wilhelmina Hospital, Nijmegen, Netherlands
P204. The First Case of Candida auris Fungemia in Thailand
Case Report:
Objectives:
Candida auris is an emerging fungal species resistant to several antifungal agents.
There is an increasing worldwide awareness of C. auris. We therefore aim to report
the first case of C. auris infection in Thailand.
Methods: We present the first case of C. auris fungemia in an elderly woman from Thailand.
Results: A 81-year-old woman was admitted in a private hospital in January 2018 due
to status epilepticus which required ICU support with prolonged mechanical ventilation.
In addition, she had chronic kidney disease requiring hemodialysis through her right
subclavian central venous catheter. During 4 months in the hospital, she experienced
catheter-related bacterial blood stream infection, nosocomial pneumonia, urinary tract
infection and tracheobronchitis which was treated with several regimens of broad-spectrum
antibiotics. On day 110 of hospitalization, she had hypotension with low-grade fever
during hemodialysis. Blood cultures from her central venous catheter grew Candida
spp. While peripheral line cultures remained negative. The central catheter was removed
and she was treated with micafungin. The Candida isolate was subsequently identified
as Candida auris by MALDI-TOF and sequencing. Swab cultures from the central catheter
exit site also grew C. auris. She denied any travel outside of Thailand prior to admission.
Despite intravascular catheter removal, she had again candidemia from C. auris three
weeks later and micafungin was re-introduced. However, the patient died 6 days after
restart of treatment. After diagnosis, strict contact precautions were installed with
no further intra-hospital transmission. CLSI microbroth dilution antifungal susceptibility
testing of all isolates showed resistance to fluconazole (32 mg/L) and voriconazole
(0.25 mg/L), higher MICs of amphotericin B (1 mg/L) and low MICs to echinocandins
(0.063 mg/L). Isavuconazole had low MICs of < 0.016 mg/L. By using AFLP and MLVA genotyping
the isolates had the same genotype and were shown to belong to the south-Asian C.
auris clade.
Conclusion: We report the first case of C. auris (south-Asian clade I) fungemia from
Thailand. The index case for this C. auris introduction into Thailand remained unclear
and no secondary cases were discovered. Active surveillance for this emerging antifungal-resistant
Candida species should be brought into attention to prevent healthcare-associated
outbreaks.
Farooqi
J.
1
Moin
S.
1
Mahmood
S.F.
2
Idress
R.
1
Naqvi
F.
1
Zafar
A.
1
1Pathology and Laboratory Medicine, Aga Khan University, Karachi-KHI, Pakistan
2Medicine, Aga Khan University, Karachi, Pakistan
P205. Disseminated phaeohyphomycotic lymphadenitis with Cladophialophora carrionii
Case Report:
Objective: This study describes a case of disseminated phaeohyphomycotic lymphadenitis
in a young woman with delayed diagnosis and good clinical response after appropriate
treatment.
Methods: A 32 year old lady presented with erythematous to violaceous papules, with
oozing discharge bilaterally in her inguinal region, for a few months. The history
revealed that she had a past history of tuberculous meningitis, treated with first
line antituberculous therapy for eighteen months, and two years back she developed
pigmented discharging lymph nodes bilaterally in her axillae. The histopathology of
the biopsy showed chronic granulomatous inflammation with multiple branching septate
fungal hyphae. She received amphotericin B for 21 days but without improvement. Now,
sample from these inguinal lesions was sent for histopathology and culture.
Results: Histopathology of the biopsy material showed dense inflammation and well-formed
granuloma comprising of epithelioid cells and multinucleate giant cells. Multiple
branching septate fungal hyphae noted in the inflammatory debri and in and around
granuloma. PAS stain highlighted septate fungal hyphae. Gram stain revealed moderate
septate hyphae with numerous pus cells. Culture on Sabouraud dextrose agar yielded
velvety olive-black colony in the fourth week. Microscopic slide examination of culture
material was suggestive of Cladophialophora carrionii. The patient was started on
voriconazole and was continued this for 6 months and the patient improved. On discontinuing
voriconazole after six months, there was a relapse in the disease. Voriconazole was
restarted and the patient improved.
Conclusion: Incomplete investigation of infectious lesions may delay diagnosis. Both
histopathological and microbiological assessments are equally important for making
a conclusive diagnosis. Antifungal therapy may delay growth of fungi that normally
grow within a week, hence a longer incubation time may be warranted for fungal smear
positive samples. In phaeohyphomycosis, the infection is usually introduced by traumatic
inoculation or inhalation of the etiologic agent; and clinical presentations are greatly
influenced by the immune status of the host. In our case there was no known immune
deficiency and no trauma that the patient could recall.
Bharat
A.
1
Alexander
D.
2
Dingle
T.C.
3
Dufresne
P.J.
4
Hoang
L.M.
5
Kus
J.V.
6
Schwartz
I.S.
7
Gade
L.
8
Litvintseva
A.P.
8
Mcgeer
A.
9
Mulvey
M.R.
1
1Antimicrobial Resistance and Nosocomial Infections, National Microbiology Laboratory
Canada, Winnipeg, Canada
2Cadham Provincial Laboratory, Winnipeg, Canada
3Alberta Public Laboratories, Edmonton, Canada
4Laboratoire de santé publique du Québec, Sainte-Anne-de-Bellevue, Canada
5BC Centres for Disease Control, Vancouver, Canada
6Public Health Ontario, Toronto, Canada
7University of Alberta, Edmonton, Canada
8Mycotic Diseases Branch, US Centers for Disease Control and Prevention, Atlanta,
United States of America
9Mount Sinai Hospital, Toronto, Canada
P206. Genomic sequencing of Candida auris cases in Canada, 2012 - Feb 2019
Objectives:
Candida auris is an emerging yeast that is associated with high rates of antifungal
resistance and healthcare-associated outbreaks that can be difficult to control. Our
objective was to carry out genomic characterization of all known C. auris cases in
Canada to monitor the emergence of this species.
Methods: Nineteen isolates of C. auris identified from 2012 - Feb 2019 were submitted
to the Canadian Mycology Reference Centre which is in development at Canada’s National
Microbiology Laboratory (NML). Isolates from all 19 cases were subjected to whole
genome sequencing (WGS) on the Illumina Nextseq platform. Phylogenetic analysis based
on nucleotide differences in the core genome was carried out with the SNVPhyl v1.1
pipeline and assemblies were performed with SPAdes v1.6.
Results: The isolates were obtained from axilla/groin (n = 7), blood (n = 4), ear
(n = 3), and other sites (n = 5). All Canadian isolates of C. auris fell within the
four known genomic lineages (clades), which were initially named after the continents
where they were identified and then renamed Clades I - IV. Canadian cases were linked
to the Clade I (South Asian clade, n = 10), Clade IV (South American clade, n = 4),
Clade III (African clade, n = 3), and Clade II (East Asian clade, n = 1). The four
geographical clades were highly divergent with approximately 15,000 - 60,000 single
nucleotide variants (SNV) between clades. Canadian isolates within a clade were often
clonal, for example, isolates from Clade IV (South American clade) differed by only
14-26 SNVs. Canadian isolates from Clade I (South Asian clade) differed by an average
of 79 SNVs, however, epidemiologically-linked isolates within this clade differed
by only 1-19 SNVs. We identified mutations associated with fluconazole resistance
(ERG11 Y143R) and voriconazole resistance (ERG11 Y132F) but not echinocandin resistance
(FKS1 hotspot mutations).
Conclusion: The number of known cases of C. auris in Canada remains low and all cases
were susceptible to echinocandins. Whole genome sequencing confirmed the global origins
of Canadian cases. Isolates tended to be clonal within each clade but high resolution
WGS may be helpful in discriminating between patient transmission and separate introductions
into a healthcare facility.
Jurado
I.
1
Varela-Echegaray
E.
1
Marcos-Arias
C.
1
Groot
P. De
2
Quindós
G.
1
Eraso
E.
1
1Inmunology, Microbiology, Parasitology, University of the Basque Country (UVP/EHU),
Bilbao, Spain
2Regional Center For Biomedical Research, Castilla-la Mancha Science & Technology
Park, University of Castilla-La Mancha, Albacete, Spain
P207. Misidentification of Candida duobushaemulonii associated to superficial infection
and reduced susceptibility to azoles
Objectives: Candidiasis by Candida haemulonii complex (Candida haemulonii and Candida
duobushaemulonii) and closely related species, such as Candida auris and Candida pseudohaemulonii
that show reduced susceptibility to antifungal drugs are increasing. Conventional
phenotypic diagnostic methods are unable to identify these species. Therefore, their
prevalence may have been underestimated. The aim was to use novel molecular techniques
to identify isolates from a stock culture collection with dubious identities, which
could belong to emerging and multi-resistant species related to C. haemulonii.
Methods: The study included 150 clinical isolates from the yeast stock collection
of the Medical Mycology Laboratory at the University of the Basque Country (UPV/EHU)
collected between 1993 and 2017 that could be inaccurately identified by API®ID 32C
(bioMérieux, France). Three clinical confirmed isolates (two C. auris and one C. duobushaemulonii)
and one C. pseudohaemulonii reference strain (CBS 10004) were included as positive
controls. All isolates were analysed by two different PCR techniques: a C. auris-specific
PCR1 and a C. haemulonii complex-tetraplex PCR2. Species identities were confirmed
by sequencing the ITS1-5.8S-ITS2 region. Finally, in vitro susceptibility to nine
antifungal agents (anidulafungin, micafungin, caspofungin, posaconazole, voriconazole,
itraconazole, fluconazole, amphotericin B and 5-fluorocytosine) was tested using SensititreTM
YeastOneYO10 (Thermo Scientific, USA).
Results:
C. auris-specific PCR accurately identified both confirmed isolates of C. auris and
no bands were detected for the other isolates. Eight isolates previously identified
as C. haemulonii were confirmed by tetraplex PCR. Neither C. auris nor C. pseudohaemulonii
were detected in the collection. One isolate from a toenail previously identified
in 1996 by API®ID 32C panel as Candida intermedia produced a 115 bp sized band in
the tetraplex PCR, corresponding to C. duobushaemulonii. All identifications were
confirmed by ITS sequencing. The C. duobushaemulonii isolate was re-analysed by the
current API®ID 32C panel, which identified it as Candida sake. The C. duobushaemulonii
isolate was susceptible dose-dependent to itraconazole (MIC 0.5 µg/mL) and fluconazole
(MIC 32 µg/mL). All C. haemulonii were susceptible dose-dependent to itraconazole
(MIC 0.5 µg/mL) and fluconazole (MIC 16 µg/mL) except two, who were resistant (MIC
1 and 64 µg/mL, respectively), and their susceptibility to amphotericin B was reduced
(MIC 2->8 µg/mL). The C. pseudohaemulonii reference strain CBS 10004 was susceptible
to all antifungal drugs. Both C. auris isolates showed resistance to fluconazole (MIC
>256 µg/mL), dose-dependent susceptibility to itraconazole (MIC 0.25 µg/mL), and one
of them was also susceptible dose-dependent to voriconazole (MIC 2 µg/mL).
Conclusion:
Candida haemulonii complex and closely related species may have been associated to
superficial infections prior to its emergence related to invasive infections, as indicated
by the identification of a C. duobushaemulonii isolate from before 2012, when current
classification of the complex was established. This complex shows reduced susceptibility
to azoles and amphotericin B. Funding: GIC15/78 IT-990-16 (Gobierno Vasco) and SAF2017-86188-P
(MINECO, Spain). 1. Ruiz-Gaitán et al. Molecular identification of Candida auris by
PCR amplification of species-specific GPI protein-encoding genes. Int J Med Microbiol.
2018;308:812-818. 2. Arastehfar et al. Low-cost tetraplex PCR for the global spreading
multi-drug resistant fungus, Candida auris and its phylogenetic relatives. Front Microbiol.
2018;29;9:1119.
Testa
J.
1
2
Calori
A.
3
Nicolini
L.A.
4
Pizzi
M.G.
2
Zeroli
C.G.
2
Martegani
R.
2
Menzaghi
B.
2
1Biotechnologies and Life Sciences Department, Center for Clinical Ethics, University
of Insubria, Varese, Italy
2Busto Arsizio Hospital, Asst-valleolona, Infectious Diseases Unit, Busto Arsizio
(VA), Italy
3Busto Arsizio Hospital, Asst-valleolona, Urology Unit, Busto Arsizio, Italy
4Department of Health Science (dissal), Infectious Diseases Unit, Genova, Italy
P208. Acute fungal obstructive pyelonephritis, echinocandin is a possible treatment?
A case report
Case Report:
Objectives/Introduction: Urinary tract candidiasis is the most frequent nosocomial
fungal infection worldwide; ascending acute pyelonephritis (AP) due to Candida species
are uncommon complications. Nowadays we observe a change in the distribution of Candida
species, with an increasing number of non-albicans Candidaisolations. In this setting
Fluconazole is the treatment of choice; the role of echinocandin is unknow, because
of the limited repository of antifungals that achieve adequate urinary concentrations.
Methods: We report a Candida tropicalis complicated urinary tract infection (cUTI)
in a patient with diabetes mellitus who successfully treated by high dosage of Caspofungin
and oral fluconazole (FLC). The infection was triggered by the placement of a ureteral
double J stent and bladder catheter, arranged for kidney stones.
Results: A 67-year-old Italian woman affected by diabetes mellitus, metabolic syndrome
and recent breast cancer, was transferred in the autumn of 2018 from an outside institution
after double J stent positioning for left ureteral stone complicated by grade III
hydronephrosis. At the admission fever and increase inflammatory values were present,
so blood and urine cultures were performed. Empiric antibiotic therapy with carbapenems
was started, without clinic improvement in 5-6 days, so we interrupted this therapy.
We isolated only pan-sensitive Candida tropicalis in urine sample but with few colonies,
so we considered as colonization. She therefore underwent to CT-scan with urological
and thoracic studies: we found left AP complicated by micro abscesses and pulmonary
thrombo-embolism. We decided to introduce high dosage of FLC and to remove ureteral
catheter double J and bladder catheter; we isolated from them another time the same
type of Candida tropicalis. Considering a diffuse disease, trans-thoracic echocardiography
and fundus oculi exam were performed, excluding fungal manifestations. After few days
she remained febrile and blood inflammation biomarkers not decreased very well, so
we decided to switch therapy with an alternative one, suspending FLC, we started Caspofungin
at the dosage of 70 mg/die. Immediately after initiation of treatment we observed
a rapid improvement with disappearance of the fever. After two weeks, abdomen CT-scan
showed an inflammation reduction and she was discharged home with maintenance high
dosage FLC (800 mg/die), concluding antifungal therapy after 3 overall months. Finally,
after therapy, CT-scan and urine culture were prescribed with no evidence of recurrence.
The patient is currently alive, without urinary problems.
Conclusion:
Candida tropicalis is a rare cause of AP. Echinocandins have been traditionally avoided
in the treatment of cUTI because of their poor urinary concentrations; their use with
high dosage, owes an achieved high renal tissue concentration. This case shows that
in fungal cUTI we can use high dosage echinocandin if fluconazole is no possible for
any reasons. We want also to stress that is necessary a prolonged therapy in fungal
AP.
Vogelzang
E.
1
Weersink
A.
2
Meis
J.
3
Dijk
K. Van
1
1Medical Microbiology and Infection Control, AUmc, location VUmc, Amsterdam, Netherlands
2Medical Microbiology and Immunology, Meander Medical Center, Amersfoort, Netherlands
3Department of Medical Microbiology and Infectious Diseases, Excellence Center For
Medical Mycology (ecmm), Canisius Wilhelmina Hospital, Nijmegen, Netherlands
P209. The first two cases of Candida auris in The Netherlands
Objectives:
Candida auris is a rapidly emerging, multidrug resistant pathogenic yeast. In recent
years an increasing number of Candida auris invasive infections and colonized patients
have been reported from several continents, including the USA, Africa, Europe and
Asia. Here, we describe the first two cases of Candida auris in The Netherlands.
Methods: Case 1 was a middle aged patient repatriated from an Indian hospital to a
Dutch intensive care unit (ICU). He had been hospitalized for five weeks in an Indian
hospital for treatment of sepsis with multiple organ failure due to pneumonia. He
was mechanically ventilated and repatriated with a tracheostomy tube and continuous
renal replacement therapy for which he had a central venous catheter. Routine admission
screening cultures for MRSA and multidrug-resistant gram-negative bacteria showed
yeast growth from Continuous renal replacement therapya removed central venous catheter
which the Bruker MALDI-TOF MS identified as Candida auris. Case 2 was a middle aged
patient, referred by the Amsterdam airport medical services to the hospital due to
shortness of breath and inability to stand without assistance. He was in Amsterdam
airport for a layover flying from India to the USA. Due to renal insufficiency, he
had received hemodialysis in an Indian healthcare facility up to two days before presentation
in our hospital. Due to subfebrile temperature a urine sample was cultured which grew
10^4-5 CFU yeasts. The Bruker MALDI-TOF MS identified the yeast as Candida auris.
Results: In both cases contact precautions were instituted since patients had been
previously admitted to an Indian healthcare facility. Routine screening cultures were
taken from nose, throat and perineum to identify potential multidrug-resistant gram-negative
bacteria and MRSA. At present no routine screening for Candida auris is performed
in the Netherlands. Screening failed to detect Candida auris, but groins and axilla
screens were not included. Molecular typing with AFLP and MLVA showed that both isolates
had closely similar genotypes and both belonged to the South Asian Candida auris Clade
I. Broth microdilution showed that both Candida auris isolates had high fluconazole
(>64 mg/L) and voriconazole (4 mg/L) MICs. Amphotericin B (0.5-1 mg/L), anidulafungin
(<0.016-0.063 mg/L) and micafungin (0.063 mg/L) had low MICs. In both cases antifungal
therapy was not indicated since there were no signs of an infection. Due to installment
of contact precautions no transmission of Candida auris was observed.
Conclusion: We report the first two cases of Candida auris in The Netherlands in patients
who were recently admitted to an Indian healthcare facility. The patients were immediately
placed in contact isolation after admission and no transmission of Candida auris occurred.
Concern is raised regarding surveillance for multidrug-resistant organisms, since
routine screening of patients from foreign hospitals is still focused on multidrug-resistant
gram-negative bacteria and MRSA and not yet on (multi)drug resistant yeast, such as
Candida auris.
López-Saiz
A.
Sevillano
E.
Guridi
A.
De-La-Fuente
I.
Mateo
E.
Eraso
E.
Quindós
G.
Immunology, Microbiology and Parasitology, University of the Basque Country (UPV/EHU),
Bilbao, Spain
P210. rEvaluation of the disinfectant activity of a portable ultraviolet C equipment
Objectives: Healthcare-associated infections are caused by different microorganisms,
including bacteria, viruses and fungi. In the recent years, Candida auris has emerged
as a multidrug-resistant fungal pathogen difficult to eradicate from hospital environment.
It has been isolated from medical equipment, and from different objects that hardly
ever are disinfected and can act as fomites (bed hand-controllers or mobile phones).
In some cases, routine chemical and physical decontamination approaches are used but
not all the above-mentioned objects can be treated with them due to possible deterioration.
Ultraviolet C radiation could represent an alternative, which does not cause aesthetic
or functional alterations in inanimate objects. Therefore, the objective of this study
was to evaluate the disinfection capacity of a portable ultraviolet C (UV Sanitizer
Corvent®) equipment.
Methods: The disinfectant effect of the UV sanitizer equipment was evaluated using
different exposure times (30 s, 1 min, 2 min, 3 min and 5 min), against five Candida
isolates: three clinical strains of C. auris, and two reference strains, Candida albicans
SC5314 and Candida parapsilosis ATCC 22019. These microorganisms were inoculated onto
slides, starting from a suspension of 1.5-5.0 x 109 ufc/mL, that was mixed with an
interference solution to analyze the disinfecting effect in both clean and dirty conditions
(presence of organic matter). Slides were exposed to ultraviolet C radiation inside
the equipment. The results were compared with those obtained after the disinfection
of the slides by immersion in 70% ethanol following the protocol described in EN 14562:2006
standard.
Results: Disinfection, considered as a logarithmic reduction equal to or greater than
5 from the initial inoculum, was achieved at a minimum exposition time of 3 min, in
both clean and dirty conditions. The presence of organic matter interfered in the
disinfection process to some extent, since the final values were slightly higher after
the treatment of the samples in dirty conditions. The effectivity of 3 min immersion
in 70% ethanol was very high against all microorganisms, obtaining a total reduction
in all cases.
Conclusion: The ultraviolet C radiation applied for 3 minutes using the portable ultraviolet
C UV Sanitizer Corvent® equipment was effective in the disinfection of slides inoculated
with C. auris, C. albicans and C. parapsilosis. This equipment is a promising alternative
for implementing disinfection protocols in hospitals and laboratories with inanimate
objects of common use (mobile phones, TV or bed controllers) reducing risk of infection
transmission.
Arastehfar
A.
1
Daneshnia
F.
1
Hilmioğlu-Polat
S.
2
Fang
W.
3
Yaşar
M.
2
Polat
F.
2
Metin
D. Yeşim
2
Zomorodian
K.
4
Coenye
T.
5
Ilkit
M.
6
Pan
W.
3
Liao
W.
3
Hagen
F.
1
Kostrzewa
M.
7
Lass-Flörl
C.
8
Boekhout
T.
1
1Westerdijk Fungal Biodiversity Institute, Utrecht, Netherlands
2Department of Microbiology, Faculty of Medicine, University of Ege, Izmir, Turkey
3Medical Mycology, Shanghai Changzheng Hospital, Second Military Medical University,
Shanghai, China
4Basic Sciences In Infectious Diseases Research Center, Shiraz University of Medical
Sciences, Shiraz, Iran
5UGent, Ggent, Belgium
6Division of Mycology, Department of Microbiology, Faculty of Medicine, University
of Çukurova, Adana, Turkey
7Research and Development, Microbiology and Diagnostics, Bruker Daltonik GmbH, Bremen,
Germany
8Div Hygiene & Med. Microbiology, Med. Univ. Innsbruck, Innsbruck, Austria
P211. Emergence of ERG11 Y132F azole resistant Candida parapsilosis blood isolates
in a Turkish hospital: A 10 year experience (2009-2019)
Objectives: The recent emergence of azole resistant C. parapsilosis isolates carrying
Y132F mutation in ERG11 is a matter of concern in India, Kuwait, South Korea, and
USA. Given the horizontal transfer of C. parapsilosis and the propensity of Y132F
mutants to colonize the hospital environments, their prompt identification followed
by application of appropriate infection control strategies are of importance.
Methods: During 10 years (2009-2019), 228 non-duplicate isolates of C. parapsilosis
were recovered from the blood samples of patients admitted to Ege University Hospital,
Izmir, Turkey. All isolates were primarily identified by API 20C AUX and re-identified
via MALDI-TOF MS. Regardless of fluconazole MIC values, ERG11 sequencing was performed
for all 228 isolates. In the next step, MRR1, TAC1, and UPC2 sequencing was performed
for 90 isolates containing all resistant isolates with or without ERG11 Y132F mutation
and randomly selected susceptible dose-dependent and susceptible isolates.
Results: Surprisingly, Y132F was the most predominant mutation in ERG11 (n = 46, 20.2%),
followed by K143R (n = 21, 9.2%), and G458S (n = 6, 2.6%), all implicated in fluconazole
resistance. Moreover, Q250K and G307A were two newly suggestive mutations exclusively
occurred in fluconazole resistant isolates. Among isolates harboring ERG11 Y132F,
43.5%, 17.3%, and 10.8% of isolates carried nonsynonymous mutations in UPC2, TAC1,
and MRR1, which is inconsistent to what was previously reported from a South Korean
study. The first isolate carrying Y132F mutation emerged in 2012 and isolates carrying
this mutation persistently recovered from the blood samples onwards (2012-2019). Strikingly,
the number of isolates harboring ERG11 Y132F, doubled and increased 5 fold between
2009-2015 and 2009-2019, respectively (Figure 1). Future studies are ongoing to determine
the corresponding genotype of each isolate using microsatellite typing and the antifungal
susceptibility data for voriconazole, micafungin, and anidulafungin remain to be completed.
Conclusion: We observed a significant and persistent isolation of emerging ERG11 Y132F
fluconazole-resistant C. parapsilosis isolates, which is a worrisome complication
in our hospital requiring implementation of typing and sequencing of target genes
followed by establishing appropriate infection control strategies to confine further
spread of such isolates. The widespread use of azoles in clinical settings, especially
developing countries, highlights the importance of worldwide surveillance studies
to evaluate the prevalence and spread of ERG11 Y132F isolates. Figure 1. Accumulation
of ERG11 Y132F mutation in C. parapsilosis isolates over 10 years of study from the
blood samples of patients admitted to Ege University Hospital, Izmir, Turkey.
Kale
P.
1
Khillan
V.
1
Sarin
S.K.
2
1Clinical Microbiology, Institute of Liver and Biliary Sciences, New Delhi, India
2Hepatology, Institute of Liver and Biliary Sciences, DELHI, India
P212. Candida auris: Epidemiology, Risk Factor Analysis and Management of Invasive
Infections in Tertiary Care Hepatobiliary Centre
Objectives:
Candida auris involvement in candidemia and other deep-seated invasive infections
is associated with very high mortality. The inability of several available commercial
identification systems to quickly diagnose C. auris remains a challenge to early therapy.
The data is limited especially in patients with hepatobiliary diseases, in whom the
risk factors and mangement differs due to the risk of antifungal toxicity. We aimed
to study the epidemiology, risk factors and therapeutic management of invasive infections
caused by C. auris in patients with hepatobiliary diseases.
Methods: Single-center, prospective study of patients with suspected invasive fungal
infections between January2017-December 2018. the patients with hepatobiliary diseases
were included inthe study based on vlinical diagnosis and radiological features. Demographics,
comorbidities, and laboratory variables were recorded. The samples recieved in the
microbiology laboratory as a part of clinical diagnostic protocols were processed
accordingly. The positive yeast cultures were subjected to identification by Vitek
2(Biomerieux, India) with updated software using YST cards, and antifungal sensitivity
confirmed by Broth microdilution in accordance with CLSI guidelines. The final outcome
considered were mortality within one month after diagnosis or discharge of the patient
with stable parameters.
Results: Total of 109 isolates of C. auris from 55 patients, blood 14(12.8%), abdominal
fluids 14(12.8%), urine 58(53.21%), respiratory 4(3.6%), liver abscess 2 (1.83%),
pancreatic abscess 1(0.9%) and wound infections 3 (2.75%). Underlying disease was
chronic liver disease 37 (68%), 10 (20%) post liver transplant patients, acute on
chronic liver failure 4 (7%), acute liver failure 2 (3%), acute pancreatitis 1 (1.8%)
and pancreatic neuroendocrine tumor 1 (1.8%). 14 (25%) patients were discharged, mortality
was 41 (74.04%). Risk factors were MELD 40 (p0.04), Child C (p 0.05) and CTP score
above 12. Prior use of steroids (p 0.02), neutropenia (p 0.03), prolonged hospital
stay (0.029), use of broad spectrum antibiotics more than 7 days (p 0.05) were the
risk factors significantly associated with development of Candida auris infections
and higher mortality. Co-morbidities, acute renal failure, diabetes, hepatitis infection
were not significantly associated with mortality. The antifungal resistance: fluconazole
68.42%, voriconazole 14.03%, flucytosine 59.64%, amphotericin B 28 % with no resistance
to caspofungin and micafungin.
Conclusion:
C.auris infections are associated with misidentification, intrahospital transmission,
poor treatment outcomes and higher mortalities. Prompt detection, earlier initiation
of therapy and effective surveillance can curtail C. auris in hospitals. Our study
depicts the spectrum of invasive infections caused by C. auris, its prevalence, risk
factors and therapeutic options. The presence of risk factors like steroid use, neutropenia,
broad spectrum antibiotic use and hospital stay of more than 7 days should prompt
towards escalating diagnostic measures for rapid identification of C.auris for early
initiation of therapy. Active screening of patients with risk factors can also reduce
mortality. The study results also help to guide empiric therapy with echinocandins
as azoles and amphotericin B show high resistance in these isolates. This study will
guide prophylactic and therapeutic options in hepatobiliary disease patients with
underlying risk factors to reduce mortality.
Poirier
W.
Giraud
S.
Bouchara
J.-P.
Host-Pathogen Interaction Study Group, SFR ICAT 4208, Univ Angers, Univ Brest, Institute
of Biology in Health, IRIS, University Hospital Center, Angers, France, Angers Cedex,
France
P213. Lignin degradation pathway by Scedosporium species
Objectives:
Scedosporium are usually soil saprophytes. Among the ten species recognized within
the genus, three have been regularly reported as causing human infections, particularly
in patients with cystic fibrosis (CF): Scedosporium apiospermum, Scedosporium aurantiacum
and Scedosporium boydii. Because of their low sensitivity to almost all available
antifungal drugs, a better understanding of the pathogenic mechanisms of these fungi
is required in order to identify new therapeutic targets. Likewise, identification
of the origin of the contamination of patients may be helpful to propose prophylactic
measures. In this aim, environmental studies were conducted on the ecology of these
opportunistic molds. Scedosporium species are abundant in human-made environments,
in relation with specific traits of the fungi: ability to survive at low oxygen pressure
and to degrade hydrocarbons. Recent studies have demonstrated the correlation between
the lignocellulolytic properties of filamentous fungi and their ability to degrade
environmental organic pollutants such as aromatic hydrocarbons. Among the lignocellulosic
compounds, the particular properties of lignin make it an interesting study molecule.
In this context, we attempted to elucidate the mechanisms developed by Scedosporium
species to degrade this polyphenolic molecule, with a special emphasis on the opening
of aromatic rings.
Methods: Culture in specific media and biochemical analyses were performed to characterize
Scedosporium properties. Orthologue enzymes from the literature were used to screen
Scedosporium genomic data by tBLASTn (Basic Local Alignment Search Tool) searches
and real-time PCR experiments were then conducted to analyze the relative expression
of these candidate genes.
Results: The lignolytic properties of Scedosporium species were first confirmed by
cultivation of the fungi on agar media containing the different components of lignocellulose
as carbon source. According to literature, the early steps in the lignin degradation
pathway involve peroxidases and oxidases. Biochemical analyses allowed us to detect
these enzyme activities in the culture supernatant of these fungi grown in lignin-containing
media. Fifteen ortologue genes were identified by tBLASTn searches, four encoding
members of the peroxidase family and the others encoding oxidase. However real-time
PCR experiments revealed that only four of these genes encoding peroxidases (2) and
oxidases (2) were over-expressed in the presence of lignin. Based on an exhaustive
bibliography, at least three different pathways, i.e the β-ketoadipate, the oxoadipate
and the gentisate pathways, were identified as potential routes for the degradation
of molecules resulting from the early oxidation steps and the opening of the aromatic
rings. In all Scedosporium species, enzymes involved in the gentisate pathway are
encoded by genes organized into a six membered gene cluster as revealed by bioinformatic
analyses, and real-time PCR experiments demonstrated the over-expression of all these
genes in culture media containing dihydroxybenzoate.
Conclusion: Results obtained in this study confirm that Scedosporium species exhibit
the enzymatic arsenal to degrade natural complex molecules and are capable to open
aromatic rings. This may explain their presence in polluted environments, but one
may also consider these enzymes as virulence factors since it has been suggested a
link between the ability to use aromatic compounds and the propensity of these fungi
to cause cerebral infections in receptive hosts.
Chouaieb
H.
1
Yaacoub
A.
1
Mili
W.
2
Ismail
S.
1
Ghorbel
M.
2
Fathallah
A.
1
1Parasitology Mycologie Laboratory, Farhat Hached university hospital, Sousse, Tunisia
2Ophthalmology Department, Farhat Hached University Hospital, Sousse, Tunisia
P214. Fusarium keratitis with poor prognosis: a report of four cases.
Case Report:
Introduction and objective Keratitis caused by Fusarium species, a sight-threatening
disease, is difficult to treat because Fusarium spp are highly resistant to most antifungals.
Our objective was to describe four cases of Fusarium keratitis with poor prognosis.
Methods Direct microscopic examination of corneal scrapings and culture on Sabouraud-chloramphenicol
medium were performed. The identification of fungi was based on macroscopic and microscopic
characteristics of culture.
Results All patients were male and the median age was 53, 5 years (range: 38-70).
The predisposing factors were corneal trauma by a vegetative matter in three cases
(75%), past history of herpetic keratitis in one case (25%) and steroid use in one
case (25%). Direct microscopic examination was positive in 75% of cases and culture
was positive in all cases. Culture grew Fusarium solani (50%) and Fusarium solani/Fusarium
chlamydosporium (50%). It is important to mention that the two patients with Fusarium
solani/Fusarium chlamydosporium keratitis were hospitalized in the same room; the
first one was referred to ophthalmology department for Fusarium keratitis, when the
second one developed the infection during his hospitalization. On the other hand,
the antifungal susceptibility of the two strains were identical, so, it is quiet likely
that the second patient was contaminated by the first one. Three patients underwent
surgery and received antifungal therapy. One patient was treated by topical Amphotericin
B associated to oral Itraconazole, the second received topical Amphotericin B, topical
Betadine and oral Voriconazole and the third patient was treated by diluted Betadine,
topical and oral Voriconazole. The fourth patient was lost to follow-up; he was discharged
against medical advice. In most cases, the outcome was adverse.
Conclusion Keratomycosis is a severe disease that can lead to severe visual loss and
even loss of the eye. Prompt diagnosis and early treatment are needed to avoid complications.
Borzova
Y.
1
Voronina
O.
2
Ryzhova
N.
2
Kunda
M.
2
Aksenova
E.
2
Simonova
O.
3
Davletgareeva
D.
4
Suslova
I.
4
Bogomolova
T.
1
Skorbunova
O.
4
Klimko
N.
5
Vasilyeva
N.
4
1Department of Medical Microbiology, North-Western State Medical University named
after. I.I. Mechnikov, Sant-Petersburg, Russian Federation
2N.F. Gamaleya National Research Center of Epidemiology and Microbiology, Ministry
of Health of Russia, Moscow, Russian Federation
3National Medical Research Center for Children’s Health, Ministry of Health, Moscow,
Russian Federation
4Kashkin Research Institute of Medical Mycology, North-Western State Medical University
named after. I.I. Mechnikov, Sant-Petersburg, Russian Federation
5Department of Clinical Mycology, Allergy and Immunology, North-Western State Medical
University named after. I.I. Mechnikov, Sant-Petersburg, Russian Federation
P215. Candida blankii as an emerging pathogen of cystic fibrosis patients
Objectives:
Candida blankii is emerging pathogen for children, and can be important for patients
with cystic fibrosis (CF). Publications about C. blankii in CF patients are limited.
Methods: Tracheal aspirate and sputum of 255 CF children 0-18 years old were analyzed
by DNA extraction, amplification of ITS1-5.8SrDNA-ITS2 fragments of Fungi genomes,
Sanger sequencing and identification of sequences using BLAST NCBI. Massively parallel
sequencing of amplicons based on the MiSeq Illumina platform was used for some samples.
Bioproject PRJNA545010 was published in GenBank.
Results:
C. blankii was revealed in the samples of two patients (P). P1 was 12 years old when
C. blankii was detected in microbiome containing Pseudomonas aeruginosa, Mycobacteroides
abscessus, but dominating bacteria were Streptococcus spp. The month later C. blankii
was replaced by C. sake. In response to the therapy microbiome cleared of M. abscessus,
but saved P. aeruginosa. The year later fungi and P. aeruginosa were not revealed
in the low airway sample of P1. P2 has C. albicans and P. aeruginosa in the low airway
sample at 7 years. Dominating bacteria were Streptococcus spp. At 8 years, when P2’s
conditions worsened, C. blankii, P. aeruginosa and Capnocytophaga spp. as main bacteria
were revealed. Active therapy eliminated P. aeruginosa, but did not influence on C.
blankii. The year later C. blankii accompanied Staphylococcus aureus and P. aeruginosa
in P2 microbiome.
Conclusion: Repeated isolation of C. blankii from low airway samples of CF patient
suggests the possibility of respiratory tract colonization by C. blankii.
Moreno
C. Alvarez
1
Morales-López
S.E.
2
Rodriguez
G.J.
3
Rodriguez
J.Y.
3
Robert
E.
4
Picot
C.
5
Parra-Giraldo
C.M.
6
Pape
P. Le
7
1Universidad Nacional De Colombia, Bogotá, Clínica Universitaria Colombia, Colsanitas,
Colombia, bogota, Colombia
2Universidad Popular del Cesar, Valledupar, Colombia, valledupar, Colombia
3Cesar, Cimce, Cl. c #d -, Valledupar, Cesar, Colombia
4Ea1155 Iicimed, Nantes University Hospital, NANTES, France
5Pontificia Département de Parasitologie-Mycologie, Université de Nantes, Nantes Atlantique
Universités, EA1155-IICiMed, Faculté de Pharmacie, Nantes, France, Dantes, France
6Unidad de Proteómica y Micosis Humanas, Grupo de Enfermedades Infecciosas Departamento
de Microbiología, Facultad de Ciencias, Pontificia Universidad Javeriana, Bogotá D.C,
Colombia, Bogota, Colombia
7Ea1155 Parasitology and Mycology Department, Nantes University, Nantes, France
P216. The mortality attributable in Candidemia to C. auris is higher than other Candida
species: Myth or or reality?
Objectives:
Candida auris has emerged as a major threat in the healthcare setting, having caused
invasive infections outbreaks around the world. Besides, it has been proved difficult
to treat due to its multidrug-resistant nature and mortality rates have varied significantly
among 30-60% in candidemia (1-2). However, the overall attributable mortality rate
related to candidemia is unclear, nor is there any difference with other candida species
(Candida non-auris). We investigated epidemiological, clinical and laboratory features
of patients with candidemia to evaluate the mortality by C. auris or C. non-auris.
Methods: A case-control study was conducted between 2013 and 2017 in 12 hospitals
of Valledupar, Colombia comparing 22 cases of C. auris candidemia with 52 of C. non-auris.
All isolates of candidemia were recovered to confirm identification by automated matrix-assisted
laser desorption/ionization time of flight (MALDI-TOF MS) (Bruker Daltonik, Bremen,
Germany) and susceptibility to widely used antifungals was determined with Sensititre
Yeast One® AST plates (Thermo Fisher Scientific). Appropriate antifungal therapy was
defined according to the antimicrobial susceptibility tests, for the case of C. non-auris
the CLSI M27-A3 breakpoints were used, in the case of AmB, resistant isolates were
defined as isolates with MIC> 1 μg / mL. For C. auris, breakpoints recommended by
the CDC were used. The risk factors, clinical and microbiological characteristics
and outcomes of patients with C. auris and C. non-auris candidemia (CAC) were compared.
Odds ratio and corresponding 95% confidence intervals were calculated using EPI Info
7™.
Results: Seventy four patients of candidemia were enrolled. Total number of 22 cases
(29.7%) and 52 controls (C. albicans, 21.6%; C. parasilopsis, 21.6%; C. tropicalis,
21.6%; C. glabrata, 1.4% ) were included and analyzed in this study. The comparison
of the main clinical and epidemiological variables is summarized in Table 1. Previous
fluconazole exposure was significantly higher in C. auris candidemia patients (OR
3.3: 1.15-9,5). In vitro antifungal susceptibility of Candida species isolates is
presented in Table 2. Most C. auris isolates were resistant to fluconazole (86.3%)
and amphotericin B (59%) while C. non-auris isolates were in general susceptible (fluconazole
resistant, 1.9%; Amphotericine B resistant, 3.8%). No isolates resistant to echinocandins
were detected. The average time to start antifungal therapy was 3.6 days. Sixty three
(85.1%) patients received adequate antifungal therapy, without significant differences
between the two groups (C. auris Vs C. non-auris; 86.4%vs 84.6%, respectively). The
crude mortality at 30 and 90 days of candidemia was up to 37.8% and 40.5%, respectively.
However, there was no difference in mortality both at 30 and 90 days, between the
group with candidemia by C. auris and by C. non-auris, 31.8% vs 42.3% (OR 0.6; 95%
IC 0.24-1.97) and 36.4% and 42.3% 0,77 (0,27-2,1).
Conclusion: Mortality due to candidemia is similar between C. auris and C. non-auris
in this study. Appropriate antifungal therapy in both groups could contribute to finding
no differences in outcomes. A high resistance to Anfo B was evidenced as previously
described in Colombia(3).
Vasilyeva
N.
1
Pinegina
O.
2
Vybornova
I.
1
Chilina
G.
3
Apalko
S.
4
Scherbak
S.
4
Pchelin
I.
5
Taraskina
A.
5
1Kashkin Research Institute of Medical Mycology, North-Western State Medical University
named after I.I.Mechnikov, St. Petersburg, Russian Federation
2Pavlov First Saint Petersburg State Medical University, Saint Petersburg, Russian
Federation
3Kaschkin Research Institute of Medical Mycology, North-Western State Medical University
named after I.I. Mechnikov, Saint-Petersburg, Russian Federation
4City Hospital No. 40, Saint Petersburg, Russian Federation
5Kashkin Research Institute of Medical Mycology, North-Western State Medical University
named after I.I. Mechnikov, Saint Petersburg, Russian Federation
P217. Genetic characteristic and susceptibility profiles of Candida auris clinical
isolates from Russia
Objectives: The yeast Candida auris is an emergent nosocomial pathogen. In October,
2017, the first case of candidaemia due to C. auris in Russia has been diagnosed.
A large series of isolates was published from Moscow. However, the source of the pathogen
and its spreading to other Russian cities remained obscure. Our objective was to provide
genetic characteristic and describe antifungal drug susceptibility profiles of C.
auris clinical isolates from Russia.
Methods: For the study, a total of six C. auris strains were available. Four originated
from Moscow and two originated from St. Petersburg. We identified isolates by ribosomal
ITS region sequencing. Whole genome sequence of one isolate was obtained by Illumina
MiSeq technology. Antifungal susceptibility testing was performed using colorimetric
Sensititre YeastOne YO10 broth dilution panels.
Results: For the first Russian C. auris isolate from Moscow, we obtained whole genome
sequence. It clustered within Indian/Pakistani clade with isolates from Indian subcontinent.
The earliest C. auris isolate from outside Moscow was obtained in St. Petersburg in
March, 2019. All six studied isolates, including two strains from St. Petersburg,
harbored exactly the same ITS region genotype (MK829041). Also, antifungal drug susceptibility
profiles were highly similar. All isolates were resistant to fluconazole, but susceptible
to echinocandins and amphotericin B.
Conclusion: Currenty, C. auris is being detected in two Russian cities. The spreading
clone has Indian/Pakistani origin, probably independent from known outbreaks outside
Indian subcontinent.
Liang
G.
Department of Medical Mycology, Institute of Dermatology, Chinese Academy of Medical
Science, Nanjing, China
P218. Candida auris in China
Objectives: Since 2009, Candida auris has been spreading globally, which is a serious
threat to human health and has attracted the international attention. We analyzed
the epidemiological trend and pathogenic characteristics of Candida auris in China
and try to discover the problem behind and put forward the reasonable strategies to
provide useful information for prevention and treatment of the diseases.
Methods: We reviewed all Candida auris cases reported from China by searching Pubmed,
EMbase, Wanfang and CNKI; also deeply speculated the hidden clinical problems in China.
Results: First, there are more than 19 cases has been recorded in China. Second, immunosuppressor
and Long-term use of broad-spectrum antibiotics may be the high risk. Thirdly, fifteen
cases was reidentified as Candida auris by PCR and sequencing the ITS and D1/D2 regions
which was misidentified as C. haemulonii by Vitek. Fourth, both Azole-resistant and
non-Azole-resistant Candida auris was found. Fiveth, the infection by Candida auris
can not be effective diagnosed and identified timely 3 years ago in the most third-grade
class-A hospital in China, but now it is being improved because of popularized MALDI-TOF
equipment.
Conclusion: The situation of Candida auris infection may be much severer than expected
because of the misidentification especially in high-risk groups and more attention
has been paid and better medical conditions has been strengthened for the Challenges
ahead.
Marak
R.S.K.
1
Yadav
S.
2
Dixit
A.K.
2
1Microbiology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow,
India
2Microbiology, sanjay Gandhi Postgraduate Institute of Medical Sceinces, Lucknow,
India
P219. Candida auris Candidemia in a Tertiary Care Centre in North India: Rapid Identification
from Direct Blood Culture Positive Samples by MALDI-TOF MS and Analysis of Risk Factors
Objectives: To identify the Candida auris from direct positive blood cultures by MALDI-TOF
MS and analyse the risk factors associated with this multidrug resistant yeast- Candida
auris
Methods: A prospective study was conducted from February 2017 to January 2018 by taking
8 ml of positive blood cultures. After multiple washings and treating with triton
X, it was directly subjected to the MALDI-TOF MS assay. The clinical data of candidemia
cases due to C. auris were determined for significant associated risk factors with
C. auris infection.
Results: of the 122 candidemia cases reported during the study, 23 (18.85%) were due
to C. auris; being the 3rd most common species after C. tropicalis and C. parapsilosis.
The age ranged from 4-day old neonate to 80 years old and there was female preponderance
(n = 13). MALDI-TOF MS was able to rapidly identify all Candida auris species on the
same day when the blood culture was flagged positive thereby shortening the time for
species identification by MALDI-TOF MS from culture. The underlying risk factors significantly
associated with C. auris candidemia were diabetes mellitus, underlying respiratory
illness, gastrointestinal surgery, prior antifungal exposure. Mortality was seen in
60.8%. All isolates were resistant to fluconazole (MIC ≥ 64µ/ml).
Conclusion:
Candida auris is a recently described agent of fungemia that poses a serious global
health threat. C. auris infection has been observed across India and notable for its
antifungal resistance; often multidrug-resistant. C. auris is difficult to identify;
polymerase chain reaction, DNA sequencing and MALDI-TOF MS can correctly identify
it. C. auris known to cause outbreaks in healthcare settings and therefore, it is
important to quickly identify C. auris in a hospitalized patient and the healthcare
facilities can take special precautions to prevent its spread.
Odegbemi
O.B.
1
Dada-Adegbola
H.O.
2
Adeoye
I.
1
Fayemiwo
S.A.
3
1Epidemiology and Medical Statistics, University of Ibadan, Ibadan, Nigeria
2Medical Microbiology and Parasitology, University of Ibadan, Ibadan, Nigeria
3Medical Mycology Unit, Division of Infection, Immunity and Respiratory Medicine,
School of Biological Sciences, University of Manchester, Manchester, United Kingdom
P220. rEpidemiology of Cryptococcal antigenemia among HIV infected patients in southwestern,
Nigeria 2018
Objectives: Cryptococcal antigenemia occurs in Nigeria, but the magnitude of this
disease remains unclear. This study was carried out i. To determine the prevalence
of Cryptococcal antigen (CrAg) among HIV-seropositive subjects ii. To determine the
prevalence of CrAg among HIV-seronegative subjects and iii. To assess the relationship
between CD4 count and CrAg in HIV-seropositive subjects attending Adeoyo Maternity
Teaching Hospital, Yemetu, Ibadan
Methods: It was hospital based case-control study using simple random sampling. Semi-structured
interviewer administered questionnaire was used to collect data from subjects and
retrospective review of CD4 count records in HIV infected subjects. Five millilitres
venous blood was collected from each participant. Serum CrAg testing was done using
CrAg Lateral Flow Assay. Data was analyzed using descriptive statistics and bivariate
analysis at 5% confidence level.
Results: One hundred and fourteen HIV-seropositive individuals (cases) and 228 HIV-seropositive
individuals (controls) were recruited. Mean age of cases was 41.2 ± 10.0 years and
85 (74.6%) were females while mean age of controls was 38.9 ± 13.7 years 156 (68.4%)
were females. The prevalence of CrAg among cases was 11.4% and 7.0% among controls.
Cases were about two times more likely to test positive for CrAg. However, the association
was not statistically significant (OR: 1.71, 95%CI: 0.79 - 3.68). Individuals with
CD4 counts of ≤100 cells/µl were 20 times more likely to have positive serum cryptococcal
antigen than individuals with CD4 counts >100 cells/µl (OR: 20.3, 95%CI: 5.23-78.9).
Conclusion: This study demonstrated significant prevalence of Cryptococcal antigenemia
among the study population; however, prevalence was higher among cases. Screening
for CrAg should therefore be part of routine tests amongst all confirmed HIV-seropositive
subjects, since asymptomatic cryptococcal antigenemia predicts impending cryptococcal
infection with probable mortality.
Haghani
I.
1
Shams-Ghahfarokhi
M.
2
Asl
A. Dalimi
3
Abastabar
M.
4
Shokohi
T.
4
Hedayati
M.T.
4
Fathi
M.
3
1Medical Mycology, Mazandaran university of Medical sciences, Sari, Iran
2Department of Mycology, Faculty of Medical Sciences, Tarbiat Modares University,
Tehran, Iran
3Department of Parasitology, Faculty of Medical Sciences, Tarbiat Modares University,
Tehran, Iran
4Department of Medical Mycology, Invasive Fungi Research Center, School of Medicine,
Mazandaran University of Medical Sciences, Sari, Iran, Sari, Iran
P221. High prevalence of Fusarium proliferatum isolates from onychomycosis and antifungal
susceptibility pattern
Objectives: Onychomycosis refers to any fungal infection of the nail and is usually
caused by dermatophytes; however, non-dermatophyte molds (NDM) and yeasts are increasingly
recognized as the pathogens accounting for nail disease. Regarding this, the present
study was conducted to describe the molecular epidemiology of Fusarium onychomycosis
in the North of Iran.
Methods: To this end, 257 nail samples collected from the Iranian patients clinically
suspected of onychomycosis were subjected to direct microscopy, calcofluor white staining,
and fungal culture. The characteristics of Fusarium isolates were further identified
at a species level by determining multi-locus sequences for internal transcribed spacer
and translation elongation factor 1 alpha
Results: According to the results, Fusarium species were isolated from 27 patients
with onychomycosis. Based on previous partial genes analysis, the recognized species
in our study were among the members of F. fujikuroi species complex (n = 14), F. solani
species complex (n = 12), and Fusarium incarnatum-equiseti species complex (n = 1).
In the present study, F. proliferatum was the dominant Fusarium species isolated from
the samples. With regard to the increased prevalence of Fusarium onychomycosis and
the intrinsic resistance of these agents to a broad range of antifungals, it is necessary
to correctly identify Fusarium species. Our results were indicative of a change in
the epidemiology of Fusarium species isolated from onychomycosis. The minimum inhibitory
concentration (MIC) of luliconazole and lanoconazole ranged within 0.001 to - 1 μg/mL
and their geometric mean MICs were 0.0103 and 0.0343 μg/mL against Fusarium species,
respectively.
Conclusion: The increased prevalence of Fusarium onychomycosis and the intrinsic resistance
of these infectious agents to a broad range of antifungals have highlighted the importance
of the accurate identification of Fusarium species. According to the results of the
present study, it seems that the epidemiology of Fusarium species isolated from onychomycosis
has been changed. In addition we conclude that luliconazole and lanoconazole demonstrate
potent activity against Fusarium species isolated from onychomycosis. These compounds
are accordingly promising for the treatment of superficial fusariosis such as onychomycosis.
Koffi
D.
1
Ira
B.
1
Toure
A.O.
1
Jambou
R.
1
Denning
D.
2
1Parasitology and Mycology, ABIDJAN, Côte d’Ivoire
2University of Manchester, Manchester, United Kingdom
P222. Fungal disease burden: an underestimated health challenge in Cote d’Ivoire
Objectives: Due to limited access to more powerful diagnostic tools, there are few
data on the burden of fungal infections in Cote d’Ivoire, despite a high HIV and TB
burden and many cutaneous diseases. Here we estimate the burden of serious fungal
infections in this sub-Saharan country.
Methods: National demographics were used to perform a PubMed search and retrieve all
published articles on fungal infections in Cote d’ivoire and countries bordering West
Africa. When no data existed, risk populations were used to estimate frequencies of
fungal infections, using previously described methodology by LIFE (www.LIFE-Worldwide.org).
Results: The population of Cote d’ivoire is around 24 millions; 37% are children,
and 9% are >65 years. Tinea capitis in children is common, measured at 13,9% in the
last epidemiological study (2013). Considering the prevalence of HIV infection (2.7%
of the population, a total of ~500,000) and a hospital incidence of 6% of cryptococcosis,
it is estimated that 3726 patients per year develop cryptococcosis. For pneumocystosis,
it is suggested that 6023 new cases occur each year with the pevalence of 14,1% in
paediatric HIV infection. An estimated 1567 new cases of chronic pulmonary aspergillosis
occur after pulmonary tuberculosis (a 5 year prevalence of 4938 cases (20.3/100,000).
Allergic bronchopulmonary aspergillosis (ABPA) and severe asthma with fungal sensitisation
(SAFS) were estimated in 104/100,000 and 151/100,000 respectively, in 1,100,000 adult
asthmatics. Vulvovaginal candidiasis (VVC) is common and recurrent VVC affects ~6%
of women in their fertile years - 407,000 women. An unknown number develop candidaemia
and invasive aspergillosis. There are no incidence data on fungal keratitis, histoplasmosis
and chromoblastomycosis, although some cases of mycetoma and histoplasmosis have been
reported.
Conclusion: The present study indicates that around to 6.8% (1.6 million) of the population
is affected by a serious fungal infection, predominently tinea capitis in children
and rVVC in women. These data should be used to inform epidemiological studies, diagnostic
needs and therapeutic strategies in Cote d’Ivoire.
Oliveira
M.
1
2
Simões
H.
3
Verissimo
C.
1
Sabino
R.
1
1Infectious Diseases, National Health Institute Dr. Ricardo Jorge, Lisbon, Portugal
2Animal Biology Department, Faculty of Sciences of the University of Lisbon, Lisbon,
Portugal
3Infectious Diseases, National Health Institute, Lisbon, Portugal
P223. Frequency and molecular epidemiology of Aspergillus isolated from patients with
suspicion of respiratory fungal infection
Objectives: The aim of this study was to determine the frequency of Aspergillus detected
in respiratory samples from a cohort of patients with suspicion of fungal infection
of the respiratory tract as well as to determine the susceptibility to azoles of the
isolates from the Fumigati section.
Methods: A retrospective study was performed involving samples obtained from 16 hospitals
covering different districts of continental Portugal and Azores islands. One hundred
and eighty-seven respiratory samples (101 bronchoalveolar lavage fluids, 52 bronchial
lavages, 27 bronchial secretions, 6 expectorations and 1 bronchial aspirate) were
collected between November 2011 and December 2017 from a cohort of 146 patients with
suspicion of respiratory fungal infection (ages ranging from 20 to 87 years old).
Demographic and clinical data were recorded. Detection of Aspergillus was done by
culture, immunoenzimatic assay and/or molecular techniques. Aspergillus molecular
identification to species level was performed by sequencing of the calmodulin and
β-tubulin genes. To detect possible resistance to azoles, isolates belonging to section
Fumigati were inoculated into Sabouraud dextrose agar media supplemented with 1 µg/ml
or 4 µg/ml of voriconazole, 4 µg/ml of itraconazole and 0.5 µg/ml of posaconazole
and their growth was observed and recorded after 7 days of incubation at 27ºC. Doubtful
results were confirmed when possible by E-test and by real-time multiplex PCR for
the detection of mutations in the Cyp51A gene.
Results: Fifty-seven (39.0%) of the studied patients were positive for Aspergillus.
From the cases with a positive culture (n = 58) the species were identified by sequencing
and belonged to six different sections. The most frequently isolated was the section
Nigri (42.1%) followed by the Fumigati (33.3%) and Flavi sections (8.6%). Regarding
the species, the most frequent was A. niger sensu stricto (33.9%) followed by A. fumigatus
sensu stricto (32.1%). Nine cryptic species were also identified which frequency was
21.4%. In order to study the frequency of azole resistance in Fumigati isolates collected
from the samples of this cohort as well from other biological products, 52 isolates
- Aspergillus fumigatus sensu stricto (n = 45), A. lentulus (n = 4), A. udagawae (n
= 2) and A. pseudofelis (n = 1) – were tested. The tested A. fumigatus sensu stricto
isolates did not show resistance to azoles. An A. udagawae strain revealed low susceptibility
to voriconazole (MIC was not determined due to loss of strain viability). An A. pseudofelis
strain also showed decreased susceptibility to voriconazole (MIC = 1 μg/ml) as well
as to and itraconazole (MIC = 2 μg/ml).
Conclusion: In this study, the genus Aspergillus was frequently isolated in the respiratory
samples tested and a high number of cryptic species was detected. Although resistance
to azoles was not a problem identified in the tested isolates, determination of the
in vitro susceptibility profile and molecular identification of the Aspergillus species
is essential to improve the diagnosis and management of aspergillosis since several
cryptic species have intrinsic resistance to antifungal drugs.
Brun
S.
Vingataramin
Y.
Akhoundi
M.
Lintanf
M.
Bruel
C.
Izri
A.
Parasitology-mycology, Avicenne Hospital, Bobigny, France
P224. Epidemiological analysis of a large Tinea epidemic in schools of a city in the
northern suburbs of Paris, France
Objectives: The main goals of this investigation were i) to identify the causative
agents of a large Tinea epidemic in several schools of a city in the northern suburbs
of Paris, France, and ii) to determine the epidemiological factors which played an
important role in dissemination of this dermatophyte infection.
Methods: Following a report to the Parasitology-Mycology Department of Avicenne hospital
(Bobigny, France) regarding a possible Tinea epidemic in two schools of Clichy-sous-Bois
city (northern-suburbs of Paris), all the children of these schools were screened
for dermatophytosis by clinical and mycological examinations. Some children from other
schools of this city and members of their families came to the same laboratory for
dermatophytosis screening. Suspected lesions of hair or skin were sampled by scraping
and swabbing for mycological diagnosis and demographic data were recorded in parallel.
A dermatophytosis case was determined by positive direct microscopic examination (presence
of fungal filaments or spores in hair or scales) and/or positive dermatophyte culture.
A map of the cases repartition was realised. Next, in order to evaluate the intra-
and interspecific genotypic relationships among T. tonsurans isolates, they were subjected
to sequencing of two fragments of the alkalin protease 1 (ALP1) gene. The obtained
sequences were aligned using the Basic Local Alignment Search Tool (BLAST) and compared
to the sequences deposited in GenBank.
Results: During 15 months, 122 individuals (coming from 12 schools of the city and
members of their families) were sampled and 139 samples were analysed for mycological
direct microscopic examinations and mycological cultures. Ninety-three (70%) samples
(79 scalp and 14 skin samples) were positive for dermatophyte culture or direct examination
in 82 (67%) individuals. Trichophyton tonsurans was isolated in 60 samples (87%),
followed by Microsporum audouinii (4 isolates), Trichophyton rubrum (4), Trichophyton
soudanense (1) and Microsporum canis (1). The infected individuals had an age ranging
from 3 months to 53 years with an average of 8 years old. All of these infected individuals
were living in houses that, together with their schools, are located in an area of
less than 1 km2. Most of them were originated or had parents coming from sub-Saharan
Africa, followed by Haiti and North Africa. According to the sequence analysis of
ALP1 gene, the T. tonsurans isolates all shared the same nucleotide sequences, but
were different from the only sequence available in GenBank coming from USA.
Conclusion: This study presents likely the largest Tinea epidemic recorded not only
in France but also in Europe, with a large majority of infections caused by T. tonsurans.
The genotypic analysis of the T. tonsurans isolates revealed the circulation of a
unique strain in this city of the northern suburbs of Paris, distinct from the strain
described in USA. The high population density together with the high poverty rate
of this city are of the major factors, beside the high transmission rate of T. tonsurans,
that have facilitated the transmission of the dermatophytes between the individuals
in a same family and a same district, as well as in schools.
Moreira
R.T.F.
1
Silva
D.P.
1
Maranhão
F.C.A.
2
Moreira
I.F.
3
Farias
F.I.R.
3
Alvarez
J.
4
Coutinho
S.D.A.
4
1Nursery School, Federal University of Alagoas, Maceió, Brazil
2Biological Sciences Institute, Federal University of Alagoas, Maceió, Brazil
3Medicine Faculty, Federal University of Alagoas, Maceió, Brazil
4Post-graduation Program In Environmental and Experimental Pathology, Paulista University,
São Paulo, Brazil
P226. Presence of Malassezia furfur in the cutaneous microbiome of preterm infants
in a Neonatal Intensive Care Unit
Objectives: In humans, the colonization of the skin by yeasts belonging to the genus
Malassezia starts from the birth. They usually live in commensalism but may become
pathogenic depending on predisposing factors and may cause superficial mycoses. Although
in a lower percentage, M. pachydermatis and M. furfur are responsible for serious
invasive infections in man. In outbreaks of fungemia reported in neonatal intensive
care units (NICU), the neonates showed prematurity, low birth weight, multiple comorbidities
and parenteral nutrition with lipid supplementation. The aim of this study was to
investigate the occurrence of Malassezia spp. in the cutaneous microbiome of low and
extremely low birth weight neonates hospitalized in a neonatal intensive care unit.
Methods: Forty newborns, gestational age between 21 and 34 weeks, weight between 405
and 1,430 grams, hospitalized in a NICU of a public hospital in the city of Maceió,
Brazil, were evaluated. Using sterile swabs, samples from the oral and nasal cavities,
axilla, inguinal and anal areas of the neonates were collected at the time of admission
to the unit, which did not exceed 12 hours postpartum. The samples from the same areas
were collected after 48 h, 96 h, and then twice a week until the discharge resulting
from clinical improvement or death, making a total of 192 clinical samples. The samples
were seeded on Dixon’s medium and microbial colonies were phenotypically characterized
using standard biochemical tests. After extraction of DNA, the strains were submitted
for 26S rDNA gene PCR amplification. Subsequently, the RFLP analysis using the restriction
enzymes BtsCI and HhaI was performed on PCR products. The standard strain Malassezia
furfur CBS-1878 was used as a positive control.
Results: Eight preterm infants (20% - 8/40), weighing between 585 and 1,344 g, presented
the colonization by Malassezia sp from 48 h of life. Some newborns showed colonization
from the 1st to the 6th week, before they left the NICU. There was a higher frequency
of Malassezia in the axillae and anal region, followed by the nasal cavity and inguinal
area. The difficulty in culture maintenance was observed and some strains failed to
survive after the first isolation, making a total of 12 characterized strains. The
biochemical phenotypic behavior of the 12 strains was similar and consistent with
M. furfur. These strains submitted to PCR showed bands between 500 and 600 bp and
presented the same pattern of bands in the RFLP analysis indicating M. furfur.
Conclusion: This study demonstrated the colonization by M. furfur in different sites
of the cutaneous microbiome of preterm infants in NICU. Since the infections caused
by these yeasts are less frequent, these agents are mostly neglected. However, our
findings reveal the need for monitoring low and extremely low birth weight neonates,
as this yeast may be pathogenic, as described in malasseziosis outbreaks in NICUs.
It is worth to note that adequate isolation and cultivation techniques should be employed
since most species of the genus Malassezia are lipid-dependent and do not grow in
the media usually employed for fungal isolation.
Rakotoarivelo
R.A.
1
Raberahona
M.
2
Rasamoelina
T.
3
Rabezanahary
A.
4
Rakotomalala
F.A.
3
Razafinambinintsoa
T.
2
Bénet
T.
5
Vanhems
P.
6
Randria
M.J.D.D.
2
Romanò
L.
7
Cogliati
M.
8
Cornet
M.
9
Andrianarivelo
M.R.
3
1Service des Maladies Infectieuses, Hôpital Universitaire Tambohobe, Faculté de Médecine,
Université de Fianarantsoa, Fianarantsoa, Madagascar
2Service des Maladies Infectieuses, Hôpital Universitaire Joseph Raseta Befelatanana,
Antananarivo, Madagascar
3Centre d’Infectiologie Charles Mérieux, Université Ankatso, Antananarivo, Madagascar
4Service de Pneumologie, Hôpital Régional de Reference, Toamasina, Madagascar
5Service d’Hygiène, Epidémiologie et Prévention, Hospices Civils de Lyon, Lyon, France
6Laboratoire des Pathogènes Emergents, Fondation Mérieux, Centre International de
Recherche en Infectiologie, INSERM U1111, CNRS UMR5308, ENS de Lyon, UCBL1, Lyon,
France
7Laboratorio di Micologia Medica, Dipartimento di Scienze Biomediche per la Salute,
Universita degli Studi di Milano, Milano, Italy, Milano, Italy
8Laboratorio di Micologia Medica, Dipartimento di Scienze Biomediche per la Salute,
Universita degli Studi di Milano, Milano, Italy
9Université Grenoble Alpes, CNRS, CHU Grenoble Alpes, Grenoble INP, TIMC-IMAG, Grenoble,
France, Grenoble, France
P227. Cryptococcosis in HIV-infected patients in Madagascar: high prevalence and mortality
Objectives: In Madagascar, the epidemiology of cryptococcosis is poorly documented.
The main objective was to estimate its prevalence in HIV-infected patients and to
describe the fungal isolates and the patient outcome
Methods: An observational study was conducted in the hospitals of Antananarivo (central
highlands) and Toamasina (eastern coast). Consecutive HIV-infected adults with CD4≤
200/µl were enrolled between November 2014 and December 2016. The serum crytococcocal
antigen (CrAg) was screened using a lateral flow immunoassay (IMMYCrAg ®). In case
of positive result and clinically suspected cryptococcal meningoencephalitis (CM),
a lumbar puncture was performed to evaluate the presence of CrAg in the cerebrospinal
fluid (CSF), as well as India ink staining and culture. The isolates were identified
using MALDI-ToF and genotyped. Antifungal susceptibility was evaluated using the E-test.
A high-dose oral fluconazole (at least 1200/day) was given for 10-12 weeks followed
by 200 mg/day. Adjusted prevalence ratios (aPR) based on multivariate robust variance
Poisson regression, and 90-days survival based on Kaplan-Meier method were used
Results: Overall, 129 patients were included, median age was 37 years. At baseline,
the median CD4 count was 83 cells/µl, 62.8% had a CD4 count <100 cells/µl and 38.1%
were on ART. Overall, 17 patients had a serum CrAg-positive (CrAg+) and 112 CrAg-negative
(CrAg-). CrAg+ patients were more likely than CrAg- patients to present low CD4 cells
count 38 Vs 86; p = 0.018). Among the 17 CrAg+ in serum, 14 had a CSF CrAg+ of which
13 yielded Cryptococcus spp. in culture. After multivariate regression, adjusted on
CD4 count, previous ART initiation (aPR = 0.4, 95% CI: 0.2-0.9) and presence of neck
pain (aPR = 15.7, 95% CI: 3.4-72.1) were associated with CM. The prevalence of cryptococcal
infection was 13.2% (95%CI 7.9–20.3) and that of CM was 10.9% (95%CI 6.1-17.5). The
prevalence of cryptococcal infection in Toamasina (20%) was not different from the
one in Antananarivo (11.1%) (p = 0.21). The mortality rates were 70.6% and 71.4% for
cryptococcal infection and CM, respectively. Survival was significantly higher for
CrAg- patients as compared with CrAg+ (p = 0.049, Log-rank test). All 17 clinical
isolates obtained from CSF (13 at baseline and 4 during follow-up) were identified
as Cn var. grubii. The 13 initial isolates were genotyped and belonged to the VNI
type (3 isolates), the rarer but also globally distributed VNII type (4 isolates)
and a hybrid intra-varietal type VNI/VNII (6 isolates). All three molecular types
were found in Antananarivo whereas only VNI/VNII was found in Toamasina. Among the
13 Cn var. grubii strains, 2 acquired fluconazole resistance after 4 and 5 months
of exposure, respectively.
Conclusion: Prevalence of cryptococcal infections and CM in HIV-infected patients
in Madagascar were 13.2% and 10.9% respectively. The point-of-care LFA CrAg test confirmed
to be reliable and cost effective for the diagnosis. Challenges to facilitate access
to more effective molecules to treat patients with CM include reducing administrative
formalities linked to drug importation and increasing priorities to implement national
control programs.
Bobokhojaev
O.
1
Osmanov
A.
2
Denning
D.
2
1Department of Phthisiopulmonology, Tajik State Medical University, Dushanbe, Tajikistan
2Global Action Fund for Fungal Infections, Geneva, Switzerland
P228. The Burden of Serious Fungal Infections in Tajikistan
Objectives: The aim of this work is to estimate the burden of serious fungal infections
in Tajikistan.
Methods: Initially, we identified all published data on serious fungal infections
in the country. We have used the model proposed by LIFE (Leading International Fungal
Education) to estimate the burden of infections where no local data exist.
Results: Tajikistan is the low-income (GDP Per Capita is 800 USD) country in Middle
Asia with a population of 8.9 million people. 5% of the population lives on less than
1.9 USD a day and 54% live on less than 5.5 USD a day. There are 2,813,903 adult (15-50
yrs.) women in Tajikistan who comprise 32% of the population. It was estimated that
168,834 Tajik women develop recurrent vulvovaginal candidiasis ( = > 4 episodes/year).
The number of HIV positive patients is 15,000; 10,067 (67%) of them do not receive
antiretroviral therapy. We estimate 1,253 patients with oesophageal candidiasis and
4,530 patients with oral candidiasis annually, 27 cases of cryptococcal meningitis
and PCP is approximated at 216 cases annually. According to our estimations there
are 1,040 cases of chronic pulmonary aspergillosis (CPA) as a sequel of TB. Due to
the fact, that CPA may occur as a consequence of multiple other pulmonary conditions,
the total prevalence of 4,161 cases was estimated. We have estimated 6,008 cased of
ABPA and 7,930 cases of SAFS; 196 annual deaths from asthma provides us with an estimation
of 137 fungal asthma deaths annually. We have estimated 445 cases of candidemia a
year applying a low European rate. There are approximately 205 cases of invasive aspergillosis
in immunocompromised patients annually and 1,388 cases in patients with COPD which
gives us a total of 1,593 cases of invasive aspergillosis annually.
Conclusion: According to our estimations, there are 196,778 (2.21% of the population)
people suffering from serious fungal infections in Tajikistan. Hence, improving diagnostics
is the first step of understanding a scale of the fungal burden and the foundation
of addressing the problem of serious fungal infections.
Zeeshan
M.
1
Fatima
S.
1
Arif
M.O.
2
Farooqi
J.
1
3
Arsalan
A.
1
Jabeen
K.
1
2
4
Zafar
A.
3
1Pathology and Laboratory Medicine, Aga Khan University, Karachi, Pakistan
2Dow University of Health Science, Karachi, Pakistan
3Pathology and Laboratory Medicine, Aga Khan University, Karachi-KHI, Pakistan
4Pathology and Laboratory Medicine, Aga Khan University, Karachi, Pakistan, Pakistan
P229. Cases of Mycetoma reported from a Histopathology laboratory of Pakistan: Two
years’ Experience
Objectives: Mycetoma is a chronic granulomatous infection that is found worldwide
and is endemic in tropical and subtropical regions. Occurrence of mycetoma varies
in different regions as per environmental temperature and rainfall in that particular
area. The exact prevalence of this disease is largely unknown, though estimates are
available for various countries. (van de Sande. 2013). Pakistan is a tropical country
with diverse climatic conditions. Our green and lush north-eastern border consists
of agricultural land is shared with Indian Punjab. South eastern part is close to
Indian desert of Rajasthan, and the western border with Iran and Afghanistan is arid.
There are multiple reports published from various regions of India as well as Iran.
There is dearth of information regarding the frequency of mycetoma, both eumycetoma
and actinomycetoma, from Pakistan. The objective of this study was to find out the
number of cases reported during year 2017 and 2018 from histopathology department
of Aga Khan University Hospital laboratory, a tertiary care hospital laboratory also
providing diagnostic services to outside patients
Methods: Retrospective data of subcutaneous surgical tissue biopsies with positive
findings of mycetoma were retrieved from hospital laboratory database from the years
2017-2018. Site of lesion, age, gender, type of mycetoma and geographical location
of patients were analyzed The data was entered and descriptive epidemiologic assessment
was carried out using MS excel 2013
Results: Total 30 cases of mycetoma reported in these two years. Eumycetoma was reported
in the majority of the cases 25/30 (83%) while actinomycetoma in 4/30 (13%). In one
case, both entities of mycetoma were detected in a single lesion (3%). Our cohort
was all adults with age range of 25-60 years. Eumycetoma cases were mostly observed
in male 18/25 (72%) whereas Actinomycetoma cases were mostly seen in females 3/4 (75%).
In eumycetoma anatomical site involvement were predominantly lower extremities 22/25
(88%) followed by hand in rest of the other cases. All the reported cases of actinomycetoma
had foot involvement only. In majority of cases tissue specimen culture was not performed
23/30 (77%). Out of 7 specimens only 2 were culture positive for fungal etiological
agents (29%)
Conclusion: In view of our data, we can definitely describe the presence of mycetoma
in Pakistan. However, there is a desperate need of national registry to report cases
and creating strong liaison between dermatologists, radiologist, surgeons and clinical
microbiology laboratory and Histopathologist, so that burden of the disease may be
estimated. Only then can we stress upon our national health authorities to prioritize
this neglected disease by improving patients’ access to specialized facilities for
better management.
Irfan
M.
1
Iqbal
N.
1
2
Jabeen
K.
3
Awan
S.
4
Zubairi
A.B.S.
4
1Medicine, Aga Khan University, Karachi, Karachi, Pakistan
2Medicine, Jinnah Medical and Dental College, Karachi, Pakistan, Pakistan
3Pathology and Laboratory Medicine, Aga Khan University, Karachi, Pakistan, Pakistan
4Medicine, Aga Khan University, Karachi, Karachi, Pakistan, Pakistan
P231. Radiological and microbiological profile of patients with allergic bronchopulmonary
aspergillosis presented in outpatient pulmonary clinics of Karachi-Pakistan
Objectives: There is limited data available on microbiology and radiographic features
in patients with allergic bronchopulmonary aspergillosis (ABPA) from Pakistan. The
aim of the study is to determine the radiological and microbiological profile of patients
visited outpatient pulmonary clinic of a tertiary care hospital of Karachi Pakistan
Methods: A retrospective study was conducted at pulmonary outpatient clinics, Aga
Khan University Hospital, Karachi, Pakistan from January 2017 to December 2018. Patient
with ABPA was enrolled using ISHAM criteria, data was collected on microbiology and
radiology features.
Results: A total 7759 asthmatic patients presented at outpatient pulmonology clinics,
245 were labelled as ABPA, 167 patients fulfilled the inclusion criteria and 91(54.5%)
were female (mean age 41.9 ± 13.0 years). High resolution CT scan (HRCT) chest was
done in 126, out of these 104(82.5%) patients had bronchiectasis. Central bronchiectasis
was noted in 98(94.2%), Mucus plugging in 71(56.3%) and hyperinflation seen in 30(23.4%)
patients. Microbiological testing was available in 103(61.7%). The commonest bacterial
pathogen isolated was Pseudomonas aeruginosa 32(31.1%) followed by Hemophilus influenzae
16(15.5%) and Morexella catarrahlis 7(9.7%). While among fungal cultures commonest
isolated fungas was Aspergillus fumigatus 17(23.6%) followed by Aspergillus flavus
16(22.2%) and Aspergillus Niger 11(15.3%). Co infection (bacterial and fungi) isolated
in 18(17.45%) patients.
Conclusion: Extensive bronchiectasis was noted in our cohort of patients with ABPA
that leads to frequent bacterial exacerbation. Pseudomonas aeruginosa was found to
be common among bacterial pathogens. Early diagnosis can prevent lung damage.
Böhlin-Wiener
A.
1
2
Szakos
A.
3
Klingspor
L.
2
1Clinical Microbiology, Karolinska University Hospital, Stockholm, Sweden
2Laboratory Medicine, Karolinska Insitutet, Stockholm, Sweden
3Pathology and Cytology, Karolinska University Hospital, Stockholm, Sweden
P232. Epidemiology and outcomes of patients with invasive mould infections: a retrospective
observational study at Karolinska University Hospital, 2010 - 2014
Objectives: To investigate the epidemiology and outcomes of invasive mould infections
(IMI) during 2010-2014, compared to 2005-2009 (1).
Methods: Patients with proven or probable IMI were included. Data regarding patient
baseline characteristics, underlying diseases, types of transplant, infecting mould
species, sites of infection, antifungal therapies, etc were extracted from patient
journals. Outcome was 90-days and 1-year survival post diagnosis.
Results: A total of 127 (vs 100) patients were included, an increase by 27% compared
to the previous period. The rise in incidence occurred mainly during 2010-2011 (Fig
1). The majority, 54% (vs 66%) were in the age group 18-65 and 30% (vs 24%) were over
65 years old. Males predominated with 57% (vs 60%). Most isolates were Aspergillus
spp, n = 59 (vs 75) followed by Mucorales spp, n = 14 (vs 9) and Fusarium spp, n =
8 (vs 3). In n = 7 cases (vs 10), more than one mould was isolated. An additional
n = 45 had a positive galactomannan antigen test. Lungs were the most often affected
organ, 92% (vs 88%) and haematological malignancies (HM) were the most common underlying
diseases, 71% (vs 70%). In total, 431 (vs 354) allogeneic Haematopoietic Stem Cell
Transplants (HSCT) were performed during the study period, of which 6.5% (vs 7.6%)
developed IMIs. Following diagnosis, 75% (vs 87%) received antifungal therapy, usually
voriconazole, 53% and liposomal amphotericin B, 38%. Breakthrough infections occurred
in 33% of the cases in spite of prophylaxis, mainly posaconazole, 59% and caspofungin,
24%. The 90-days overall survival remained basically unchanged, 48% (vs 49%), but
with significant yearly variations (Fig 2). The allogeneic HSCT and HM cohorts had
highest overall survival rates, 68% respectively 55% (vs 37% respectively 43%). The
1-year overall survival declined to 35% (vs 46%) with lowest rate 13% in 2012.
Conclusion: There were no significant changes in patient characteristics, mould epidemiology
or underlying diseases. Survival rates improved for the HM and allogeneic HSCT cohorts.
The yearly variations in mortality rates coincide with construction work around the
hospital, notably with periods of intense blasting. During these periods there was
also an increase in the incidence of IMIs.
(1). Klingspor L, et al: Epidemiology and outcomes of patients with invasive mould
infections: a retrospective observational study from a single center (2005-2009).
Mycoses. 2015 Aug; 58(8):470-7
Vannini
M.
1
Lieutier
F.
2
Emery
S.
2
Retur
N.
2
Hasseine
L.
1
1Laboratoire De Parasitologie Mycologie, CHU DE NICE, NICE, France
2Service Pharmacie, CHU DE NICE, NICE, France
P233. Epidemiology of candidemia and their antifungal susceptibility in our University
Hospital from 2014 to 2018
Objectives: Given the differences between local epidemiologies, we have to know our
fungal epidemiology, in order to adapt our antifungal strategies. The purpose of this
study is: 1. to identify the epidemiology and antifungal susceptibilities of candidemia
over 5 years; 2. to evaluate the anti-fungal treatment and to see the survival in
the third month (M3) of the patients after the diagnosis of fungemia.
Methods: This study was retrospective, conducted from January 2014 to December 2018.
All patients who had at least one positive fungal blood culture were included. A single
blood culture per patient was taken into account with a study of the MICs (Molecules
tested: amphotericin B, caspofungin, voriconazole and fluconazole).
Results: In total, 306 Candida blood culture isolates were obtained from 280 patients
(39 in 2014, 62 in 2015, 72 in 2016, 60 in 2017 and 47 in 2018). The three most common
Candida spp isolated from blood cultures were Candida albicans (44%), Candida glabrata
(22%) and Candida; parapsilosis (13%). Any resistance to the four tested antifungals
is observed for C. albicans and C.parapsilosis. However, resistance was observed for
C. glabrata in 10 % for amphotericin B, 28% for voriconazole, and 15% for fluconazole.
In 2 % and 75%, there is respectively a resistance and an intermediate susceptibility
to caspofungin. For the time being, the antifungal treatment was recovered for 170
patients. Among them, 67%, 26% and 5% received respectively caspofungin, fluconazole
and ambisome. 2% have not received antifungal treatment, because they were at the
end of their life. Evolution at M3 was reported and the mortality rate was 32%. Candidemia
has probably a role in 51% of them.
Conclusion: We report an overall increase in candidemia but a constant distribution
of C albicans, C paraspsilosis and C glabrata. The lack of antifungal resistance for
C. albicans and C. parapsilosis for candidemia suggests a possibility of treatment
with fluconazole as a first-line in our hospital, without waiting for the results
of the antifungal susceptibilities. We have also to analyse the possible nosocomial
etiology, and the link between antifungal resistance, and the cause of death of those
patients.
Alvarez
J.
1
Camargo
L.
2
Corsini
C.
2
Neves
J.
1
Coutinho
S.D.A.
1
1Post-graduation Program In Environmental and Experimental Pathology, Paulista University,
São Paulo, Brazil
2Veterinary Hospital, Paulista University, São José dos Campos, Brazil
P234. Presence of Malassezia pachydermatis in the cutaneous microbiome of humans cohabiting
with dogs
Objectives:
Malassezia species are yeasts that are inhabitants of the cutaneous microbiome of
animals and men; however, they can eventually cause infections. In dogs, M. pachydermatis,
a zoophilic and non-lipid-dependent species, particularly causes otitis and dermatitis.
Although malasseziosis is not considered zoonotic, it was suggested the disease may
be transmitted to neonates by the professionals of intensive care units (ICU) who
have dogs. No data on the exchange of Malassezia species between animals and men who
have close contact are available. The aim of this study was to isolate and identify
M. pachydermatis from the cutaneous microbiome of dog owners and compare with that
of people who do not have contact with animals.
Methods: A total of 15 dogs with otitis and/or dermatitis caused by M. pachydermatis
and their owners were selected. We collected three clinical samples of each owner
(total 45 samples) from the upper trunk, scalp, and behind the ears. At the same time,
three clinical samples were collected from 24 people (total 72 samples) of the same
regions, who did not have any contact with animals. The samples were seeded on Dixon’s
medium and the isolates obtained were inoculated on Sabouraud dextrose agar. Cultures
that grew only on the Dixon’s medium were considered lipid-dependent Malassezia species
and those on Sabouraud dextrose agar were considered as M. pachydermatis. For genus
confirmation, DNA was extracted and 26S rDNA was amplified using the polymerase chain
reaction. The standard strain M. pachydermatis CBS-1879 was used as a positive control.
Results: The presence of Malassezia spp. in the people living with dogs (80.0% - 12/15)
and those who not living with animals (83.3% - 20/24) was similar (Table 1). Malassezia
spp. were isolated from 37.8% (17/45) and 58.3% (42/72) of clinical samples from those
cohabiting with dogs and those who were not, respectively (Table 1). Malassezia pachydermatis
was the most common species in the cutaneous microbiome of the owners found in 52.9%
(9/17) of the isolates, whereas lipid-dependent Malassezia spp. were isolated from
47.1% (8/17) (Table 1). Malassezia pachydermatis was isolated from the three sites
sampled, scalp, upper trunk, and behind the ears. Only lipid-dependent Malassezia
(100.0% of the isolates) was found in people who did not cohabit with dogs, M. pachydermatis
was not isolated from these people (Table 1). "Dog cohabitation" has a statistically
significant effect on the presence of M. pachydermatis in the cutaneous microbiome
of the owners (p < 0.05).
Conclusion:
Malassezia pachydermatis was associated with the nosocomial outbreaks of fungemia
in neonates hospitalized in ICUs, with the possibility of transmission occurring by
health professionals who were having dogs and carried the yeast to the ICU. However,
limited literature exists on the association of close cohabitation with dogs or other
pets with the human microbiome. Our results show that there is a change in the Malassezia
spp. in the cutaneous microbiome of people cohabiting with dogs, thus significantly
increasing the presence of a zoophilic strain of M. pachydermatis.
Skiada
A.
1
Drogari-Apiranthitou
M.
2
Petrikkos
G.
3
11st Dpt. of Medicine, Laiko Hospital, Athens University, Athens, Greece
2Infectious Diseases Research Laboratory, 4th Department of Internal Medicine, General
University Hospital “Attikon”, School of Medicine, University of Athens, Athens, Greece
3Infectious Diseases, School of Medicine, European University of Cyprus, Nikosia,
Cyprus
P235. “zygomyco.net”: 15 years of a global mucormycosis registry of the joined ECMM/ISHAM
Working Group on zygomycosis.
Objectives: Taking into consideration the challenges related to epidemiology, treatment
and outcome of mucormycosis, a Working Group (WG) on Zygomycosis was initially formed
in ECMM, in 2004. It aimed to create a registry by collecting mucormycosis cases from
European countries. From 2008 onwards the WG expanded under also the auspices of the
ISHAM and it now includes participants from all over the world. The aims of this project
are: 1) to conduct a prospective international registration of patients with mucormycosis
using a well-established global network, 2) to collect and molecularly identify mucormycetes
and tissue specimens from the patients enrolled in order to establish an international
archive that will be used in future studies and 3) to provide information about this
rare but devastating disease and enhance the collaboration of physicians and researchers
all over the world.
Methods: Definitive and probable mucormycosis cases from each participating country
were initially prospectively recorded in standardized case report forms (CRFs) and
sent to the study coordinator. Since the creation of the registry platform (www.zygomyco.net)
participants can submit cases directly. Isolated mucormycetes are identified at the
participating institutions and molecular identification, if not available, is performed
at an appointed laboratory (National Centre for Mycology, Madrid, Spain). In absence
of culture data, paraffin-embedded tissues are sent to an appointed laboratory for
PCR. Appropriate statistical methods are used for univariate and multivariate analysis.
Results: The WG on zygomycosis has organized 3 international fora on zygomycosis,
co-organized international workshops, and meetings are regularly held. As an ongoing
study, results are being presented at conferences and related papers are published.
During 2005-2007, 230 cases were submitted and analyzed. The first analysis (A. Skiada
et al., Clin Microbiol Infect 2011;17:1859–1867) has shown a wide variation regarding
underlying diseases, clinical presentations, fungal species and treatment modalities
in different countries. These observations have increased our understanding of the
risk for transmission and diversity of this disease, which may lead to increased suspicion,
earlier diagnosis and appropriate management. The analysis has also contributed to
issuing guidelines on the diagnosis and treatment of mucormycosis. More than 300 further
cases have been added during 2008-2018, from more than 15 countries, Brazil, India,
Iran, Lebanon and the USA included.
Conclusion: The existence of the ECMM/ISHAM Working Group on Zygomycosis and its official
website and registration platform, provide the means for a network of investigators,
including many experts, to interact, exchange information and hopefully improve the
outcome of mucormycosis. Focus on more detailed information on the development of
the disease according to various underlying conditions and mucoralean species involved
is a future perspective, as a tool for the construction of predictive risk models
for patients at risk. This project can also be a powerful tool for the assessment
of novel diagnostic procedures, therapeutic modalities and efficacy of new antifungals.
Uhrlass
S.
1
Rimek
D.
2
Hubka
V.
3
Schroedl
W.
4
Reuschel
M.
5
Muetze
H.
1
Koch
D.
1
Krueger
C.
1
Graeser
Y.
6
Nenoff
P.
1
1Partnership Dr. C. Krueger & Prof. P. Nenoff, Laboratory of medical microbiology,
Roetha OT Moelbis, Germany
2Gesundheitsschutz, Thueringer Landesamt für Verbraucherschutz, Bad Langensalza, Germany
3Laboratory of Fungal Genetics and Metabolism, Institute of Microbiology ASCR, Prague,
Czech Republic
4Ag Mykologie, Institut für Bakteriologie und Mykologie, Leipzig, Germany
5Klinik Für Heimtiere, Reptilien, Zier- Und Wildvögel, Stiftung Tierärztliche Hochschule,
Hannover, Germany
6Institut Für Mikrobiologie Und Hygiene, Universitätsmedizin – Charité, Berlin, Germany
P236. First report on the isolation of five new geophilic Arthroderma species in hedgehogs
Objectives: During the last years an increase of rare and new dermatophytes in human
beings, but also in animals has been observed in routine diagnostics of mycological
laboratories, not at least due to the significantly improved molecular identification
methods. The conventional diagnostics includes native preparation plus fungal culture.
Today, molecular methods, e. g. polymerase chain reaction (PCR), in particular an
in-house- PCR-ELISA, are completing mycological diagnostics.
Methods: Recently, between April and September 2018, a survey of the health of hedgehogs
was performed in the city of Hanover, Germany. Altogether 50 European hedgehogs were
included in this study. Skin and sting samples from the animals were collected, and
mycological investigation was done as described before. A minor part of hedgehogs
showed clinical signs of a dermatomycosis, most animals were, however, asymptomatic.
For confirmation of cultural dermatophyte isolates, sequencing of the Internal Transcribed
Spacer (ITS) region of the rDNA, and the Translation Elongation Factor (TEF)-1α gene
was performed.
Results: The following dermatophytes were isolated from the hedgehogs: Trichophyton
erinacei (8), Arthroderma (A.) crocatum (4), A. quadrifidum (2), A. insingulare (1),
A. chiloniense (1) and A. tuberculatum (1). Based on their macro architecture, their
microscopic features, and their genetic characteristics, all from hedgehogs isolated
strains were compared with each one or two clinical isolates of the respective Arthroderma
species. These reference strains were isolated from human skin samples, e.g. scrapings,
nail samples, and hair roots, achieved between 2016 to 2018. Clinical diagnoses were
tinea manuum, tinea corporis, tinea capitis, and tinea unguium.
Conclusion: Meanwhile, both the here described new animal isolates from hedgehogs,
but also the human strains of different Arthroderma species are deposited at the deposited
at the „Deutsche Sammlung von Mikroorganismen und Zellkulturen” (DSMZ, German Collection
of Microorganisms and Cell cultures), Brunswick, Germany. The DNA sequences (ITS and
TEF-1α) of all strains are available now at the gene bank of the National Center for
Biotechnology Information (NCBI) in Bethesda, Maryland, USA.
Zhang
L.
1
Liu
Z.
1
Li
T.
1
Xiao
M.
2
Wang
Y.
2
Xu
Y.
2
1Department of Infectious Disease, Peking Union Medical College Hospital, Beijing,
China
2Department of Clinical Laboratory, Peking Union Medical College Hospital, Beijing,
China
P237. Epidemiology, Clinical characteristics, and Outcome of adult Candidemia in a
Chinese tertiary hospital: from 2008 to 2015
Objectives: The aim of this study was to assess the epidemiology, clinical characteristics,
risk factors and outcome of candidemia patients in a tertiary hospital.
Methods: A retrospective observational study of all cases of candidemia which occurred
during hospitalization was carried out in a tertiary hospital including 6 intensive
care units (ICUs), 22 medical and 24 surgical wards in Beijing, China(2008-2015).
Demographic and clinical data were collected from the database of the patient’s medical
records. Chi Square test (or Fisher’s Exact Test when expected frequencies were less
than 5) and multivariate logistic regression models were used to identify the risk
factors associated with the higher mortality during hospitalization.
Results: A total of 192 episodes of candidemia were identified in 181 patients(male
102,female 79, mean age 58.96±17.86). All cause mortality during hospitalization was
64/181 (35.4%), and attributable mortality was 50/181 (27.6%). Multivariate analysis
showed that age≥65 years, ICU hospitalization and polymicrobial bloodstream infections
were independently associated with a higher risk of crude mortality. Candida albicans
was the predominant specie 100/192(52.1%) followed by C.glabrata 31/192(16.1%), C.parapsilosis
28/192(14.6%) and C.tropicalis 26/192(13.5%). Species distribution differed significantly
according to different wards and sources. Candida albicans was predominant in obstetrics
gynecological ward 9/12(75%), emergency ward 16/23(69.5%) and ICU 38/70(54.3%). Non-albcan
candida spp. were predominant in surgical wards 23/37(62.2%) and internal medical
wards 27/50(54%). Intra-abdominal infection34/192 (17.7%) was the most frequent infective
source of candidemia, followed by pulmonary infection 34/192(16.1%) and CVC-related
candidemia 27/192(14.1%), however, the source of infection was not identified in 71/192(40.0%)
episodes. Meanwhile, we also found higher percentages of C.albicans in candidemia
secondary to abdominal infection 22/34(64.7%) and multifocal infections 7/11(63.6%),
but higher percentages of non-albicans in skin and soft tissue infection 4/6(66.7%)
and CVC-related candidemia 17/27(63.0%) (Figure 2).Fluconazole resistance accounted
for 1.0%, 3.6%,3.8% and 6.5% of isolates of C.albicans (1/100), C.parapsilosis (1/28),
C.tropcalis (1/26) and C.glabrata (2/31), respectively.
Previous antibiotic exposure (<30 days)154/181(85.1%), reservation of central venous
catheter 150/181(82.3%), previous hospitalization history 140/181(77.3%) and retention
of Foley catheter 122/181(67.4%) were the most frequent risk factors.
Conclusion: Our study showed that candiemia was associated with poor outcome and higher
mortality. Identification of risk factors associated with candidemia diagnostic and
prognositic may lead to better management in terms of preventive and therapeutic measures.
Given the difference of candida species distribution in different wards and different
infecive sources, improving the awareness of epidemiology are in urgent need in candidemia
treament.
Jenks
J.
Reed
S.
Hoenigl
M.
Medicine, University of California San Diego, San Diego, United States of America
P238. Epidemiology and Outcome of Culture-Proven Coccidioides immitis Infections in
San Diego, California, United States
Objectives:
Coccidioides are dimorphic fungi endemic to the Southwestern United States (Coccidioides
immitis) and parts of Central and South America (Coccidioides posadasii). Infection
from these fungi, coccidioidomycosis, can cause a range of disease from self-limited
acute pneumonia to severe, disseminated disease. The objective of this study was to
describe clinical manifestations, treatment and outcome of patients with culture-proven
Coccidioides immitis infections in San Diego, California, United States, an area endemic
to Coccidioides immitis.
Methods: We performed a retrospective chart review of medical records of all patients
with culture-proven Coccidioides immitis infection at UCSD between 5/01/2017 - 12/31/2018
(1 1/2 year period). The study was approved by the UCSD Human Research Protection
Program.
Results: A total of 11 cases with detection of Coccidioides immitis in culture were
identified (Table). of 11 cases, 10 were evaluable, and of those 1 had chronic coccidioidomycosis
and 9 had proven acute infection. of those with acute infection, 56% (5/9) had traditional
risk factors for coccidioidomycosis infection including African American race, immunosuppressive
state, lung transplant status, HIV/AIDS, and type II diabetes mellitus, while the
remaining 4/9 lacked traditional risk factors. Five of 9 (56%) had primary coccidiodomycosis
of the lungs and 4/9 (44%) disseminated disease. of those who received antifungal
therapy, 3/7 (43%) received combination therapy and 4/7 (57%) monotherapy with fluconazole.
At 180 days, 5/9 (56%) were alive, 3/9 (33%) deceased, and 1/9 (11%) lost to follow-up.
of those who were not alive at 180 days, 1/3 (33%) had disseminated disease and 2/3
(67%) primary coccidioidomycosis, both who were on immunosuppressive therapy.
Conclusion: Culture-proved Coccidioides immitis infection occurred in a variety of
hosts, with 44% lacking typical risk factors for infection. Disseminated coccidioidomycosis
infection occurred in 44%; of these, 50% lacked traditional risk factors and 75% were
alive at 180 days.
Jabeen
K.
1
Umar
S.
2
Shaheen
N.
2
Khan
M.
3
Farooqi
J.
2
1Pathology and Laboratory Medicine, Aga Khan University, Karachi, Pakistan, Pakistan
2Pathology and Laboratory Medicine, Aga Khan University, Karachi, Pakistan
3Medical College, Aga Khan University, Karachi, Pakistan
P239. Positive fungal culture in tissue specimens from burns patients: Data from a
tertiary care hospital laboratory in Pakistan
Objectives: Due to defective skin barrier burns patients are at great risk of development
of fungal wound infections. Fungi are frequently cultured from tissue specimens from
these patients either singly or in combination with bacteria. Spectrum of fungal wound
infection in burns patients from Pakistan has not been reported previously. We evaluated
positive cultures from burn wounds samples received at a tertiary care hospital laboratory
in Karachi, Pakistan. Positivity rate of fungal isolation within these cases and spectrum
of yeast and filamentous mold was reported.
Methods: We reviewed laboratory data of tissue and wound samples received for bacteriological
assessment from burn patients at the Aga Khan University Hospital laboratory, Karachi,
Pakistan from September 2017-March 2018. Specimens were inoculated on chocolate agar
and sheep blood agar with colistin and nalidixic acid incubated in 5% CO2, a triplate
with MacConkey agar, Mannitol salt agar and Sabouraud’s dextrose agar incubated in
ambient environment, and sheep blood agar incubated in anaerobic environment. All
cases were incubated at 37 °C and pus and tissue samples were also enriched by inoculating
in cooked meat broth. Clinical and demographic information recorded on the worksheet
at the time of clinical reporting was collected for the study. Frequencies were computed
to describe the data.
Results: A total of seventy positive tissue cultures from burn patients were identified
over the study period. These specimens were received for 36 male patients and 34 female
patients with age ranging from eight months to eighty five years (average: 15.5 year).
of these, 26 (37%) had growth of either filamentous mold (17 cases) or Candida species
(11 cases). Two cases had growth of both mold and yeast. Aspergillus flavus was the
most common mold (9 cases) followed by Fusarium species (3 cases) and Lichtheimia
corymbifera (2 cases). Candida tropicalis was the most common yeast (5 cases) followed
by Candida parapsilosis (3 cases). There was a concomitant bacterial growth in 19
cases predominantly of Staphylococcus aureus (13 cases), Pseudomonas aeruginosa (8
cases) and other Gram negative rods (6 cases).
Conclusion: High rate of fungal isolation from burn wound infection was seen in this
study despite less optimal culture techniques. The findings in our study are consistent
with the global increase in incidence of fungal infections in burn wounds. High index
of suspicion of fungal infections by clinicians and revision of culture protocols
in burn patients may be warranted for optimal management of these patients.
Farooqi
J.
1
Ladak
A.
2
Shaheen
N.
3
Jabeen
K.
4
1Pathology and Laboratory Medicine, Aga Khan University, Karachi-KHI, Pakistan
2Medical College, Aga Khan University, Karachi, Pakistan
3Pathology and Laboratory Medicine, Aga Khan University, Karachi, Pakistan
4Pathology and Laboratory Medicine, Aga Khan University, Karachi, Pakistan, Pakistan
P240. rFungal isolation from wound samples submitted for culture at a tertiary care
hospital laboratory in Karachi, Pakistan
Objectives: Skin and Soft tissue infections rank the fourth most common infections
worldwide. These often emerge from chronic wounds, traumatic implantation and surgical
wounds. Bacteria are the most common isolated organisms from such wounds. However,
recently, many centers across the world have started to report an increase in fungal
isolates, possibly due to excessive use of antibiotics and expansion in host risk
factors. This study aims to present the frequency of reported fungal isolates in patients
with skin and soft tissue infections and relate the incidence of these infections
with concomitant risk factors.
Methods: We reviewed laboratory slips of all tissue and pus samples received for bacteriological
assessment at the Aga Khan University, Hospital clinical laboratory from mid-September
till to mid-October 2018. All samples were inoculated on Chocolate Agar and Sheep
Blood Agar with colistin and nalidixic acid incubated in 5% CO2, a triplate with MacConkey
Agar, Mannitol Salt Agar and Sabouraud’s Dextrose Agar incubated in ambient environment,
and Sheep Blood Agar incubated in anaerobic environment. All cases were incubated
at 37 °C and also enriched by inoculating in Cooked Meat Broth. Clinical information
recorded on the worksheet at the time of clinical reporting was collected for the
study. Any missing information was filled in from the in-house hospital information
management software if available. Variables such as patient age, location, gender,
relevant medical history, type and site of wound, microbial isolates and drug resistance
were collected. Frequencies were computed to describe the data.
Results: We reviewed a total of 140 patients’ information over the study period. Our
study population consisted of 48.6% (n = 68) females and 51.4% (n = 72) males with
the median age of 30.5 years. of the 140 samples submitted, 76.4% (n = 107) were culture
positive; pathogens were isolated from 81.3% of these (n = 87), and 9.3% yielded fungi.
The categories of lesions included accidental wounds [8.6% (n = 12)], burn and blast
injuries [2.9% (n = 4)], diabetic wounds [7.9% (n = 11)], nosocomial infections [15%
(n = 21)], skin and soft tissue infections [57% (n = 80)] and unknown [8.6% (n = 12)].
The fungal isolates were spread across all categories of lesions. Skin and soft tissue
infections had the highest number of fungal isolates (n = 4) while diabetic wounds
had none. Yeasts (Candida species) were most commonly isolated (n = 4), followed by
Fusarium species (n = 3), Aspergillus species (n = 2), Bipolaris species (n = 2) and
Curvularia species (n = 1).
Conclusion: Our center has reported a high number of fungal isolates over the short
study period. Our study raises the question of identifying underlying causes that
has led to this high number of fungal isolates reported in just one month from samples
sent only for bacterial culture. Moreover, centers across the region should be educated
about the rising fungal load in the population especially wound samples. Setting an
additional fungal isolation medium may be life saving for patients with severe necrotizing
fungal wound infections.
Sebastian
C.
1
V
R.
1
Mathew
J.
2
Savio
J.
2
1Microbiology, St.John’s Medical College, Bangalore, India
2Microbiology, St. John’s Medical College, Bangalore, India
P241. Subcutaneous Mucormycosis – Beyond Rhizopus and Mucor species
Case Report: Introduction The Mucorales, Apohysomyces and Saksenaea species are unusual
human pathogens that are reported with increasing frequency as a cause of subcutaneous
infection in previously healthy patients following trauma or invasive procedures.Identification
of these species in a routine laboratory is challenging due to their difficulty in
sporulation. Special techniques for inducing sporulation must be employed for their
identification. Objective To report 4 cases of subcutaneous mucormycosis due to the
mucorales - Apohysomyces and Saksenaea species Case 1 25 years old farmer presented
with necrotising fasciitis of the entire back following religious self-flagellation.
Microscopic examination of the tissue revealed aseptate hyphae. Culture yielded a
mucorale that was identified as Apophysomyces variabilis. Patient condition improved
with wound debridement and liposomal Amphotericin B. He was discharged on Posaconazole.
Case 2 A 30 year old male patient, diabetic since 6 months presented with submandibular
swelling of 10 days duration following an injury to the neck. Pus sample revealed
aseptate hyphae. Culture isolate was identified as Apophysomyces species on culture.
Case 3 55 years old patient presented with persistent fever and necrotising fasciitis
of the right leg. It all started following a bite by a pig 15 days back. He was not
a known case of diabetes mellitus but was diagnosed on admission. The tissue sample
yielded Apophysomyces elegans on culture. Case 4 25 years old retro positive patient
presented with a gluteal abscess following an intramuscular injection. The fungal
culture of the tissue sample yielded Saksenaea vasiformis.
Conclusion Mucormycosis is an angio-invasive infection which is rapidly progressive
and associated with a high morbidity and mortality. Rhizopus species is the most common
mucoralean worldwide causing mainly rhino-orbito-cerebral mucormycosis. Genera other
than Rhizopus like Apophysomyces and Saksenaeae are associated with cutaneous and
subcutaneous manifestations. More and more cases are presently being diagnosed in
developing countries like India where the at-risk population of diabetic patients
and other immunocompromised states are continually on the rise. 39 cases of mucormycosis
were reported from our laboratory in the last 2 and a half years, from January 2017
to May 2019. 34 were rhino-orbito-cerebral mucormycosis caused by Rhizopus species.
of the 5 cases of subcutaneous mucormycosis, 3 were caused by Apophysomyces species
and one each by Saksenaea vasiformis and Rhizopus arrhizus respectively. All three
Apophysomyces species were identified as Apophysomyces elegans in our laboratory.
However sequencing at PGIMER Chandigarh has confirmed one of them as A.variabilis.
Two of the four cases were diabetic patients and one was retro positive. All the four
patients gave a history of trauma. In a country like India, mucormycosis should be
considered in patients presenting with non healing wound especially following a trauma,
even in young adults as they may be silent diabetics.
Gulmez
D.
Sığ
A.K.
Akar
N.
Duyan
S.
Arikan-Akdagli
S.
Medical Microbiology, Mycology Laboratory, Hacettepe University Medical School, ANKARA,
Turkey
P242. Changing trends in genus and species distributions of clinical fungal strains:
An evaluation of ten-year data from a tertiary care center in Turkey
Objectives: Opportunistic fungal infections may develop due to a wide variety of fungal
species. Changes in species distribution profiles are reported from many centers and
occasionally reflect an increase in rates of antifungal drug-resistant or -less susceptible
species. The aim of this retrospective evaluation is to determine the fungal species
and their relative isolation rates in the last ten years in our center with a specific
focus on bloodstream yeast isolates and mould growth from lower respiratory tract
specimens.
Methods: Fungal strains isolated from clinical samples from 2009 to 2018 were included.
Isolation and identification were done by standard mycological methods. Fungal growth
in all sample types was evaluated and repetetive growths from the same type of specimens
were excluded. Isolated fungal species were analysed in course of time and with respect
to the specimen types. Distribution of species in five-year periods (2009-2013 vs.
2014-2018) was compared by z test.
Results: A total of 23046 specimens sent to the laboratory during the study period
revealed fungal growth. Excluding the episodes of repetetive growth, 17239 fungal
strains were isolated from 15465 clinical samples. Total number of specimens and isolates
markedly increased in time, from 757 and 819 in 2009 to 2511 and 2875 in 2018, respectively.
Urinary tract (n = 7164, 46%) and lower respiratory tract (n = 4523, 29%) were the
most common fungal isolation sites followed by blood (specimens from peripheral vein
and catheter, n = 1076, 7%). For all specimen types, most common species were Candida
albicans (58%) and Candida glabrata species complex (SC) (12%). Among moulds, Aspergillus
fumigatus SC (51%) was the most common. Per the yeast species isolated from blood
cultures (Figure 1), isolation rate of C.albicans decreased from 48% to 44% (p = 0.186)
and that of C.glabrata SC increased from 10% to 15% (p = 0.014) in 2014-2018 as compared
to 2009-2013. No significant change was observed in species distribution of isolates
from sterile specimens other than blood. Distribution of mould genera from lower respiratory
tract specimens is shown in Figure 2. Although A.fumigatus SC was always the most
common, its rate of isolation decreased from 63% in 2009-2013 to 46% in 2014-2018
(p<0.001). In the same time intervals, isolation of Aspergillus species other than
A.fumigatus SC increased from 26% to 34% (p = 0.145) and moulds other than Aspergillus
(Penicillium spp., Order Mucorales, Scedosporium spp., Alternaria spp., Paecilomyces
spp., dematiaceous fungi and unidentified moulds) increased from 11% to 20% (p = 0.185).
Conclusion: In our center, while the most commonly isolated yeast and mould species
display a usual profile, species distribution is diverse. Evaluation of bloodstream
yeast isolates revealed a decrease of C.albicans and an increase in C.glabrata SC,
which appears as a recent change. Significance of increase in rate of C.glabrata SC
remains noteworthy also with respect to potential resistance or decreased susceptibility
to antifungal agents. Isolation of less common mould genera from lower respiratory
tract samples remains remarkable.
Sympardi
S.
1
Drogari-Apiranthitou
M.
2
Zervakakis
I.
3
Kalofonou
M.
3
Minogiannis
N.
3
Papakonstantinou
H.
4
Liapi
G.
4
Arvaniti
A.
1
Karaiskou
A.
1
Giannopoulou
P.
5
Trikka-Grafakos
E.
5
1Infection Control Team, Thriasio General Hospital of Elefsis, Elefsis, Athens, Greece
2Infectious Diseases Research Laboratory, 4th Department of Internal Medicine, General
University Hospital “Attikon”, School of Medicine, University of Athens, Athens, Greece
3Department of Plastic Surgery, Thriasio General Hospital of Elefsis, Elefsis, Athens,
Greece
4Department of Pathology, Thriasio General Hospital of Elefsis, Elefsis, Athens, Greece
5Laboratory of Microbiology, Thriasio General Hospital of Elefsis, Elefsis, Athens,
Greece
P243. A mucormycosis case during the catastrophic flood in Mandra, Attica, Greece,
November 2017
Case Report:
Objectives: Cutaneous mucormycosis after major trauma may cause devastating morbidity
and death if not treated promptly and appropriately. The presentation of this case
report aims to sensitize health care professionals for the potential occurrence of
cutaneous mucormycosis after natural disasters.
Methods: Case report: A previous healthy 75-year-old woman was trapped in her flooded
home during the flood in Mandra, Attica, Greece, which occurred on 15/11/2017. She
remained four hours under water until rescued. She was transferred to the Emergency
department (ED) of a General Hospital with dyspnoea and type-I acute respiratory failure
(ARF) due to near-drowning. The patient had also multiple blunt injuries with skin
rupture in both lower extremities. At the ED she received treatment for ARF consisting
of oxygenation, tracheal aspiration, bronchodilators and antibiotics. Her wounds were
surgically cleaned and sutured and tetanus immune globulin was administered. The Glasgow
Coma Scale score (GCS) was evaluated at 13/15 and the patient was admitted to the
Pneumology department for further treatment. At day 2 post-admission clinical improvement
was documented, but on day 3 her consciousness level deteriorated (qSOFA score >2)
with onset of fever and low blood pressure. The antibiotic scheme was changed. Upon
re-evaluation of the leg wounds, development of necrotic eschars at the wound margins
was observed. On day 4 the patient was put on mechanical ventilation (GCS<8/15), underwent
extensive tissue debridement and liposomal amphotericin B 10mg /kg /24h was added
to the antibiotic scheme on suspicion of mucormycosis. Excised tissue was sent to
the Pathology and Clinical Microbiology departments for histological examination and
culture.
Results: The patient was transferred to a high dependency unit (HDU), however, on
day 5 she suffered severe cardiovascular collapse while the infection on both legs
had progressed over a large area. The patient’s family denied consent for bilateral
hip disarticulation. The patient died on day 6 after hospital admission. Histology
revealed fungal hyphae typical for Mucorales.
Conclusions: Mucormycosis should be always suspected in victims of natural disasters.
Infection can progress rapidly, requiring early therapy with antifungals, frequent
inspection of the wounds and repeated surgical debridement. Early primary wound closure
should be avoided.
Albirair
M.T.
1
Fahal
A.H.
2
Denning
D.
3
4
1Blue Nile National Institute for Communicable Diseases (BNNICD), Wad Madani, Sudan
2Mycetoma Research Center, University of Khartoum, Khartoum, Sudan
3University of Manchester, Manchester, United Kingdom
4National Aspergillosis Centre, Manchester University NHS Foundation Trust, Manchester,
United Kingdom
P244. The burden of serious fungal diseases in Sudan
Objectives: Sudan is a large north-African country with a population of about 40 million.
Apart from mycetoma which is a substantial problem, the burden of fungal infections
in Sudan is not well documented. Serious fungal infections are increasing with more
immunocompromised patients, longer lives and a high frequency of respiratory diseases.
The aim of this study was to estimate the burden of serious fungal infections in Sudan.
Methods: Epidemiological data reporting fungal infection rates in Sudan and nearby
countries together with population estimates for general and specific at-risk patient
groups were acquired. We used previously described deterministic modelling to estimate
national incidence or prevalence.
Results: HIV rates are low in Sudan and we estimated 231, 1,378 and 3,568 cases with
cryptococcal meningites, Pneumocystis pneumonia and oesophageal candidiasis, respectively.
Asthma affects an estimated 480,000 adults, and 11,400 and 15,048 people were estimated
to have allergic bronchopulmonary aspergillosis (ABPA) and severe asthma with fungal
sensitisation (SAFS) respectively, probably with some overlap between them. We estimated
1,085 cases of all forms chronic pulmonary aspergillosis (CPA) with 50% assumed to
occur post tuberculosis or are mis-diagnosed as TB. We estimated 354 cases of invasive
aspergillosis complicating leukaemia, HIV and lung cancer. There are no COPD prevalence
data. In the absence of local data, we have estimated candidaemia at 5/100,000 or
2,000 cases. We estimated ~530,000 women to be affected by recurrent vulvovaginal
candidiasis (RVVC). As many as 3.6 million school-age children suffer from tinea capitis,
based on a 21% rate. Mycetoma is a particular problem in Sudan and a minimum of 1.81/100,000
inhabitants or about 700 estimated cases are found there, predominantly eumycetoma.
No reliable data exist on fungal keratitis, histoplasmosis or mucormycosis.
Conclusion: Our study revealed about 4.1 million (10.5%) of the Sudanese population
suffer from serious fungal infections yearly with about 15,633 affected by life-threatening
invasive fungal infections. Improvements in fungal disease diagnosis and management
is required in Sudan.
Skiada
A.
1
Chander
J.
2
Klimko
N.
3
Hamal
P.
4
Dolatabadi
S.
5
Drogari-Apiranthitou
M.
6
Lass-Flörl
C.
7
Kanj
S.
8
Lagrou
K.
9
Arsic-Arsenijevic
V.
10
Zimmerli
S.
11
Petrikkos
G.
12
11st Dpt. of Medicine, Laiko Hospital, Athens University, Athens, Greece
2Department of Microbiology, Government Medical College Hospital, Chandigarh, India
3Department of Clinical Mycology, Allergology and Immunology, North-Western State
Medical University n.a. I.I. Mechnikov, Saint-Petersburg, Russian Federation
4Department of Microbiology, Palacký University, Faculty of Medicine and Dentistry
and University Hospital Olomouc, Olomouc, Czech Republic
5Faculty of Engineering, Sabzevar University of New Technology, Sabzevar, Iran
6Infectious Diseases Research Laboratory, 4th Department of Internal Medicine, General
University Hospital “Attikon”, School of Medicine, University of Athens, Athens, Greece
7Div Hygiene & Med. Microbiology, Med. Univ. Innsbruck, Innsbruck, Austria
8Division of Infectious Diseases, American University of Beirut Medical Center, Beirut,
Lebanon
9Laboratory of Clinical Bacteriology and Mycology, Department of Microbiology and
Immunology, Excellence Center For Medical Mycology (ecmm), KU Leuven, Leuven, Belgium
10Medical Mycology Reference Laboratory, Faculty of Medicine University of Belgrade,
Belgrade, Serbia
11Department of Infectious Diseases, Inselspital, Bern University Hospital, University
of Bern, University Hospital of Bern, Bern, Switzerland
12Infectious Diseases, School of Medicine, European University of Cyprus, Nikosia,
Cyprus
P245. Global epidemiology of mucormycosis: Preliminary analysis of the cases submitted
to the zygomyco.net registry during the last 10 years
Objectives: Mucormycosis is an invasive fungal infection with high morbidity and mortality.
Due to its rarity, registries are an important tool, used to collect information about
risk factors, diagnosis and treatment of this potentially lethal infection. The aim
of this study was to perform a preliminary analysis of the cases submitted to zygomyco.net,
the ECMM/ISHAM Working Group on Zygomycosis global registry, during a 10-year period
(2008-2018).
Methods: In order to submit a case, a participant must register to the site (www.zygomyco.net),
giving his name, country of origin and e-mail address. Participants in the study can
submit cases on-line in electronical CRFs. Alternatively, they can download a paper
form and send it to the coordinators. The required data include demographics, underlying
conditions, mode of transmission, clinical presentation, diagnosis, treatment and
outcome. In order to be included in the study the case must pertain to a probable
or proven case of mucormycosis. A data review committee reviewed all submitted cases.
Results: During the study period 365 cases were submitted from 16 countries (Austria,
Belgium, Brasil, Czech Republic, Greece, India, Iran, Italy, Lebanon, Netherlands,
Romania, Russia, Serbia, Switzerland, United Kingdom and USA). The largest proportion
of cases (34%) were submitted from India. The analysis was done in the 330 cases which
were complete. Sixty-two % of the patients were male. The mean age was 45y (range
1 month to 81 years). Underlying conditions were diabetes mellitus (37%), hematological
malignancies (30%), other malignancies (7%), bone marrow transplantation (9%), solid
organ transplantation (6%) and trauma (9%). Neutropenia was present in 25%. The main
sites of infection were lungs (35%), sinus/orbital/cerebral region (34%), soft tissue
(16%), disseminated (12%) and gastrointestinal (3%). Diagnosis was made only by histopathology
in 11%, only by direct examination in 3%, only by culture in 7%, and with combined
methods in the rest. Cultures were positive in 74.5% of cases (Rhizopus spp. 56%,
Lichtheimia spp. 15%, Mucor spp. 8%, Apophysomyces spp. 6%, Rhizomucor spp. 4%, Saksenaea
spp. 2%, Syncephalastrum spp. 2%, Cunninghamella spp. 1% and unspecified Mucorales
6%. Treatment was performed only by surgery in 3%, only by antifungals in 37% and
by combined approach in 44%. In the remaining 16% of patients, either the diagnosis
was made post-mortem or the patients declined treatment and left the hospital against
medical advice. Crude mortality was 41%.
Conclusion: This is a preliminary analysis of the cases submitted to zygomyco.net
in the period 2008 to 2018. A wide range of clinical presentations, underlying diseases
and isolated species was observed. Diagnosis is still difficult in many parts of the
world and mortality remains high. Further analysis of the data is going to highlight
the differences occurring in various countries and the risk factors associated with
mortality.
Maschio-Lima
T.
1
Marques
M.
2
Almeida
B. De
1
Lemes
T.
1
Bianco
L.
1
Caetano
M.
1
Brizzotti
N.
2
Silva
C.
2
Fernandes
J.
2
Castilho
E.
2
Siqueira
J.P.
2
Almeida
M.
2
1UNESP, São José do Rio Preto, Brazil
2FAMERP, São José do Rio Preto, Brazil
P246. Isolation and phenotypic characterization of Sporothrix brasiliensis from cats
with suspected sporotrichosis
Objectives: To confirm cases of suspected sporotrichosis in felines in the municipality
of São José do Rio Preto, Brazil, by isolating and identifying the Sporothrix spp.
in clinical samples of symptomatic animals; and to evaluate the clinical and epidemiological
factors associated with the disease.
Methods: In a 15 months period (August 2017 to November 2018), 114 samples of secretion
and/or biopsy of lesions from symptomatic cats were collected at the Center for Zoonoses
Control and analyzed using traditional mycological techniques. Phenotypic features
of the isolates were used to differentiate the species of the Sporothrix schenckii
complex (S. schenckii var. schenckii, S. brasiliensis, S. globosa, S. mexicana, S.
luriei, S. albicans). These features include presence or not of sessile pigmented
conidia, colony growth rates at 30ºC and 37 ºC, and assimilation of sucrose and/or
raffinose. Epidemiological aspects of the cats, which all had clinical suspicion of
the disease, were analyzed based on the information presented in research sheets filled
in at the time of collection by veterinarians.
Results: Regarding the origin of the animals, 65.79% (75) of the cats were erring,
23.68% (27) semi-domiciled, and 10.53% (12) fully domiciled. Most of the animals (99,
86.84%) were adults; 91 (79.82%) were males, and 23 (20.17%) were castrated. Multiple
skin lesions were present in 82 (71.92%) animals, 67% (55) of them exclusively ulcerated.
Indication of euthanasia was given to 66 (57.89%) cats. In relation to the mycological
analyses, 83 (72.80%) samples were positive for fungus, exhibiting characteristics
of the genus Sporothrix, i.e. creamy wrinkled colonies that become blackened due to
the production of melanin, and septate hyphae with conidiophores giving rise to conidia
in the shape of a daisy flower. The analyses revealed that the 83 isolates showed
morphophysiological profiles characteristic of S. brasiliensis.
Conclusion: Considering the short period of analysis, and that the samples were provided
from a single center, it is evident the high occurrence of feline sporotrichosis in
the municipality of São José do Rio Preto, Brazil, caused by S. brasiliensis. The
high percentage of positivity in adult, uncastrated and erring male felines highlights
the need to create control measures in this city.
Then
E.
1
Jong
A. De
1
Ruiz-Gaitan
A.
2
3
Al-Hatmi
A.
1
4
Ben-Ami
R.
5
6
Kurzai
O.
7
8
Ende
B. Gerrits Van Den
1
Hagen
F.
1
9
10
1Medical Mycology, Westerdijk Fungal Biodiversity Institute, Utrecht, Netherlands
2Severe Infection Research Group, Medical Research Institute La Fe, Valencia, Spain
3Department of Clinical Microbiology, La Fe University and Polytechnic Hospital, Valencia,
Spain
4Ministry of Health, Directory General of Health Services, Ibri, Oman
5Tel Aviv Sourasky Medical Centre, Tel Aviv, Israel
6Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
7National Reference Center For Invasive Mycoses, Leibniz Institute for Natural Product
Research and Infection Biology-Hans Knoell Institute, Jena, Germany
8Institute For Hygiene and Microbiology, Julius Maximilian University of Wuerzburg,
Wuerzburg, Germany
9Medical Microbiology, University Medical Center Utrecht, Utrecht, Netherlands
10Department of Dermatology, Jining No. 1 People’s Hospital, Jining, China
P247. CauSTR – A rapid multiplex microsatellite typing tool for the multi-drug resistant
yeast Candida auris
Objectives: Since the first description of Candida auris as a novel yeast pathogen
it has quickly conquered hospital environments causing colonization of patients that
ultimately can lead to bloodstream infections. During the past decade C. auris has
been reported from 25 countries and a development of multi-drug resistance has been
observed. Molecular epidemiological typing studies using whole genome sequencing,
amplified fragment length polymorphism fingerprinting and multi-locus sequencing typing
all identified four lineages linked to geographic regions. The aim was to develop
a rapid and cost-effective molecular typing tool that relies on genetic diverse microsatellite
loci.
Methods: The genome of C. auris strain B8441 (GenBank GCA_002759435.2) was used to
search for repetitive loci using Tandem Repeat Finder (https://tandem.bu.edu/trf/trf.html).
This resulted in 34 candidate loci that were tested by conventional PCRs using the
CDC C. auris reference set. Nine microsatellite loci remained and were subsequently
tested on a larger set of 92 C. auris isolates (India n = 28, Oman n = 24, Spain n
= 13, Israel n = 11, Germany n = 6, Korea and Saudi Arabia n = 2 each, and n = 1 for
Austria, Belgium, Japan, Kuwait, Switzerland and the Netherlands). Three multiplex
PCRs were developed using fluorescently labeled primers to detect and differentiate
the loci when analyzed on an ABI3730xL Genetic Analyzer.
Results: The genome size of C. auris strain B8441 is 12.366 Mbp and contains 2,374
microsatellites ranging from 1 bp repeat units up to a 461 bp repeat unit. Based on
other fungal genomes it was expected that the smaller microsatellite units of 1–10
bp represent the majority of the microsatellites. But surprisingly, as can be observed
in Figure 1, those with a repeat unit of 12, 15 and 18bp are more common than those
containing 2–10bp repeat units. The final panel consisted of six tri-nucleotide, one
with tetra-nucleotide, one nona-nucleotide and one undeca-nucleotide loci. The Simpson’s
diversity index D ranged from 0.5387–0.8120 for individual loci, when all nine loci
were considered. Fifty-two genotypes were observed with a Simpson’s D of 0.9656. The
isolates from the endemic regions (India, Oman) were found to be clonal, while those
from nosocomial outbreaks (Israel, Spain) could be linked to two different endemic
regions. Single reported cases from Europe could be mostly linked to the South East
C. auris population.
Figure 1. A selection of microsatellite types that occur at least ten times in the
C. auris genome are shown. The number in each box stands for the size of the repeat
unit and the size of the box represents the relative abundance of these specific repeat
units.
Conclusion: Microsatellite loci are abundantly present in the C. auris genome but
surprisingly the most variable repeat units (2–10bp) are sparsely distributed. The
developed nine-loci microsatellite typing panel ‘CauSTR’ in this study is able to
rapidly differentiate the four recognized lineages previously identified by other
molecular methods and to observe subtle differences within C. auris.
Carbia
M.
1
Ende
B. Gerrits Van Den
2
Fernández
N.
3
Setropawiro
M.
2
Hagen
F.
2
4
5
1Parasitology and Mycology, Instituto de Higiene, Montevideo, Uruguay
2Medical Mycology, Westerdijk Fungal Biodiversity Institute, Utrecht, Netherlands
3Sección Micología De La División Infectología, Hospital de Clínicas “José de San
Martín”, Buenos Aires, Argentina
4Medical Microbiology, University Medical Center Utrecht, Utrecht, Netherlands
5Department of Dermatology, Jining No. 1 People’s Hospital, Jining, China
P248. Molecular characterization and epidemiology of Histoplasma isolates from Uruguay
Objectives: Histoplasmosis is an invasive fungal infection, often encountered in HIV-positive
subjects, caused by members of the genus Histoplasma. Historically three varieties
were recognized within the single known pathogenic Histoplasma species, namely H.
capsulatum variety capsulatum, variety duboisii (African histoplasmosis) and variety
farciminosum (Equine histoplasmosis). However, a recent taxonomic revision based on
whole genome sequencing, phenotypic and clinical data differentiated H. capsulatum
var. capsulatum into four species: H. capsulatum sensu stricto, H. mississippiense,
H. ohiense and H. suramericana. This study was initiated to retrospectively investigate
the epidemiology of Histoplasma in Uruguay.
Methods: Forty-two clinical Histoplasma isolates obtained from 42 cases, collected
during 1963-2019 and preserved at the Instituto de Higiene, Montevideo, Uruguay, were
included. A set of 25 isolates that includes the four newly recognized Histoplasma
species was used as reference. Fourteen patients (33.3%) were found to be HIV-positive,
12 (28.6%) had other underlying diseases/predisposing factors, 1 (2.4%) was immunocompetent
and for the remaining 15 cases (35.7%) no clinical data was available. Histoplasma
DNA was extracted using the CTAB-protocol and subsequently subjected to multi-locus
sequence typing (partly sequencing of ITS, ARF, OLE, H-anti, M-anti and 60S) and amplified
fragment length polymorphism fingerprinting. The mating-type MAT1-1 and MAT1-2 was
determined by conventional PCR.
Results: There was a nearly equal distribution of mating-type MAT1-1 and MAT1-2 isolates,
randomly distributed over time and among Uruguayan patients. By AFLP fingerprinting
and multi-locus sequence typing it was observed that all Uruguayan isolates clustered
together. However, based on the former molecular typing tool it appears that all these
isolates were clonal while with the sequence-based approach more subtle clustering
was observed. With both molecular tools the Uruguyuan Histoplasma isolates clustered
closely together with the reference strain of H. suramericana.
Conclusion: Using AFLP fingerprinting and multi-locus sequencing typing a set of 42
Histoplasma isolates from Uruguay was found to belong to the newly described species
H. suramericana. There was no link observed between the clinical background versus
the molecular characteristics of the studied isolates.
Osmanov
A.
1
Denning
D.
1
2
Huseynov
R.
3
1Global Action Fund for Fungal Infections, Geneva, Switzerland
2Manchester Academic Health Science Centre, Manchester, United Kingdom
3Azerbaijan Medical University, Baku, Azerbaijan
P249. The Burden of Serious Fungal Infections in Azerbaijan
Objectives: We aim to calculate the burden of serious fungal infections in Azerbaijan.
Methods: We have performed search on published papers of serious fungal infections.
Where no published data existed, we used population at-risk to identify burden of
serious fungal infections. Our estimations were based on the model developed by LIFE
(Leading International Fungal Education).
Results: Azerbaijan is in the South Caucasus region with the population of 9.8 M people
and GDP per capita of 5,805 USD. There are 4,051,310 adult women (15-55 yrs) in Azerbaijan,
so it was estimated that 176,961 women suffer from recurrent vulvovaginal candidiasis
(rVVC) which is defined as = > 4 episodes/year. There are 8,000 PLHIV which is most
likely an underestimate; 54% of them do not receive antiretroviral therapy. Hence,
we estimate 2,673 and 1,291 patients with oral and oesophageal candidiasis respectively.
There are probably 16 cases of cryptococcal meningitis and 64 cases of Pneumocystis
pneumonia annually which is likely to be an underestimate. We have estimated 902 cases
of chronic pulmonary aspergillosis (CPA) as a sequel of TB and 2,707 cases of CPA
complicating other conditions such as pneumothorax, emphysema, and sarcoidosis; which
results in the total of 3,609 cases of CPA. Based on asthma prevalence there are approximately
4,188 patients with allergic bronchopulmonary aspergillosis (ABPA) and 5,528 with
severe asthma with fungal sensitisation (SAFS). We used a low European rate to estimate
invasive candidiasis cases and there are approximately 493 and 74 with candidemia
and invasive candidiasis respectively. We have estimated approximately 111 patients
with invasive aspergillosis and 20 patients with mucormycosis.
Conclusion: We have estimated 195,026 (2% of the population) people in Azerbaijan
with serious fungal infections. Providing diagnostics tools for fungal infection will
lead to better understanding of this problem and will allow to highlight this problem
at the national level.
Grant
I.H.
1
Geller
J.
2
Sabath
H.
3
1Community Medicine, New York Medical College, Tarrytown, United States of America
2Information Revelations, Montclair NJ, United States of America
3International Environmental Diagnostics Inc, Irvington NYw, United States of America
P250. rDisability and disease after indoor macrocyclic Trichothecenes mycotoxin and
microfungal exposure: exposure variable analysis indicates unexpected significant
relationship between immunosuppression and MTHFR detoxification defects.
Objectives: Macrocyclic trichothecene mycotoxins are extremely toxic nanoparticulate
exotoxins dangerous for all mammals, binding to surface dust where mold grow, aerosolized
and inhaled if disturbed. They damage intracellular protein/DNA/RNA production and
mitochondrial function causing premature cell death (apoptosis) on contact. A exotoxins,
they remain toxic despite disinfection. They cause damage to mammalian skin, mucosa,
phagocytes, neurons, and organs via systemic circulation after inhalation. Produced
by Stachybotrys and Trichoderma in water-damaged indoor environments, they are more
potently toxic than T2-toxin and doxynivalenol (DON, vomitoxin) mainly produced by
Fusarium on agricultural grains. Objectives: Determine: - Severe illness frequency
due to indoor mold/mycotoxin exposure. - Specific exposure risks - The impact of genetically-impaired
intracellular MTHFR detoxification capacity. - Conditions that predispose to opportunistic
fungal infection with such exposures (e.g. mucosal injury, immunosuppression, malnutrition)
Methods: Prospectively monitored 51(21M,30F) adults and children exposed to 30 Trichothecenes-contaminated
indoor-environments with comprehensive environmental-mycological and medical investigations,
fungal IGGs, urine mycotoxins(MCTs: Aflatoxin, Gliotoxin, Ochratoxin A, Trichothecenes),
immune parameters(neutrophils, lymphocyte subsets, monocytes, IGG,IGA,IGM,IGE,subclasses),
immunosuppressants, nutritional deficiencies (protein, Vitamin D,zinc), genetic Methylenetetrahydrofolate
reductase (MTHFR) single-nucleotide-polymorphisms C677T&A1298C. Disease Severity Outcomes(DS),
Environmental Mold-Contamination Severity(EMCS), Hazardous Exposure Activities(HEA),
Exposure Duration (ED), MCT/Trichothecenes exposure(MTC/TE), and clinical findings
were stratified and analyzed. Biostatistical analysis(Pearson Correlation Coefficients)
correlated DS with EMCS, HEA, ED, MCT/TE, mucosal Injury(MI), Neurological disease,
Cell-mediated Immunodeficiencies(CMIs), combined cellular-immunoglobulin immunodeficiencies,
mucosal injury (MI), mold-specific IGG response(M-IGG), MCT excretion and MTHFR defects.
Results:
CORRELATIONS:
Disease Severity based on formal disability classification/physical injury strongly
correlated with Hazardous Activities (p = 0.000043), Neurological disease (p = 0.000263),
Immunoglobulin deficiencies (p = 0.000923), Combined Cellular-Immoglobulin Deficiencies
(p = 0.00502), Environmental Mold Contamination (p = 0.007531) and Mucosal Injury-the
main expected injury from Trichothecenes inhalation (p = 0.018034). Mucosal injury
strongly correlated with Neurological damage (p = 0.026842) and combined cellular-immune
defects (p = 0.026842). Combined Cellular-Immunoglobulin Deficiencies strongly correlated
Hazardous Activities (p = 0.009464). Development of mold-specific IGGs correlated
with environmental mold-contamination (p = 0.26842) and Urine Trichothecenes(p<.00001).
Females were more often adversely affected. Environmental Exposure Hazardous Activities
(especially unprotected mold-disturbance, uncontained construction/repairs, basement-living,
HVAC-contamination and sewage-overflow) correlated with enviromental Trichotehcenes
level (p = 0.046588), Combined Cellular Deficiencies (p = 0.038993) and immunoglobulin
deficiencies (p = 0.005952). Environmental Mold Contamination correlated with Disease
Severity (p = 007531), Hazardous Activities (p = 0.002153), Environmental Trichothecenes
level (p = 0.00502), multiple environmental mycotoxins (p = 0.02207), Combined Cellular-Immunoglobulin
Deficiencies (p = 0.04658),immunoglobulin deficiencies (p = 0.032448) and elevated
mold IGGs (p = 0.026842). Environmental mycotoxin contamination correlated with urine
mycotoxin excretion (p = 0.046588). Environmental Trichothecenes contamination significantly
correlated with Environmental Mold Contamination (p = 0.00502), Hazardous Activity
(p = 0.046588) and Exposure Duration (p = 0.046588) Urine Macrocyclic Mycotoxin Score
strongly correlated with the Environmental Mycotoxin Score (p = 0.046588).
Conclusion: Indoor exposure to macrocyclic Trichothecenes appears extremely hazardous.
The high frequency of bleeding (90%), mucosal injury (82%), neurological damage (75%),
pain (64%), chronic fatigue (63%), burning (57%), with expected respiratory/Gi /Dermatologic
symptomatology is consistent with known Trichothecenes effects in animal models and
military data. The high (94%)MTHFR methylation-defect incidence is striking compared
to estimated USA 50-60% prevalence. MTHFR is a key intracellular detoxifying-enzyme
protecting ribosomes/mitochondria RNA/DNA damage from Trichothecene injury via glutathione
metabolism. Further investigation is needed to determine if Trichothecenes trigger
genetic damage or autoimmunity. Urine macrocyclic Trichothecenes detection appears
a useful marker for environmental macrocyclic Trichothecenes exposure. Public health
education and epidemiological research are urgently needed to investigate correlation
between environmental mold/mycotoxin exposure, multisystem illness, immunodeficiencies
and female susceptibility.
Hesstvedt
L.
1
Gaustad
P.
2
Andersen
C. Torp
2
Muller
F.
2
Nordoy
I.
1
1Dept. of Infectious Diseases, Oslo University Hospital, Oslo, Norway
2Dept. of Microbiology, Oslo University Hospital, Oslo, Norway
P251. Survivors of candidemia without antifungal treatment
Objectives: Candidemia is associated with high mortality, which increases without
antifungal treatment. The aim of this nationwide, retrospective study was to examine
factors associated with 30-day survival in a subpopulation of candidemia patients
who did not receive antifungal treatment.
Methods: We collected data by reviewing medical records from patients who were diagnosed
with candidemia between January 1st 2008 and December 31st 2012. Age (at or below
and above 65 years), sex, Candida species (C. albians vs. C. glabrata), predisposing
illnesses (malignancy; gastrointestinal cancer, hematological malignancy or all other
malignancy, organ transplantation, chronic obstructive pulmonary disease, diabetes
mellitus, inflammatory bowel disease, intravenous drug users – IVDU) and health-care
related risk factors (central venous catheter - CVC, urine catheter, mechanical ventilation,
dialysis, abdominal surgery, neutropenia – neutrophils <0.5 x109/L > 5 days, use of
antibiotics, corticosteroids, chemotherapy, proton pump inhibitor –PPI or total parenteral
nutrition-TPN) were recorded.
Results: In 144 candidemia patients (60.4% men, age 63.9 ± 16.2 years) not receiving
any antifungal treatment, the overall 30-day survival rate was 36.8%. No significant
difference was found between genders of untreated survivors (n = 53, 54.7% men, age
63.6±20.4) and untreated non-survivors (n = 91, 63.7% men, age 74.2±11.7) Overall
significantly more of the patients < 65 years than ≥ 65 years survived without treatment
(55.8% vs. 26.5%, p = 0.015). of predisposing illnesses malignancies excluding hematological
and gastro-intestinal cancers was significantly less common in untreated survivors
compared to non-survivors (23.5% vs. 76.5%, p = 0.02). of healthcare related risk
factors the untreated survivors significantly less often were mechanically ventilated
(34.5% vs. 65.5%, p = 0.049), less often had urine catheters (27.0% vs. 73.0%, p<0.001),
received TPN (23.9% vs. 76.1%, p = 0.04) or PPI (28.4% vs. 71.6%, p = 0.03) compared
to untreated non-survivors. Central venous catheters were more often removed in untreated
survivors than in non-survivors (29.2% vs. 10.9%, p = 0.04). Untreated intravenous
drug users (IVDU) (n = 9) all survived candidemia. No significant difference was seen
between untreated survivors and non-survivors related to C. albicans or C. glabrata
candidemia.
Conclusion: Patients surviving untreated candidemia were significantly younger, had
less underlying malignancy and fewer risk factors for candidemia, in addition more
often having their CVC removed than the patients who died without treatment. The prognosis
in untreated candidemia is influenced by comorbidity and its subsequent treatment.
OSullivan
M.
Clinical Microbiology, Chrisitan Medical College, Vellore, Tamil Nadu, India
P252. Mucormycosis – A clinicoepidemiological review of cases over 10 year
Objectives: Objectives: To study the epidemiology and outcomes of various agents causing
mucormycosis in different clinical settings in a tertiary care hospital from South
India.
Methods: Patients and methods: We reviewed details of 184 consecutive patients with
culture-proven mucormycosis with consistent clinical syndrome and supporting features
from September 2005 to September 2015.
Results: Results: The mean age of patients was 50.42 years; 70.97% were male. Unlike
developed countries, Rhizopus microsporus (29/184; 15.7%) and Apophysomyces elegans
(20/184; 10.8%) also evolved as important pathogens in addition to Rhizopus arrhizus
in our setting. Paranasal sinuses (136/184; 73.9%) followed by musculoskeletal system
(28/184; 15.2%) were the common areas of involvement. A. elegans typically produced
skin and musculoskeletal disease in immune-competent individuals with trauma (12/20;
60%) and caused significantly lower mortality (P = 0.03). R. microsporus was more
common in patients with haematological conditions (25% vs 15.7%) and was less frequently
a cause for sinusitis than R. arrhizus (27.58% vs 10.9%). The overall mortality was
30.97%. Combination therapy with surgery and antifungals offered the best chance for
cure.
Conclusion: Conclusions: Agents causing mucormycosis may have unique clinical and
epidemiological characteristics.
Costales
I.
1
Milian
L.
2
Vallina
E.
1
Martínez
S.
1
Templado
A.
1
Boga
J.A.
1
Peláez
T.
1
1Servicio De Microbiología Y Parasitología, HUCA, Oviedo, Spain
2Servicio De Microbiología Y Parasitología, CAUSA, Salamanca, Spain
P253. Biliary Tract Candidiasis: an overview of the epidemiology in a general hospital
Objectives: Infections of the biliary tract are a common gastrointestinal disorder.
Infections caused by Candida spp. and other fungal species have increased their incidence
in the last few years. Knowing the autochthonous epidemiology is advisable in order
to ensure an effective empirical therapy and an optimal management of the patients.
Methods: Biliary tract samples from patients diagnosed with acute cholangitis from
January 2017 to June 2019 were included in the study. We considered only one one sample
per patient and clinical episode. All isolates were identified using MALDI-TOF Biotyper®
(Bruker Daltonics) methodology and yeast susceptibility testing were performed by
SensititreTM YeastOne® (Thermo Scientific, TREK Diagnostic systems, UK). In case of
polyfungical infections, all yeast isolates were identified and susceptibility test
weas performed, but were considered as a single sample.
Results: From a total of 299 biliary samples analyzed, 80 of them (26.8%), corresponding
to 65 patients, showed positive fungal growth. During the study period, the incidence
of yeasts isolated from biliary tract increased from 2017 (0.49/1.000 admissions)
to 2019 (0.95/1.000 admissions). The species most frequently recovered from biliary
tract candidiasis episodes were C. albicans (56.25% of cases), C. glabrata (16.25%),
C. Krusei (6.25%), C. tropicalis (6.25%), C. parapsilosis (6.25%) and other yeasts
(8.75%). The percentages of isolation of different yeasts isolated in the first and
last year of the study were respectively as follows: C. albicans (70.6% vs 53%), C.
glabrata (11.7% vs 23.6%), C. krusei (5.9% vs 11.8%), C. tropicalis (0% vs 16.7%),
C. parapsilosis (0% vs 13.3%) and other yeasts (11.8% vs 23.5%). A total of 51 cases
(78.46%) of biliary tract candidiasis were monofungal infections and 14 (21.54%) were
polyfungal infections.
Conclusion: In our hospital, the incidence of biliary tract candidiasis increased
during the study period probably due to an improvement of culture methods, as well
as greater clinical suspicion requesting a micologycal analysis of bile samples. Globally,
C. albicans was the most frequent yeast species isolated, followed by C. glabrata.
However, a significant increase in potentially fluconazole resistant species (C. glabrata
and C. krusei) occurs in the last year of the study.
Iunovidova
A.
1
Klimko
N.
2
Sobolev
A.
1
1Department of Clinical Mycology, Allergology and Immunology, North-Western State
Medical University named after I.I, Mechnikov, Saint-Petersburg, Russian Federation
2Department of Clinical Mycology, Allergology and Immunology, North-Western State
Medical University n.a. I.I. Mechnikov, Saint-Petersburg, Russian Federation
P254. Prevalence of severe asthma with fungal sensitization (SAFS) in European population.
Objectives: SAFS can be defined as a continuum of fungal sensitization with asthma
at one end andallergis bronchopulmonary aspergillosis at the other. The main goal
was to determine the prevalence of SAFS in the European population due to insufficient
data.
Methods: Incident case and population data were obtained for SAFS fron the Surveillance,
Epidemiology, and End Results between 2011 and 2018.
Results: Recent global estimates have found 10,000,000 cases of fungal asthma occur
annually. The major assumptions made were based on previous studies of SAFS; severe
asthma is thout to affect about 10% of adults and 33-70% are sensitised to fungi and
that 2.77-2.90% of population have asthma. Although the prevalence of SAFS is uncertain
as this disease has been recently described it was conservatively estimated that 30%
of patients with severe asthma have or develop SAFS. Patients with SAFS probably constitute
a significant proportion (~50% estimated) of the nearly 0.5 million asthma related
deaths worldwide. The burden of SAFS has been estimated to 4,125,428 (+-174,033) cases
in 43 countries.
Conclusion: The diagnostic gap between estimated burden and recorded cases numbers
provides a clear cut target to close, to improve patient outcomes. Therecognitionoffungalinfectionsasamajorcontributortomortalityofseveralconditionsemphasizes
the need for public health efforts in reducing the incidence and mortality of these
infectious diseases
Badiane
A.
Diongue
K.
Seck
M.C.
Ndiaye
M.
Ndiaye
D.
Parasitology and Mycology, Universite Cheikh Anta Diop de Dakar, Dakar, Senegal
P255. Epidemiological aspects of superficial mycosis among arabic school children
in two localities in Sénégal (Thies and Touba)
Objectives: The objective of this study was to determine the prevalence of superficial
mycosis in koranic schools of Thies and Touba, two cities located in western Senegal.
Methods: This study were conducted in children from seven (07) koranic schools in
two cities of Senegal (Thies and Touba). A total of 210 children were recruited. For
each child, depending of the lesions squama, hairs or nail pieces were collected using
sterile material. Fungal pathogens were identified using direct examination with 30%
Potassium Hydroxide (KOH) and culture on Sabouraud addionned with chloramphenicol
and actidone (cycloheximide).
Results: The most prevalent lesion was tinea capitis (90%). The culture was positive
for 25.1% of the pupils. The species identified were Trichophyton soudanense (85.18%),
Microsporum audouinii langeronii (9.25%), Trichophyton rubrum (3.70%), and Chrysosporium
keratinophilum (1.85%). Among infected participants, children aged 3 to 10 years were
more contaminated (53.70%), followed by the age group of 11 to 15 years (44.44%) and
participants over the age of 15 were less infected (1.85%). The prevalence of infection
was higher among boys (85.18%).
Conclusion: This study showed a fairly prevalence of superficial mycoses among koranic
school children.
Samaras
K.
Markantonatou
A.-M.
Zarkada
E.
Zachrou
E.
Vyzantiadis
T.-A.
First Department of Microbiology, Medical School, Aristotle University of Thessaloniki,
Thessaloniki, Greece
P256. Infections by Mucoraceous moulds recorded during a period of twelve years.
Objectives: Mucoraceous moulds are very common in natural environment. They may rarely
be the cause of severe or even fatal infections in immunocompromised patients, patients
with uncontrolled diabetes mellitus or patients bearing deep and dirty wounds. The
aim of this study was to record the cases of infections by mucoraceous moulds, microbiologically
diagnosed in our laboratory and to detect the existence of any possible specific feature
of these infections.
Methods: A retrospective recording was performed of all similar cases tested by our
laboratory during the last twelve years
Results: Eleven cases of mucoraceous mould infections were recorded (7 male, 4 female).
The fungal pathogens isolated were: 9 Rhizopus arrhizus, 1 Mucor circinelloides and
1 Saksenaea vasiformis. Seven patients were immunocompromised, two were suffering
from uncontrolled diabetes mellitus and two of them had deep, dirty wounds. Five of
the aforementioned cases concerned culture plates sent to the laboratory for identification
of the fungal isolate. As for the other six specimens, direct microscopy of the clinical
sample was positive for all of them. In 8 cases, antifungal susceptibility testing
was performed and the range of MICs was 0.003-16 μg/ml for amphotericin B and 0.75-12
μg/ml for posaconazole. The outcome was favourable for five patients. In nine out
of the eleven cases the infection was presented in spring or autumn, seasons that
in our country are characterized by mild to moderate temperatures and relatively high
humidity.
Conclusion: All patients had at least one risk factor for mucormycosis. A seasonal
distribution was noticed, something that has been noted by other authors too. Positive
direct microscopy in all clinical samples demonstrates the great importance of the
procedure which provides promptly very characteristic images and contributes to the
initiation of targeted treatment which may be life saving.
Wilmes
D.
Losert
H.L.
Leibhan
H.
Gerkrath
J.
Rickerts
V.
Fg16-mycology, Robert Koch Institute, Berlin, Germany
P257. Molecular Epidemiology of Histoplasmosis in Germany
Objectives: Histoplasmosis is a systemic fungal infection caused by obligate pathogenic
fungi of the genus Histoplasma. Autochthonous infections in wild and domesticated
animals document the presence of Histoplasma in the environment in Germany. The aim
of this study is to characterize the molecular epidemiology of Histoplasmosis in Germany
by multilocus sequence typing (MLST) of Histoplasma cultivated from patients diagnosed
in Germany and to compare them to strains characterized by a modified MLST from infected
animals in Germany.
Methods: Since the year 2000, we participated to the diagnosis of 76 human cases (mean
= 4/year) and 10 animal cases (7 cats, 3 badgers) of proven and probable infection.
Twenty-two Histoplasma strains cultivated at our Laboratory, including two CBS reference
strains were typed using a 4 gene scheme (arf, H-anti, ole and tub) previously described
by Kasuga (1). Concatenated sequences were aligned with reported sequences of worldwide
isolates. Then a modified MLST for formalin fixed paraffin (2) embedded samples was
performed on pathology blocks from veterinary samples. Neighbour joining trees were
constructed to demonstrate relationships using the Geneious software suite.
Results: All four genes could be sequenced for 16 of 22 isolates while the amplification
of the ole gene failed for 6 isolate. The phylogenetic analysis demonstrated clustering
of the German patient isolates with previously defined clades (African: n = 7; Latin
American: n = 7; Bat associated clade1: n = 1; Netherlands: n = 1) corresponding to
the patients travel history. of note, 3 patient isolates clustered with the Eurasian
clade, in which also 7 animal samples (5 cats and 1 badger) clustered, suggesting
related organisms. Two of the patients had a travel history to Southeast Asia, while
for one no travel history was reported.
Conclusion: Our study demonstrates that histoplasmosis in Germany is mostly imported
from endemic regions including Latin America, Africa and Southeast Asia. However,
clustering of human isolates and veterinary cases from Germany within the Eurasian
clade suggest the presence of Histoplasma in the German environment. Further molecular
studies on human and veterinary cases and environmental sampling are needed to define
environmental niches.
Lmimouni
B.E.
1
Azelmat
S.
2
Hennequin
C.
3
Denning
D.
4
1School of Medicine and Pharmacy, University Mohamed The Fifth, Parasitology and Medical
Mycology Lab, Military Teaching Hospital Mohamed the Fifth, Hay Riad, Rabat, Morocco
2Parasitology and Medical Mycology, Military Teaching hospital Mohammed the Fifth,
Rabat, Morocco
3Parasitology-mycology, APHP, Hôpital St Antoine, Paris, France
4University of Manchester, Manchester, United Kingdom
P258. Burden of fungal infections in Morocco
Objectives: We report for the first time in Morocco burden estimates of fungal infections.
The aim of this study was to estimate the incidence and prevalence of fungal infection
in Morocco.
Methods: We used the national demographic data and performed a PubMed and Scopus search
to retrieve all published papers on fungal infections from Morocco. We have also modelled
the likely burden of serious fungal infections using methodology previously published
by LIFE.
Results: We report for the first time the main causes of these diseases in our country.
Superficial mycoses and dermatophytic infection (onychomycosis, tinea capitis, others
…) are very commonly diagnosed, We have found 13,294 cases in the published papers.
Deep fungal infection are less found in the literature. Modlelling shows higher rates
for all infections where modelling is possible, especially for pulmonary fungal infections.
Literature Modelling Infection Rate/100,000 Total burden Rate/100,000 Total burden
Cryptococcal meningitis 0.32 115 0.7 252 Pneumocystis pneumonia 0.22 78 1.2 420 Invasive
aspergillosis 0.88 315 1.9 674 Mucormycosis 0.01 4 0.2 72 Chronic pulmonary aspergillosis
post TB 0.74 266 8.6 3085 Allergic bronchopulmonary aspergillosis (ABPA) 0.01 4 51
18,400 Severe asthma with fungal sensitization (SAFS) 0.28 100 67 24,292 Candidaemia
1.47 528 5 1800 Oral Candidiasis 0.25 90 22 7800 Mycetoma 0.11 40 Recurrent Candida
vaginitis (>4x/year) 0.17 30 2755 496,000 Histoplasmosis 0.01 4 - - Skin dermatophytic
infection 13.97 5031 - - Onychomycosis 14.08 5069 - - Tinea capitis 8.87 3194 - -
Deep dermatophytosis 0.02 10 - - Total burden estimated 14522 551,400
Conclusion: A focus on improving the diagnosis and epidemiological data related to
fungal infection is necessary in Morocco.
Santos
C. Oliveira Dos
1
Kolwijck
E.
1
Eggink
C.
2
Verweij
P.E.
3
1Medical Microbiology (777), Radboud University Medical Center, Nijmegen, Netherlands
2Ophthalmology, Radboud University Medical Center, Nijmegen, Netherlands
3Medical Microbiology, RadboudUMC, Nijmegen, Netherlands
P259. Fusarium keratitis in the Netherlands (2005 – 2016).
Objectives: Recognizing the clinical picture of fungal keratitis is difficult. As
a consequence, the fungal diagnosis might be delayed due to primary therapy with antibacterial
agents and topical corticosteroids, resulting in spreading of the fungus in the cornea
which ultimately may lead to more severe infection and monocular blindness. There
seems to be a trend of increasing incidence of fungal keratitis in the Netherlands,
especially caused by Fusarium species. Here, we describe the epidemiology and clinical
management of fungal keratitis in the Netherlands.
Methods: The Dutch national reference center for medical mycology has received Fusarium
strains isolated from 89 Dutch fungal keratitis patients, in the period 2005 up to
2017. The clinical data was added in retrospect by the ophthalmologists. All Fusaria
were identified up to genus level using conventional techniques and/or molecular techniques.
Antifungal susceptibility testing was performed according to the method described
by the EUCAST. The antifungal agents tested were amphotericin B, voriconazole and
natamycin. Susceptibility to the antisepticum chlorhexidine was also tested.
Results: In this period there was a mean incidence of Fusarium keratitis of 0.5 (range
0 – 1.5) per million per year. The male to female ratio was 1:3 (p = 0.014). The use
of contact lenses in this cohort was high; 92.9% of patients with reported use or
non-use of contact lenses. The time between start of symptoms to the diagnosis of
fungal keratitis was significantly longer in the group with therapy failure opposed
to the group with restored vision; respectively 22 days versus 15 days (p = 0.024).
The need of a corneal transplantation or enucleation of the affected eye occurred
in 27 cases (n = 20 [22.5%] resp. n = 7 [7,9%]). Enucleation occurred in patients
with delayed diagnosis of the fungal culprit (>14 days after start symptoms). The
mean visual acuity was moderately impaired with a logMAR of 0.8 (95% CI 0.6 – 1) at
the time of isolation of the Fusarium strain. The final visual outcome had an average
logMAR of 0.2 (95% CI 0.1 – 0.3), the equivalent of mild visual impairment. The treatment
strategies differed greatly between the cases. of note, five patients were treated
with chlorhexidine 0.02% monotherapy after Fusarium was isolated with good results;
4 patients had a successful outcome. The most frequently isolated species was F. oxysporum
(n = 22, 24.7%) followed by F. solani sensu stricto (n = 16, 18%) and F. petroliphilum
(n = 8, 9%). The lowest MICs were obtained with amphotericin B followed by natamycin,
voriconazole, and chlorhexidine.
Conclusion: In our population trauma is not the main route of infection. An important
risk factor in the Netherlands seems to be the use of contact lenses. When correct
diagnosis and antifungal treatment is delayed the risk of losing the affected eye
is great. The lack of a specific national guideline is reflected in the greatly varying
treatment strategies. Chlorhexidine seems to be a good addition to the therapeutic
options.
Zaidan
M.
1
Mechref
Z.
1
Frank-Soltysiak
M.
2
Nguyen
D.-P.
2
Durrbach
A.
1
Angoulvant
A.
3
4
1Nephrology, Hôpital Bicêtre, APHP, Le Kremlin Bicêtre, France
2Dim Hups / Aphp Wind, Hôpital Bicêtre, APHP, Le Kremlin Bicêtre, France
3Génétique Quantitative et Évolution – Le Moulon, INRA – Université Paris-Sud – CNRS
– AgroParisTech, Gif-sur-Yvette, France
4Mycology-parasitology, Hôpital Bicêtre, APHP, Le Kremlin Bicêtre, France
P260. rFungal invasive infections after kidney tranplant: a monocenter study
Objectives: Invasive fungal infections constitute an important cause of morbidity
and mortality in solid organ transplantation recipients. Epidemiology of invasive
fungal infections (IFI) after kidney transplantation in the modern era of immunosuppression
is poorly described. We aim to describe the IFI epidemiology in our centre.
Methods: We retrospectively analyzed the epidemiology of IFI in a single-center cohort
of kidney transplant adult recipients between January 2011 and December 2018.
Results: There were 1646 kidney transplanted patients hospitalized during the study
period; 68 (4%) cases of IFI were identified. Sex ratio (M/F) was 1.1 and mean age
63 years (44 – 77). IFI occurred 12 (1-144) months after transplantation, in patients
with immunosuppressive therapy containing tacrolimus, mofetil mycophenolate and corticosteroids.
100% of them had renal insufficiency, 68% had diabetes and 50% were neutropenic at
the time of the IFI diagnosis. A. fumigatus proven or probable invasive aspergillosis
(IA) was the leading cause (n = 28, 1.7 %) followed by pneumocystosis (n = 20, 1.22%),
candidemia (n = 15. 0.92%) and other IFI (n = 6 (0.3%). Urinary source was identified
in 77% of patients with candidemia. C. albicans (60%) was the leading species followed
by C. glabrata and C. parapsilosis (20% each). Loss of graft occurred in 20% of them.
Mortality was 50%, 40% and 10% for IA, candidemia and pneumocystosis respectively
Conclusion: In our centre IFIs, excepted pneumocystosis, were associated with high
mortality rates in renal transplant patients. IA was the most common and was associated
with the higher mortality. Candidemia occurred rarely but was associated with high
mortality and loss of graft rates.
Keser
S. Kotoric
1
Pilav
A.
2
Bektaš
S.
1
Hodžić
D.
1
Balihodžić
A. Obradović
1
1Microbiology, P.I. INSTITUTE FOR PUBLIC. HEALTH OF CANTON. SARAJEVO, Dr. Mustafe
Pintola 1/III 71 000 Sarajevo, Sarajevo, Bosnia and Herzegovina
2Social Medicine, P.I. INSTITUTE FOR PUBLIC. HEALTH OF CANTON. SARAJEVO, Dr. Mustafe
Pintola 1/III 71 000 Sarajevo, Sarajevo, Bosnia and Herzegovina
P261. Prevalence and biotypes of vulvovaginal Candidiasis among reproductive-age women
in Sarajevo.
Objectives: The study aimed to determine the prevalence of VVC among reproductive-age
women attending the Institute for Public Health of Canton Sarajevo. and to know the
fungal profile/biotypes of vulvovaginal candidiasis using Candida CHROMagar.
Methods: This is a 4-month cross-sectional study performed at the Department of Medical
Microbiology of the Institute for Public Health of Canton Sarajevo. A pair of high
vaginal swab and endocervical swab samples was collected from each of 213 individual
participating subjects. They were separately inoculated on Sabouraud’s dextrose agar
and incubated aerobically at 33°C for 48 hours and Candida CHROMagar. Infection with
Candida species was diagnosed by microscopy of a saline wet mount, Gram-stained smear
and colony growth on SDA and Candida CHROMagar.
Results: of the 213 sampled, 109 were positive for candidiasis giving a prevalence
rate of 51.1%. Candidiasis was observed mostly among the 20- to 30-year age-group.
The ages of the women ranged from 16 to 45 years with a mean of 26.8 (standard deviation
± 4.93).Women aged 26–30 years recorded the highest prevalence of 37.6% (41/109).
Candida albicans was the most prevalent species isolated in 85.3% of the women followed
by Candida crusei 12.8% and Candida parapsilosis 1.83%.
Conclusion: High prevalence rate of vulvovaginal candidiasis among females in Sarajevo
and the predominant agent is Candida albicans. In addition, correct identification
of Candida species could play an important role in management and treatment of VVC.
Fayemiwo
S.A.
1
Makanjuola
O.
1
Bongomin
F.
2
Nwaokenye
J.
3
Akinyemi
R.
4
Diala
S.
4
Faniyan
M.
5
Owolabi
M.
4
1Department of Medical Microbiology & Parasitology, College of Medicine, University
of Ibadan, Ibadan, Nigeria
2Department of Medical Microbiology and Immunology, Gulu University, Kampala, Uganda
3Department of Medical Microbiology & Parasitology, University College Hospital, Ibadan,
Nigeria
4Centre For Genomic and Precision Medicine, College of Medicine, University of Ibadan,
Ibadan, Nigeria
5Department of Medicine, University of Ibadan, Ibadan, Nigeria
P262. Cryptococcal antigenemia in HIV-uninfected patients with stroke in Nigeria
Objectives: Stroke continues to be a leading cause of morbidity and mortality globally.
Infections have been associated with stroke occasionally, but are not considered to
be a direct cause, and so they are not often included in the traditional stroke workup
and management. Sub-Saharan Africa also has the highest incidence, prevalence, and
fatality from stroke in the world. Cryptococcal infections occur globally and in a
wide variety of individuals, ranging from those who are severely immunocompromised
to those who are assumed to be immunocompetent. Recurrent strokes secondary to cerebral
infarction may occur and has been reported in immunocompetent persons following reactivation
of a latent infection. The study aimed to determine the prevalence of Cryptococcal
antigenaemia in stroke patients, to identify risk factors for Cryptococcal antigenaemia
in stroke patients and to compare Cryptococcal infection among stroke and non-stroke
patients.
Methods: The Stroke Investigative Research and Education Network (SIREN) project,
which this study was a part of, is a multicentre, multidisciplinary, case-control
study carried out in Ghana and Nigeria. One of the objectives of this extensive study
is to evaluate the risk factors of stroke in blacks in Sub-Saharan Africa. Cases were
adults (aged ≥18 years) with stroke confirmed by CT or MRI. Controls were age-matched
and gender-matched stroke-free adults (aged ≥18 years) recruited from the communities
in catchment areas of cases. A simple random sampling was used to select blood samples
of 50 stroke patients and 50 controls from the study population. Inclusion criteria
were stroke patients and controls who were HIV negative confirmed by HIV testing.
Serum from study participants was tested for the presence of Cryptococcal antigen
with the Cryptococcal Antigen Lateral Flow Assay (LFA) (Dynamiker Cryptococcal Antigen
Lateral Flow Assay (LFA)), a point-of-care test for the diagnosis of Cryptococcosis.
Results: Ninety-nine HIV-uninfected patients (50 cases and 49 controls) constituted
the study population. Majority of the participants were males (n = 69, 70%), with
a median age of 57 (range: 33-86) years. 36 (n = 50, 72%) for cases and 22 (n = 49,
44.9%) were hypertensive while six (n = 50, 12%) for cases and 14 (n = 49, 28.6%)
were diabetic. Overall, CrAg was positive in 16% (n = 16) of the participants. Similar
proportions of the cases and controls were CrAg positive (18% vs. 14%, p = 0.786).
8(n = 9, 89%) for cases and 3 (n = 7, 43%) hypertensive particiapnts were CrAg postive.
Death rates was the same between CrAg positive and CrAg negative patients (13% Vs
13%, p = 1.000 by Fisher’s exact test). Multinomial Logistic Regression DM (odds ratios,
0.77 (95%CI: 0.55-1.08); p = 0.130).
Conclusion: Cryptococcal Infectious causes should be considered in the differential
and work up of stroke in certain patient populations, especially in Sub-Saharan Africa,
and appropriate treatments should be initiated to minimize adverse stroke-related
outcomes.
Sow
D.
1
Ndiaye
M.
2
Sarr
L.
3
Diousse
P.
2
Ly
F.
2
Faye
B.T.
4
Sylla
K.
4
Ranque
S.
5
Faye
B.
4
1Service De Parasitologie-mycologie/section De Biologie, UFR Sciences de la Santé,
Université Gaston Berger, Saint-Louis, Senegal
2Service De Dermatologie, Faculté de médecine, UCAD, Dakar, Senegal
3Service D’orthopédie, Faculté de médecine, UCAD, Dakar, Senegal
4Service De Parasitologie, Faculté de médecine, UCAD, Dakar, Senegal
5IHU-Méditerranée Infection, Marseille, France
P263. Epidemiological, diagnosis and management of mycetoma cases in three hospital
centers from 2008 to 2018 in Senegal
Objectives: Mycetoma is a neglected tropical disease, characterized by the formation
of tumor like-swellings and grains. This disease transmitted through subcutaneous
tissue is caused by a range of actinomycetes (actinomycetoma) and fungi (eumycetoma).
Treatments for eumycetoma are unsatisfactory leading to high amputation rates. Senegal
represents one of the country where the disease is highly endemic. However, many mycetoma
cases are underdiagnosed/misdiagnosed due to the lack of informations in health workers
and at the community level. The purpose of this study is to generate accurate data
of the geographical distribution of Mycetoma spp. cases and their management in hospital
settings in Senegal.
Methods: In this retrospective study we report epidemiologic, clinical, diagnostic
and therapeutic data of mycetoma observed in three hospital centers in Senegal in
a 10 year-period (2008 to 2018).
Results: A total of 193 cases were included in the study. One hundred and sixty two
patients (83.5%) were recruited in two hospitals of the capital Dakar and the other
patients (16.5%) were diagnosed in Thies region located at 70km from Dakar. Most of
the cases (47.2%) were eumycetoma followed by 36.8% for actinomycetoma cases and 16.1%
undetermined form. The mean age was 38.3 years and the patients were predominately
aged between 15 and 45 years (68.4%). There was a male predominance with a sex ratio
of 2.94 and most patients were agricultural workers. One hundred fifty six patients
(80.8%) have used local and oral phytotherapy before attending hospital with sometimes
an evolution beyond 10 years (19.1%). The main affected localization was lower limbs
(90.6%) and most of the patients had lesions over 10cm. Grains were observed in 85%
cases including white (25.6%) and yellow (4.3%) grains. The diagnosis was difficult
in many cases without positive results in direct microscopy, culture and histopathology
explaining that 16.1% remained undetermined. In most of cases, actinomycetoma form
were treated with cotrimoxazole plus amoxicillin/clavulanic acid plus streptomycin
while eumycetoma cases were treated with terbinafin. The surgery was done in 100 cases
(51.8%) including amputation performed in 45 patients.
Conclusion: This study has shown the difficulty of the diagnostic of mycetoma in Senegal,
the delay in management cases and the lack of adequate therapeutic tools. The baseline
data provided will contribute to the establishment of a surveillance system, in the
context of advocating for much more attention.
Ceballos-Garzon
A.
1
Martinez
L.
3
Parra-Giraldo
C.M.
2
Montealegre
A.M. Sanz
3
Martinez
D.M.
3
Suarez
F. Rosso
3
1Department of Parasitology and Medical Mycology of The University of Nantes., Universite
de Nantes, Nantes, France
2Unidad de Proteómica y Micosis Humanas, Grupo de Enfermedades Infecciosas Departamento
de Microbiología, Facultad de Ciencias, Pontificia Universidad Javeriana, Bogotá D.C,
Colombia, Bogota, Colombia
3Centro de investigaciones clínicas Fundación Valle del Lili, Cali, Colombia
P264. Clinical and Microbiological Characterization of Candida parapsilosis complex
Infection in a Tertiare Care Hospital from Cali, Colombia
Objectives: To determine the association between the resistance of C. parapsilosis
complex to Fluconazole and adverse clinical outcomes in patients hospitalized in the
Valle del Lili Foundation. Methods: An observational prospective study was conducted.
The study included patients with C. parapsilosis complex (CPC) isolations attended
at Valle del Lili Foundation (FVL) from 2016 to 2017. The 55 strains were identified
by MALDI-TOF MS. Minimal inhibitory concentrations (MIC) were determined by broth
microdilution (M27 A3 CLSI). Statistical univariate analysis was performed; differences
between resistant cases and non-resistant cases were assessed through U Mann-Whitney
test, Pearson chi-2 test or Fisher exact test. Results: 55 patients had CPC isolations
during the study period: 18 newborns, 13 children, and 24 adults. Most isolations
were from blood cultures (n = 31) (14 of them newborns), bronchoalveolar lavage (n
= 9), peritoneal fluid (n = 8), and catheter tips (n = 3). The resistance was 36%.
52 strains were C. parapsilosis s.s., of them, 20 were FCZ resistant; 3 strains were
C. orthopsilosis, all of them FCZ sensitive. The MIC50 = 1 µg/ml and MIC90 = 16 µg/ml.
Patients with previous antifungal treatment had a higher risk of FCZ resistance (RR:
2. 14, 95% IC 1. 07-4. 26). The mortality brute rate was 30%, patients with renal
failure death risk (RR: 2.8, 95% CI: 1. 4-6. 9), heart disease (RR: 3.14, 95% CI:
1. 4–6. 9), and hemodialysis (RR: 2.97, 95% CI: 1.38-6.4). Candidemia was present
in 50% of deaths among children with parenteral nutrition.
Figure 1. Minimal inhibitory concentrations (MIC) to C. parapsilosis complex (n =
55). Strains ATCC 6258 and ATCC 22019 was used as a control.
Figure 2. Graphical analysis of mass spectra obteined by MALDI-TOF MS of cytoplasmic
protein extracs from clinical isolates of C. parapsilosis complex.
Conclusion: Fluconazole resistance in CPC has increased in the last decade. Newborns
receiving parenteral nutrition had a higher proportion of CPC fungemia, we also found
higher mortality rates among this population.
Tirkey
N.N.
Singh
K.
Animal Mycology Laboratory, Zoology, Mmv, Banaras Hindu University, VARANASI, India
P265. Characterisation and Antifungal Susceptibility of Cryptococcus species Isolated
from Rodents
Objectives: Rodents are recognized as intermediate hosts of zoonotic diseases that
represent a serious threat to human health. Cryptococcus is an opportunistic fungal
pathogen found worldwide and causes life-threatening meningoencephalitis especially
in immunocompromised host. In this view, the present study was conducted to investigate
the role of rodents in the epidemiology of cryptococcosis caused by Cryptococcus neoformans.
Methods: Rodents (Rattus rattus, Mus musculus castaneus, Mus musculus domesticus)
were trapped from the city of Varanasi, India (eastern Uttar Pradesh). After behavioural
and clinical examinations, they were euthanized. The organs were removed aseptically
for histopathology and isolation of fungi. For isolation, homogenates were cultured
on Sabouraud’s dextrose agar (SDA). The isolates were then grown in Staib’s and tobacco
agar medium. In addition, all isolates were identified on the basis of different biochemical
tests such as germ tube test, urease test and canavanine-glycine-bromothymol blue
(CGB) test. Further, molecular characterization was carried out by using PCR and DNA
sequence analysis. In vitro antifungal susceptibility profiles of Cryptococcus strains
were performed by using broth microdilution method recommended by CLSI (2009) M27-A3.
Results: Out of 20 screened, 2 rodents were positive for Cryptococcus spp. At the
necroscopy, there were no pathological lesions. Cryptococcus species were isolated
from the lungs, kidney, brain and blood of the animals. The isolates were subjected
to germ tube test and pseudohyphae were not observed after 24 hrs of incubation at
37°C in mammalian serum, therefore the test was considered as negative. The presence
of brown colonies on Staib’s and tobacco agar media confirmed that the isolated pathogen
is Cryptococcus. Further the isolates of Cryptococcus were tested for urease production
using Christensen urea citrate agar medium in which all isolates were found to hydrolyse
urea rapidly and reddish pink colour was obtained confirming it to be Cryptococcus
neoformans. All isolates were negative for Canavanine glycine bromothymol blue (CGB)
as they failed to produce a colour change in the medium. Molecular analyses showed
that these isolates were C. neoformans. Histological sections of lungs stained with
haematoxylin and eosin stain revealed the presence of C. neoformans like encapsulated
cells. In in vitro antifungal testing, isolates were found susceptible to amphotericin
B.
Conclusion: All the rodents screened are synanthropic. As they were found positive
for C. neoformans, they could be a potential threat for human health. Therefore wide
epidemiological survey should be conducted.
Ngwogu
A.
1
Ngwogu
K.
2
1Medical Microbiology, Abia State University Uturu, Aba, Nigeria
2Histopathology, Abia State University Uturu, Aba, Nigeria
P266. Prevalence and characterizaton of otomycosis among patients attending Ear, Nose
and Throat clinics at Abia State University Teaching Hospital, Aba, South Eastern
Nigeria
Objectives: Otomycosis or Fungal Otitis External is a superficial infection of the
outer ear canal. This could lead to complications involving the middle ear. The prevalence
of otomycosis has been studied in several parts of the world. It is seen more frequently
as an opportunistic infection in Immunocompromised people and those with ear injury.
This study was carried out to determine for the first time, the prevalence of otomycosis
in patients attending the Ear, Nose and Throat (ENT) Clinics at Abia State University
Teaching Hospital (ABSUTH), Aba, South Eastern Nigeria and to isolate and characterize
fungal agents responsible for otomycosis.
Methods: A total of 380 patients presented at the ENT Clinics during the period of
study. Out of this number, 100 samples were collected from those patients who presented
with symptoms of ear infection (pruritis, otalgia, otorrhea and hypacusis) by the
ENT Consultant Physician. Patients with other types of ear disorders other than otitis
media and otitis externa and patients under antibiotic therapy within the last 2 weeks
were excluded from the study. Demographic characteristics of the patients were noted.
The samples which consisted of swabs and scrapings were examined in 10% potassium
hydroxide solution and cultured on Sabouraud Dextrose Agar containing Chloramphenicol.
Fungal growth were identified using slide culture techniques and physiological tests
which include growth on some cspecialized media, germ tube test, urease test, growth
in Corn Meal Agar Containing Tween 80, nitrate assimilation, sugar assimilation and
sugar fermentation tests.
Results: Out of the 100 samples examined, (68% males and 32% females) were positive
for fungal structures both in microscopy and culture. Males were significantly more
affected than females in the ratio of 19: 4 (P<001). Although ear infection was highest
within the age group 1-10 years, fungal infection was highest within the age group
31- 40 years. Otomycosis was highest amongst outdoor, unskilled labourers (8%), followed
closely by students (6%) and lowest among civil servants (2%). The aetiologic agents
isolated were Candida albicans (13%), Candida tropicalis (5%), Aspergillus fumigatus
(3%) and Aspergillus niger (2%).
Conclusion: This study has shown for the first time that fungi are implicated in ear
infections in ABSUTH. The prevalence of otomycosis in the present study is high. Clinical
follow up with mycological diagnosis are important since symptoms (pruritis, otalgia,
otorrhea and hypacusis) are not specific. In fact many cases of otomycosis are clinically
misdiagnosed as otitis media leading to chronicity. Laboratory diagnosis and management
of otomycosis though challenging is very important. This is to detect the causative
organism and avoid wrong prescription of drugs. Recurrences are common in immunocompromised
patients than in immunocompetent patients. General practitioners, otologists and laboratory
personnel should remain alert for otomycosis. Diagnosis of ear infections should not
be made on clinical diagnosis only, it should be confirmed by laboratory diagnosis.
Specimens must be collected by an experienced Medical Personnel by aid of otoscope.
The frequent use of earphones, pricking the ear with hard objects, and traumatic attacks
on the ear are factors that could predispose to otomycosis.
Bauman
S.
Gibas
C.
Sanders
C.
Mccarthy
D.
Patterson
H.
Wiederhold
N.
Fungus Testing Laboratory, The University of Texas Health Science Center at San Antonio,
San Antonio, United States of America
P267. Six Year Surveillance Study of Scedosporium and Lomentospora Isolates Collected
from Humans in the United States (2012 - 2018)
Objectives:
Scedosporium species and Lomentospora prolificans are increasing causes of invasive
infections in immunocompromised hosts, and many isolates have reduced susceptibility
or are resistant to available antifungals. This is especially true for isolates of
L. prolificans, which are often resistant to all clinically available antifungal agents.
Although these fungi can be common colonizers in patients with cystic fibrosis, invasive
infections can occur, as can breakthrough infections in persistently neutropenic and
or lymphopenic patients, as well as lung transplant recipients. Our objective was
to evaluate the species distribution and antifungal susceptibility patterns of Scedosporium
and Lomentospora clinical isolates in the United States over a 6-year period.
Methods: The electronic database of our reference mycology laboratory (Fungus Testing
Laboratory, UT Health San Antonio) was queried for Scedosporium/Pseudallescheria/
Lomentospora isolates received between September 2012 and October 2018. Fungal species
identification was performed when requested by observation of phenotypic characteristics
combined with DNA sequence analysis of ITS rDNA and the calmodulin gene. Antifungal
susceptibility testing was performed by broth microdilution according to the CLSI
M38 standard.
Results: 1376 isolates were received over this 6-year period. The overall species
distribution as well as the distribution of species identified by our laboratory are
shown in Table 1. Overall, S. apiospermum was the most prevalent species, followed
by S. boydii and L. prolificans, and these 3 species accounted for ~89% of the isolates
received. Voriconazole had the most potent in vitro activity of the azoles and amphotericin
B tested against Scedosporium species (Voriconazole geometric mean [GM] MIC range
0.79 – 0.83 mg/L Table 2). In contrast, the activity of isavuconazole was markedly
reduced (GM MIC range 6.25 – 6.68 mg/L). Caspofungin and micafungin also demonstrated
in vitro activity against Scedosporium species, although the clinical relevance of
the minimum effective concentration (MEC) is unknown. The in vitro potency of all
agents was reduced against L. prolificans. However, greater variability was observed
when all isolates were included in the analysis versus those that our laboratory identified,
especially for L. prolificans, which is considered to be pan-resistant to clinically
available agents. This suggests possible misidentification of species, which may be
due to reliance on phenotypic characteristics.
Conclusion:
Scedosporium speices and Lomentospora prolificans are not uncommon fungi cultured
from patients in the United States. The species distribution we observed is similar
to those reported in previous surveillance studies from other countries. Although
voriconazole had the most potent in vitro activity, variability was observed among
the different species, with reduced activity observed for all antifungals against
L. prolificans.
Neves
J.
1
Alvarez
J.
2
Molinar
A.R.
1
Pasquetti
M.
1
Coutinho
S.D.A.
2
Peano
A.
1
1Departament of Veterinary Sciences, Sector of Parasitology, University of Turin,
Grugliasco, Italy
2Post-graduation Program In Environmental and Experimental Pathology, Paulista University,
São Paulo, Brazil
P268. Molecular typing of isolates of Microsporum canis collected in Brasil
Objectives:
Microsporum canis is the most common dermatophyte in cats and dogs. M. canis is diffused
worldwide and plays an important zoonotic role. Methods able to characterize a pathogen
at a strain level are expected to provide useful information to gain insight into
the dynamics of disease transmission. Among methods proposed for strain typing of
dermatophytes, the microsatellite (MS) multilocus typing method is considered one
of the most powerful tool. MS are tandem-repeating DNA sequences comprised of 1-6
bp per repeating unit, that are polymorphic in populations due to their propensity
for insertion/deletion mutation of multiples of the repeating unit during replication.
Multiple loci are generally used, so that a multilocus genotype is obtained (multi-locus
microsatellite typing – MLMT). This study was aimed to investigate the intra-specific
variability of strains of M. canis isolated from different animal hosts and environment
sites in Brasil, using a panel of 8 MS.
Methods: Fungal strains were collected in different locations in Brasil (mainly in
the city of Sao Paulo), from cats, dogs and environment sites. Overall, 73 strains
of Microsporum canis from 12 outbreak episodes were included in the genetic analyses.
DNA was extracted using a commercially available kit (NucleoSpin® Tissue, Macherey-Nagel,
Düren, Germany). PCR primers were designed against sequences flanking the MS detected
by a BLAST (Basic Local Alignment Search Tool) search using the nucleotide sequence
information assembled by the Microsporum canis CBS 113480 genome project (http://www.broadinstitute.org/annotation/genome/dermatophyte_comparative/MultiHome.html).
Primer sequences for the 8 MS markers recognized as the most polymorphic were custom
synthesized (Applied Biosystems UK) with a fluorescent label attached to the 5’ end
of each forward primer. Microsatellite fragment analysis was performed using an ABI
Prism 310 Genetic Analyzer (Applied Biosystems, FosterCity, CA) for capillary electrophoresis.
The ML-MS genotypes for the Brasilian isolates were compared with the genotypes available
in the database of the Departament of Veterinary Medicine of Turin, which includes
more than 300 strains mainly sampled in Europe.
Results: Out of the analyzed strains, 5 different multilocus genotypes were detected.
One genotype was more prevalent as it was shared by 11 strains coming from 9 episodes.
Thus the genetic diversity was quite low, perhaps due to the fact that a restricted
geographic area was sampled. A higher degree of diversity is instead present in the
existing database.
Conclusion: These data allow enriching the database of MS profiles already available,
which regards mainly strains isolated in Europe. Such an enrichment represents a key
step to make the database a more and more powerful reference in the study of the dynamics
of transmission of M. canis. Indeed, it must be pointed out that studies that report
the same strain among all isolates from a suspected outbreak, occurring in a geographic
region for which no baseline data on the degree of variation in the population exists,
remain uninterpretable.
Bonet
L. Goterris
1
Murillo
M. Guerrero
1
Pasic
L.
2
Pagarolas
A. Anton
1
Company
J. Aguilar
3
Ruiz-Camps
I.
3
Martin-Gomez
M.T.
1
1Microbiology, Vall d’Hebron Universitary Hospital, Barcelona, Spain
2Molecular Mycology Research Laboratory, Centre for Infectious Diseases and Microbiology,
The University of Sydney, Sydney, Australia
3Infectious Diseases, Vall d’Hebron Universitary Hospital, Barcelona, Spain
P269. Genetic diversity of Pneumocystis jirovecii in a tertiary hospital in Spain
Objectives:
Pneumocystis jirovecii (PJ) is an opportunistic fungus that can be found in the respiratory
tract of humans as an asymptomatic colonizer or as a cause of severe pneumonia (PJP)
in immunocompromised patients. Establishing associations between genetic types and
clusters of cases, clinical manifestation or underlying pathology is not easy as there
is no consensus on the better typing scheme. The aim of this study is to apply two
multi-locus sequence typing (MLST) protocols to ascertain their ability to discriminate
the genetic diversity of colonized and infected immunocompromised patients visited
at a tertiary referral hospital in Spain.
Methods:
Patients and samples: From April 2014 to April 2018, consecutive respiratory tract
samples with a positive result for P. jirovecii by PCR were included. Only 1 sample
per patient and respiratory episode was studied. Laboratory procedures: Two different
MLST protocols were carried out. Protocol A (http://mlst.mycologylab.org/pjirovecii)
targeted the B-tubulin, dihydropteroate synthase (DHPS) and mitochondrial 26S rRNA
(mt26S) loci. Protocol B is a newly developed scheme targeting the cytochrome B (cyt),
mt26s and superoxide dismutase (SOD) loci (Pasic et al. Establishment and optimization
of a combined MLST scheme for Pneumocystis jirovecii. 21th ISHAM Congress 2018, P-223).
Phylogenetic analyses: The obtained sequences were depurated and collapsed before
achieving phylogenetic analyses. The resulting trees were constructed computing genetic
distances among patients for each protocol. Patients were categorized into PJP (green)
or PJ colonized (red) groups.
Results: Samples from 35 pneumonia cases and 51 colonizations were included. Successful
amplification rate was non-significantly different for protocol A (total 58.1%; 26/35[LG1]
PJP and 24/51 colonizations) and B (total 72.1%; 26/35 PJP and 34/51 colonizations).
As shown in Figure 1, Protocol A allowed the identification of 11sequence-types (ST)
whereas protocol B was able to find 21 different ST. Both protocols agreed in finding
no apparent clustering of cases in time or space. However, whereas Protocol A grouped
73.1 %[LG2] of pneumonia cases into a group of 7 closely related ST and 61% of colonization
cases into the other 4 ST, no apparent correlation between illness and ST could be
seen with protocol B.
Conclusion: In the present study, two MLST protocols were carried out to describe
PJ genetic diversity in our cohort. The two protocols provided complementary information:
greater genetic diversity was documented with protocol B, whereas protocol A helped
us to find sequence-types mostly associated with PJP or colonization. This study allowed
us to demonstrate the absence of PJ outbreaks in our hospital during the study period.
Grant
I.H.
1
Geller
J.
2
Sabath
H.
3
1Community Medicine, New York Medical College, Tarrytown, United States of America
2Information Revelations, Montclair NJ, United States of America
3International Environmental Diagnostics Inc, Irvington NYw, United States of America
P270. rDisability and disease after hazardous indoor exposure to airborne Stachybotrys
spores: exposure variable analysis shows significant correlation with injury to mucosa,
phagocytes and neurons.
Objectives: While Stachybotrys is a mold that flourishes indoors with chronic water
intrusion, its spores usually require physical disturbance of its colony growth or
prolonged wetness to become airborne. The macrocyclic Trichothecenes mycotoxins in
its spores are extremely toxic nanoparticulates dangerous for all mammals, damaging
intracellular protein/DNA/RNA production and mitochondrial function causing premature
cell death on contact: apoptosis. As exotoxins, they remain toxic despite disinfection,
bind to dust causing damage to mammalian skin, mucosa, phagocytes, neurons, and organs
via systemic circulation, especially with inhalation. Objectives: To determine: -
The frequency of severe illness due to indoor airborne Stachybotrys spore exposure.
- Specific exposure risks (fungi, hazardous exposure activities) - The impact of genetically-impaired
intracellular MTHFR detoxification capacity. - Conditions that predispose to opportunistic
fungal infection with such exposures (e.g. mucosal injury, immunosuppression, malnutrition)
Methods: Prospectively monitored 14 (7M, 7F) adults and children exposed to 5 documented
Macrocyclic Trichothecenes-contaminated indoor-environments with airborne Stachybotrys
spores with comprehensive environmental-mycological and medical investigations, fungal
IGGs, urine mycotoxins (MCTs: Aflatoxin, Gliotoxin, Ochratoxin A, MT), immune parameters
(neutrophils, lymphocyte subsets, monocytes, IGG, IGA, IGM, IGE, subclasses), immunosuppressants,
nutritional deficiencies (protein, Vitamin D, zinc), genetic Methylenetetrahydrofolate
reductase (MTHFR) single-nucleotide-polymorphisms C677T&A1298C. Disease Severity Outcomes
(DS), Environmental Mold-Contamination Severity (EMCS), Hazardous Exposure Activities
(HEA), Exposure Duration (ED), MCT/Trichothecenes exposure (MTC/TE), and clinical
findings were stratified and analyzed. Biostatistical analysis (Pearson Correlation
Coefficients) correlated DS with EMCS, HEA, ED, MCT/TE, mucosal Injury (MI), Neurological
disease, Cell-mediated Immunodeficiencies (CMIs), combined cellular-immunoglobulin
immunodeficiencies (CCIs), mucosal injury (MI), mold-specific IGG response(M-IGG)
and genetic MTHFR deficiencies.
Results:
CORRELATIONS:
Disease Severity based on formal disability classification or physical injury strongly
correlated with Hazardous Activities (p = 0.01819), Immune deficiencies (p = 0.046229).
Environmental Exposure Exposure Hazards (especially unprotected mold disturbance,
uncontained construction/repairs, basement living, HVAC contamination and sewage overflow)
correlated with immunodeficiencies (p = 0.046229). Environmental contamination with
multiple mycotoxins correlated with multiple mycotoxin urinary excretion (p = 0.026354).
Duration of exposure correlated with urinary excretion of multiple mycotoxins (p =
0.006314) as well as Trichothecenes p = 0.00875). Urine Trichothecenes correlated
with immunoglobulin deficiencies (p = 0.037285).
Conclusion: The high frequency of immune deficiencies (93%), mucosal injury and pain
(79%), bleeding, and neurological damage (71%), burning and chronic fatigue (64%)
with expected respiratory/Gi /Dermatologic symptomatology in 50% is consistent with
known Trichothecenes effects in animal models and military data. The high frequency
of cell-mediated immune deficiency is consistent with invitro and in vivo research
on Stachybotrys spores injuring phagocytes, supporting injury from inhalation of Stachybotrys
spores. The high 94% MTHFR methylation-defect incidence is peculiarly striking compared
to estimated USA 50-60% prevalence. MTHFR is a key intracellular detoxifying enzyme
protecting ribosomes/mitochondria RNA/DNA damage from Trichothecene injury via glutathione
metabolism. Further investigation is needed to determine if Trichothecenes trigger
genetic damage. Urine macrocyclic Trichothecenes detection appears a useful marker
for environmental macrocyclic Trichothecenes exposure. Public health education and
epidemiological research are urgently needed to investigate correlation between environmental
mold/mycotoxin exposure and immunodeficiencies.
Dogan
G.
1
Tunger
O.
1
Kocoglu
F.
1
Temiz
C.
2
Temiz
P.
3
Senol
S.
1
Cetin
C.B.
1
1Infectious Diseases and Clinical Microbiology, Manisa Celal Bayar University Faculty
of Medicine, Manisa, Turkey
2Neurosurgery, Manisa Celal Bayar University Faculty of Medicine, Manisa, Turkey
3Pathology, Manisa Celal Bayar University Faculty of Medicine, Manisa, Turkey
P271. Two different involvement of Aspergillus fumigatus in the same case: endophthalmitis
and lumbar spondylodiscitis
Case Report:
Aspergillus species are mold type fungi that commonly found in nature and especially
for immunocompromised people can cause opportunistic infections. Aspergillus-induced
endogenous endophthalmitis is a very rare ophthalmic emergency that can result in
visual loss. Bone infections caused by Aspergillus species are also rare clinical
manifestation especially occurs in immunocompromised patients. In this case report,
two rare clinical conditions such as endogenous endophthalmitis and lumbar spondylodiscitis
due to Aspergillus are reviewed.
A 39-year-old woman with no known underlying disease was admitted to the Ophthalmology
Departmant with loss of vision in her left eye. Approximately two months ago, there
was a history of antibiotic use (moxifloxacin) for pneumonia. No additional physical
examination findings were detected in the patient. Laboratory tests revealed erythrocyte
sedimentation rate 59 mm / h, leukocyte count 9.81 103 / /L, hematocrit 34.2%, platelet
count 552 103 / /L, neutrophil count 7.53 103 / /L, C-reactive protein 1 mg / dl.
Vasculitis was detected in ocular angiography and corticosteroid therapy (prednisolone
32 mg) was started by consultation of Rheumatology Department. Lumbar puncture was
performed after normal result of cranial MRI that performed for differential diagnosis
of vasculitis, and CSF examination was normal. The patient had undergone vitrectomy
because the complaint did not regress despite steroid treatment. Direct examination
of the vitreous specimen revealed gram positive bacteria and fungi, and intravitreal
Liposomal AMB treatment with intravenous liposomal amphotericin-B (LAMB) and moxifloxacin
was initiated. Aspergillus fumigatus was grown in vitreous samples. Bronchoscopy was
performed and there was no cultural growth in BAL samples. Parenteral antimicrobial
treatment was completed and was discharged after 14 days. The patient who had low
back pain and morning stiffness was hospitalized in Rheumatology with the preliminary
diagnosis of ankylosing spondylitis and received 250 mg intravenous methylprednisolone
treatment for 3 consecutive days. Due to the absence of sacroileitis in MR imaging
and monitoring of L4-L5 spondylodiscitis, piperacillin tazobactam and daptomycin were
started empirically. The patient was than hospitalized in Infectious Diseases. In
the pathological examination of the lumbar spine, fungal hyphae were seen and LAMB
(3 mg / kg) was added to the treatment because of the positive galactomannan antigen
(GMA). The patient whose complaints had regressed and whose GMA was negative was discharged
with oral voriconazole after 45 days use of LAMB and 7 days use of parenteral voriconazole.
The patient underwent oral voriconazole therapy for 160 days during outpatient follow-up,
and regression was detected in control magnetic resonance imaging. CONCLUSION: In
immunosuppressive and immunocompetent patients, fungal agents should be considered
in case of treatment failure, and hidden foci such as endocardium, eye and vertebrae
must be investigated because of hematogenous transmission.
Capoor
M.
1
Kocchar
S.
2
Gupta
V.
2
1Microbiology, Vardhman Mahavir Medical College and Safdarjung Hospital, Delhi, India
2Opthalmology, Vardhman Mahavir Medical College and Safdarjung Hospital, Delhi, India
P272. Epidemiological trend, spectrum, clinical profile and outcome of fungal keratitis
from Delhi, India
Objectives: Fungal aetiology of keratitis ulcer is considered to be one of the leading
causes of ocular morbidity, particularly in developing countries including India.
More importantly, Fusarium and Aspergillus spp. are reported commonly implicating
corneal ulcer and against this background the present work was undertaken so as to
understand the current epidemiological trend, clinical profile and outcome of fungal
keratitis.
Methods: During the study period (2013-2016), the clinical and mycological characteristics
of fungal keratitis was investigated. Our aim was to discuss the predisposing factors,
clinical manifestations, and epidemiological characteristics of the causative agents
and describe the management strategies that have a high probability of successfully
treating this disease. The corneal scrapings were processed by direct microscopic
methods and standard culture techniques. All the samples were inoculated onto Sabouraud
dextrose agar (SDA), potato dextrose agar (PDA), brain- heart infusion based blood
agar in the form of a “C” streak. The SDA and PDA plates were incubated at 30°C for
7 days, and the other plates were incubated at 37°C for 7 days.
Results: A Total of 143 corneal scrapings from keratitis were processed, 72 (50.3%)
revealed growth, of which 60 were culture positive for fungi and 12 were culture positive
for bacteria. Direct microscopy revealed hyphae or budding yeasts or fungal elements
in 60 cases. Among fungal aetiologies, Aspergillus spp. (51.7%) and Fusarium spp.
(16.7%) were predominantly determined. The spectrum of mycotic keratitis cases was:
Aspergillus flavus (15), A.fumigatus (12), Fusarium solani (5), Fusarium oxysporum
(3), Curvularia lunata (3), C. tropicalis (3), C. parapsilosis (2), Fusarium dimerum
(2), Paecilomyces lilanicus (2), Alternaria alternata (2), A.terreus (2), A. tetrazonus
(1), A.niger (1), Bipolaris (1), Candida krusei (1), Schizophilum commune (1), Scedosporium
apiospermum (1), Chryseosporium keratinophillum (1), C. tropicalis (1) and Scytalidium
dimidiatum (1). of the 60 mycotic keratitis male preponderance was seen in 66.66%.
Predisposing factors included trauma with vegetative material (98.66%), contact lens
use (1.66%), and bird feather hit (1.66%). Pain, redness of eye and dimunition of
vision was most common clinical complaints. Steroids and inflammatory drugs were most
common pretreatment medication taken. The corneal examination revealed central corneal
ulcer with feathery margins, a paracentral superficial ulcer; a central ulcer with
an irregular edge, satellite infiltrations, and hypopyon etc. Majority of the patients
were given natamycin eye drop alone. Concomitant systemic antifungals and supportive
surgical intervention was done in 4 and three cases respectively. Complete loss of
vision was seen in one eye was seen in single case.
Conclusion: Our study suggests that mycotic keratitis caused by Aspergillus spp. especially
A. flavus may occur more often in a tropical climate, such as in Delhi, India. Aetiological
agents other than Aspergillus species and Fusarium species have shown an upward trend.
A combination of antifungal therapy and supportive surgical intervention may successfully
resolve the mycotic infection Proper understanding of microbiological and clinical
characteristics of keratomycosis will enable ophthalmologist to avoid unnecessary
and indiscriminate use of steroids or antimicrobials.Early stage of diagnosis and
formulation of an uncompromising management protocol can prevent profound visual morbidity.
Carvalho
J.
Terra
P.
Pinheiro
B.
Camargo
Z.P.D.
Rodrigues
A.M.
Department of Microbiology, Immunology and Parasitology, Federal University of São
Paulo, São Paulo, Brazil
P273. Molecular epidemiological investigation of zoonotic outbreaks caused by Sporothrix
species
Objectives: Sporotrichosis is a mycosis mostly caused by a group of thermodimorphic
fungi embedded in a clinical clade (i.e., Sporothrix brasiliensis, S. schenckii, S.
globosa, and S. luriei). The infection is caused after a traumatic inoculation of
Sporothrix propagules into the (sub)cutaneous tissue of mammals. Sporothrix species
have been reported around the world, mostly in regions with humid (sub)tropical and
temperate climates. During the last decades, vast zoonoses have been ongoing in Brazil
with Rio de Janeiro as the epicenter of this cat-transmitted epidemic. In this scenario,
the development of new molecular markers is mandatory to explore the genetic diversity
and track Sporothrix expansion during outbreaks. The purpose of this study was to
analyze the genetic diversity of Sporothrix originating from different geographic
regions of Brazil, using AFLP (Amplified fragment length polymorphism) markers.
Methods:
Sporothrix spp. genome sequences were retrieved from Genbank (https://www.ncbi.nlm.nih.gov/genbank/)
and were in silico-digested with EcoRI and MseI using the software AFLPinSilico. Only
fragments of 50-500 bp were considered. A total of 256 combinations of selective pairs
of primers (based on 2 selective nucleotides) were employed to generate 2.304 fingerprints
profiles with different fragment numbers and sizes (0 to 56 fragments/per combination).
Results:
In silico analysis highlighted 6 combinations to be tested in a subset of 15 medically
relevant Sporothrix isolates. In vitro, AFLP presented strict concordance with in
silico results considering the number and size of the amplicons resolved during capillary
electrophoresis. A total of 58 AFLP markers were unambiguously scored in the electropherograms
using the software BioNumerics v.7.6. Dendrograms were constructed (UPGMA, Jaccard
Distance) based on a banding match table and isolates were correctly clustered according
to the taxon name. Intraspecific variation was observed for all agents, including
S. brasiliensis, a species previously known to be clonal. Based on the data above
we recommend the use of 3 different AFLP combinations to explore genetic diversity
and taxonomy in Sporothrix.
Conclusion: Independent experiments with similar results showed that the AFLP is highly
reproducible, making this technique interesting to evaluate the genetic diversity
and the spreading of Sporothrix. The present content in this study will enable the
development of strategies to control the disease and will contribute to further studies
about diversity in Sporothrix.
Rodrigues
A.M.
1
Beale
M.
2
Hagen
F.
3
Fisher
M.
4
Terra
P.
1
Hoog
G.S. De
5
Brilhante
S.
6
Cordeiro
R.
6
Castelo-Branco
D.
6
Rocha
M.
7
Sidrim
J.
7
Camargo
Z.P.D.
1
1Department of Microbiology, Immunology and Parasitology, Federal University of São
Paulo, São Paulo, Brazil
2Parasites and Microbes Programme, Wellcome Sanger Institute, Cambridge, United Kingdom
3Westerdijk Fungal Biodiversity Institute, Utrecht, Netherlands
4Department of Infectious Disease Epidemiology, Imperial College London, London, United
Kingdom
5Westerdijk Institute, Utrecht, Netherlands
6Postgraduate Program In Medical Microbiology, Federal University of Ceará, Fortaleza,
Brazil
7Postgraduate Program In Veterinary Science, Federal University of Ceará, Fortaleza,
Brazil
P274. Epidemiological and molecular investigation of emerging Histoplasma species
in Brazil
Objectives: Histoplasmosis is a serious infectious disease in humans caused by Histoplasma
spp. (Onygenales), whose natural reservoirs are thought to be soil enriched with bird
and bat guano. The true global burden of histoplasmosis is underestimated and frequently
the pulmonary manifestations are misdiagnosed as tuberculosis. Molecular data on the
epidemiology of Histoplasma are still scarce, even though there is increasing recognition
of histoplasmosis in recent years in areas distant from the traditional endemic regions
in the Americas.
Methods: We used multi-locus sequence data from protein-coding loci (ADP-ribosylation
factor, H antigen precursor, and delta-9 fatty acid desaturase), DNA barcoding (ITS1/2+5.8s),
AFLP markers and mating type analysis to determine the genetic diversity, population
structure and recognize the existence of different phylogenetic species among 436
isolates of Histoplasma obtained globally.
Results: Our study describes new phylogenetic species and the molecular characteristics
of Histoplasma lineages causing outbreaks with a high number of severe outcomes in
Northeast Brazil between 2011 and 2015. Genetic diversity levels provide evidence
for recombination, common ancestry and clustering of Brazilian isolates at different
geographic scales with the emergence of LAm C, a new genotype assigned to a separate
population cluster in Northeast Brazil that exhibited low diversity indicative of
isolation. The global survey revealed that the high genetic variability among Brazilian
isolates along with the presence of divergent cryptic species and/or genotypes may
support the hypothesis of Brazil being the center of dispersion of Histoplasma in
South America, possibly with the contribution of migratory hosts such as birds and
bats. Outside Brazil, the predominant species depends on the region. We confirm that
histoplasmosis has significantly broadened its area of occurrence, an important feature
of emerging pathogens.
Conclusion: From a practical point of view, our data point to the emergence of histoplasmosis
caused by a plethora of genotypes and will allow the proposal of public policies to
contain the spread of histoplasmosis. Furthermore, with the description of such diversity,
we open avenues for future comparative genomic studies, which will allow progress
toward a consensus taxonomy, improve understanding of the presence of hybrids in natural
populations of medically relevant fungi, test reproductive barriers and explore the
significance of these variations.
Jandrlić
M.
1
Samarzija
M.
2
Vranješ
V. Rezo
1
Pleško
S.
3
Mareković
I.
3
Jandrlic
A.
4
1Department of Clinical and Molecular Microbiology, University Hospital Centre Zagreb,
Zagreb, Croatia
2Clinic For Lung Diseases, University Hospital Centre Zagreb, Zagreb, Croatia
3Department of Clinical and Molecular Microbiology, University Hopital Centre Zagreb,
Zagreb, Croatia
4Laboratory Biomedicine, Faculty of Pharmacy, Ljubljana, Slovenia, Zagreb, Slovenia
P275. Tuberculosis and Fungal Co-infection of Fungal Infections, University Hospital
Center Zagreb, Croatia, During Past Two Years
Objectives: Pulmonary tuberculosis is one of the most important health concerns. Pulmonary
fungal infections have clinical and radiological characteristics similar to tuberculosis
which may be easily misdiagnosed as tuberculosis. This study aimed to evaluate Mycobacterium
tuberculosis (MTB) and other non-tuberculous mycobacteria (NTM) with coinfection of
fungal infections in patients, during last 2 years.
Methods: Microbiological and clinical reports of 149 patients with confirmed mycobacteria
have been analyzed. Detection and identification of mycobacteria were performed by
the standard culture method using the BACTEC MGIT 960 system (BD) and Lowenstein–Jensen
medium. Identification of positive Mycobacterium tuberculosis complex (MTBC) was based
on positive acid-fast bacilli microscopic smear (Ziehl–Neelsen and fluorochrome),
positive niacin accumulation, MALDI-TOF and Mycobacteria PCR. TB isolates were also
tested for their sensitivity toward first-line anti-MTB drugs.
Results: Between April 2017 and April 2019, at 149 patients were detected Mycobacterial
infections (MTB 64%, NMT 36%). Mycological tests are not required in 36 24% patients.
Mycological tests are required in 113 76% patients. The majority of patients had isolated
several types of fungi, 160 episodes. The patients 113 were isolated yeasts, opportunistic
and saprophytic molds (54%, 16%, 28%). Frequent Candida were C. albicans, C. dubliniensis,
C. parapsilosis and C. glabrata respectively. Frequent Aspergillus were A. niger,
A. fumigatus and A. flavus respectively. In addition to Aspergillus, small number
of patient had Fusarium, Rhizopus and Pseudallescheria boydii. Saprophytic molds were
confirmed in 28% of patients.
Conclusion: Our findings showed the co-infection of fungi agents in patients with
tuberculosis that should be considered. Clinical data must be analyzed to be able
to distinguish infections, colonization, or part of normal flora.
Huang
S.F.
Wang
F.-D.
Internal Medicine, Taipei Veterans General Hospital, Taipei, Taiwan
P276. Prevalence of Aspergillus infection in delay resolution of community-acquired
pneumonia in high-risk population in Taiwan
Objectives: Pulmonary Aspergillosis is a world-widely distributed disease. The prevalence
of Aspergillus infection in patients with delay resolution community acquired pneumonia
(DR-CAP) or lower respiratory tract infection (LRTI) had not been investigated in
Taiwan. The aims of the study was prospectively evaluate the prevalence of Aspergillus
infection by serological testing and microbiological evidence in high risk patients
presented with DR-CAP or LRTI.
Methods: Patients presented with LRTI or DR-CAP were prospectively enrolled. Patients
with underling chronic pulmonary disease (CLD), active tuberculosis (TB), collagen
fiber disorders were study groups, and others who were healthy without underling disorders
except DR-CAP or LRTI. Delay resolution of community acquired pneumonia (DR-CAP) was
defined as clinical diagnosis with community acquired pneumonia not significantly
getting resolution after 3-4 weeks of appropriate anti-bacterial antibiotics. Serum
gamma-immunoglobulin (IgG) concentration for Aspergillus niger (A. niger) and Aspergillus
fumigatus (A. fumigatus) were measured by ELISA method. Positive results were recorded
by the cut-off-value provided by the company (ImmunoCAP@, ThermoFisher Scientic Inc.).
Microbiological evidence for mold infection was record according to the results of
fungus culture from sputum, bronchioalveloar lavage fluid (BALF), or nasal/throat
swab. Patients were excluded for pulmonary malignancy by diagnostic approach such
as bronchoscopic and transthoracic lung biopsy.
Results: From 2018 April to 2019 Jan, a total of 99 patients were enrolled. Overall,
38.3%, 25.1%, and 11.1% of patients were with CLD, collagen fiber disorders, and active
TB or old TB with complete anti-TB therapy (ATT), respectively. There were 19% of
patients were healthy but presented with LRTI not response to anti-bacterial antibiotics.
Overall, seroprevalence for A. niger and A. fumigatus were 32.3% and 22.9%, and the
patients with CLD had a highest seroprevalence with 35.4% and 36.6%, respectively.
In combination of microbiological and serological results, the prevalence for mold
infection was 59%, and CLD remained a highest-risk disorder (48.7%). Overall, concordance
between microbiological results for fungus culture and positive serological results
for A. niger and A. fumigatus were 69.6% and 60%, respectively.
Conclusion: Both the serological and microbiological evidence for mold or Aspergillus
infection in high-risk patients with DR-CAP or LRTI were high in Taiwan. We also firstly
reporeted the seroprevalence for Aspergillus spp. around 10% in patients without underling
high-risk disorders, that indicate the populational seroprevalence in north Taiwan.
Aghili
S.R.
1
Abastabar
M.
2
Soleimani
A.
3
Vosta
A. Akbari
3
Azizi
S.
4
Rayatnia
M.
5
Salmanian
B.
6
1Invasive Fungi Research Center/department of Medical Mycology, Mazandaran University
of Medical Sciences, Sari, Iran
2Department of Medical Mycology, Invasive Fungi Research Center, School of Medicine,
Mazandaran University of Medical Sciences, Sari, Iran, Sari, Iran
3Student Research Committee, Mazandaran University of Medical Sciences, Sari, Iran
4Department of Laboratory Medicine, Faculty of Allied Medical Sciences, Mazandaran
University of medical science, Sari, Iran
5Kidney Center of Imam Reza Hospital, Mazandaran University of Medical Sciences, Sari,
Iran
6Department of Scienes, Farhangian University, Seddighe tahereh branch, Sari, Iran
P280. rCo-infection Candida glabrata with other Candida species in nosocomial candiduria
Objectives:
Candida glabrata was formerly classified in the Torulopsis genera, but it is currently
considered to belong to the Candida genus. Evidence showed that it is the second causative
agent of nosocomial candidiasis, alone or coexisting with other yeasts, particularly
other Candida species. The reason for this association is often unknown and some have
suggested a synergistic pathogenesis. Due to the increasing resistance of C. glabrata
to many common antifungal drugs, treatment in patients is also accompanied by some
problems. Therefore, identification of co-infection in patients and the modification
of therapeutic protocols is essential.
Methods: This descriptive laboratory-based surveillance study was investigated the
incidence of nosocomial candiduria and it determined their etiologic agents in 555
hospitalized patients in two hospitals (253 hemodialysis patients in Hospital hemodialysis
center of Amol city and 305 hospitalized patients with heart failure in Heart center
of Sari city), Mazandaran province, Iran. The patients’ urine samples were cultured
in mycological culture media and colony counts >104 CFU/ml were considered significant
for candiduria. Species identification was done based on color of colony on CHROMagar
Candida and PCR-RFLP method, by ITS region and the MspI restriction enzyme and PCR
amplification of HWP1 gene.
Results: We found 40(15.8%) positive candiduria in hemodialysis patients and 58(18.8%)
in heart failure patients. C. glabrata(47.1%), C. albicans(17.6%), C. tropicalis(15.7%),
C. parapsilosis(15.7%) and C. kefyr(0.4%) were identified as candiduria agents in
hemodialysis patients and C. glabrata(40.3%), C. albicans(40.3%), C. tropicalis(7.5%),
C. parapsilosis(7.5%) and C. krusei(4.4%) were candiduria agents in heart failure
patients. C. glabrata, alone as a single-species candiduria agent was detected in
many patients. However, in these two patient groups, it has associated with other
Candida species as mix-infection. Nine hemodialysis patients had mix-infection by
C. glabrata associated with other Candida species (7 cases C. glabrata + C. albicans,
2 cases C. glabrata + C. albicans + C. kefyr) and 9 heart failure patients had mix-infection
by C. glabrata associated with one other species too (4 cases C. glabrata + C. albicans,
2 cases C. glabrata + C. tropicalis, 2 cases C. glabrata + C. parapsilosis and 1 case
C. glabrata + C. krusei).
Conclusion: Studies indicate increasing azole antifungal prophylaxis treatment and
invasive application lead to an increase in low susceptibility to azoles non albicans
species, and C. glabrata became second causative nosocomial infection agent now. The
data suggest that the growth of other Candida species was stimulated by the presence
of C. glabrata and this species in the biofilms more active than other species that
produce biofilms with more biomass. Although, C. glabrata is generally considered
a species of low virulence, mortality rate of patients with candidiasis caused by
this species is higher than C. albicans because of complicates the treatment. Given
the prevalence of C. glabrata and other candida species co-infection, it is necessary
to understand the mechanisms used by C. glabrata in collaboration with them and the
use of rapid identification methods for candidiasis agents can be a great help to
treat these patients, more effectively.
Kozlova
O.
1
Rubinchik
V.
2
Sobol
M.
3
Larionova
V.
4
Tyrenko
V.
5
Zhuravel
S.
6
Petrova
E.
7
Truhina
T.
8
Klimko
N.
1
1Department of Clinical Mycology, Allergology and Immunology, North-Western State
Medical University n.a. I.I. Mechnikov, Saint-Petersburg, Russian Federation
2V.A. Almazova NMI, St. Petersburg, Saint-Petersburg, Russian Federation
3Irkutsk Regional Cancer Center, Irkutsk, Irkutsk, Russian Federation
4n «N.N. Blokhin National Medical Research Center of Oncology» of the Ministry of
Health of the Russian Federation, MoscowFederal State Budgetary Institutio, Moscow,
Russian Federation
5S.M. Kirov Military Medical Academy, St. Petersburg, Saint-Petersburg, Russian Federation
6N.V. Sklifosovsky Research Institute for Emergency Medicine, Moscow, Moscow, Russian
Federation
7State Budgetary Healthcare Institution Infectious Diseases Hospital № 2 Moscow Health
Department, Moscow, Moscow, Russian Federation
8Tula Regional Oncology Center, Tula, Tula, Russian Federation
P281. An Open-Label Multicenter Observational Study of Anidulafungin For The Treatment
of Invasive Candidiasis In Russia
Objectives: Publications about “real life” using of anidulafungin are limited. Study
of anidulafungin for the treatment of invasive candidiasis (IC) in actual clinical
practice in Russia.
Methods: The primary endpoints were global response (clinical + microbiological response)
at end of treatment (EOT), and the 30 days overall survival rate. For diagnosis of
invasive candidiasis (IC) we used criteria EORTS/MSG, 2008.
Results: In 2015-2017 yy. in a prospective multicenter (n = 23) study included 300
adult patients who were treated with anidulafungin. Men were 57%, median age – 49
y (from 18 to 98). All examined patients had risk factors for IC. In 85% patients,
a fever resistant to the use of broad-spectrum antibacterial drugs for ≥ 4 days was
noted. Median APACHE II at the start of anidulafungin therapy was 18.8. Anidulafungin
was used for the empirical therapy of IC in 82% patients. Global response at EOT was
79%, the 30 days overall survival rate was 78%. Anidulafungin was generally well tolerated
with few treatment-related adverse events (hypokalemia – 1%, angioedema – 0.5%), which
were not the cause of drug withdrawal. IC was laboratory confirmed in 25% patients.
Candida non-albicans (57%) prevailed among IC pathogens. In patients with proven IC
the 30 days overall survival rate was 66%, which is significantly higher than in the
earlier multicenter study in Russia (43%).
Conclusion: The overall efficacy of anidulafungin was high (79%), the 30 days overall
survival rate was 78%. Adverse events occurred in 1,5% cases and were not the cause
of drug withdrawal. In 82% patients anidulafungin was used for empiric therapy. In
patients with proven invasive candidiasis the 30 days overall survival rate was 66%.
Hare
D.
1
Griffin
A.
1
Akasheh
N.
2
Bacon
C.L.
3
Martin-Loeches
I.
4
Rogers
T. R
1
5
Connell
B. O
1
1Department of Clinical Microbiology, St James’s Hospital, Dublin, Ireland
2Department of Medicine, St. James’s Hospital, Dublin, Ireland
3Department of Haematology, St. James’s Hospital, Dublin, Ireland
4Department of Intensive Care Medicine, St. James’s Hospital, Dublin, Ireland
5Trinity College Dublin, Dublin, Ireland
P282. Two cases of Pseudomembranous Tracheobronchitis due to Amphotericin B-resistant
Aspergillus flavus
Case Report: Introduction: Pseudomembranous Aspergillus Tracheobronchitis (PMAT) is
an uncommon manifestation of invasive pulmonary aspergillosis (IPA). Patients are
typically immunocompromised although sporadic cases have been reported in previously
immunocompetent individuals.1
Aspergillus flavus is the second most frequent Aspergillus species causing bronchopulmonary
disease and isolates typically display MICs to amphotericin B (amB) which are two-fold
dilution steps higher than A.fumigatus.
2 Although the clinical significance of in vitro antifungal susceptibility is debated,
there is evidence to suggest that A. flavus isolates demonstrating resistance to amB
are associated with increased mortality.3 We present two cases of A. flavus PMAT with
raised MICs to amB and contrasting host risk-factors. Methods: Susceptibility testing
was performed using E-test on RPMI agar and confirmed at a reference laboratory using
broth-microdilution in accordance with CLSI methodology. As no species-specific breakpoints
have been established for A. flavus, resistance was defined as ≥2mg/L based on expected
MIC ranges.3 Results: Patient 1: A 39-year-old male was admitted for an Allogeneic
Haematopoetic Stem Cell Transplant for Acute Lymphoblastic Leukaemia. Post-transplant,
his medications included prophylactic liposomal amB. On day 4, he became febrile with
an undetectable neutrophil count. Therapeutic doses of liposomal amB were subsequently
added due to persistent fevers. Chest CT showed bronchial narrowing suggestive of
mucous plugging and bronchoscopy revealed pseudomembranous tracheobronchitis. Bronchoalveolar-lavage
(BAL) cultured A. flavus with an MIC to amB of 4.0mg/L. His antifungal regimen was
changed to IV voriconazole and caspofungin. He required admission to ICU due to respiratory
failure but died at 31 days post-transplant. Patient 2: A 51-year-old previously immunocompetent
male presented to hospital with fevers and progressive shortness of breath. Chest
X-ray showed patchy infiltrates and he was commenced IV co-amoxiclav, clarithromycin
and oseltamivir. By day 2, he required admission to ICU due to respiratory failure.
A nasopharyngeal aspirate PCR returned positive for Influenza A RNA and IV hydrocortisone
was added as an adjunct to his antiviral therapy. Bronchoscopy showed a pseudomembranous
tracheobronchitis and brushings cultured A.flavus with an MIC of 2.0mg/L to amB. He
received dual antifungal therapy with IV voriconazole and amphotericin B but died
at 31 days post admission. Conclusion: These cases contrast a neutropenic patient
with classical risk-factors for IPA with a patient who was not immunocompromised but
was diagnosed with PMAT following Influenza infection. Diagnosis may be delayed by
the absence of clearly distinguishable symptoms, laboratory parameters or imaging
findings, and cases may be refractory to antifungal therapy. Our experience is consistent
with evidence demonstrating poor outcomes in patients with A.flavus infections with
in-vitro resistance to amB, and underline the importance of performing susceptibility
testing to guide antifungal therapy. References: 1. Khalid S et al. Pseudomembranous
aspergillar tracheobronchitis in a non-neutropenic critically ill patient in the intensive
care unit. J Community Hosp Intern Med Perspect. March 2017p.43-45 2. Van Der Linden,
J. W.M et al. Aspergillus species intrinsically resistant to antifungal agents. Medical
Mycology, Vol.49, Issue Supplement_1, April 2011,p.S82–S89 3. Inès Hadrich et al.
Amphotericin B in vitro resistance is associated with fatal Aspergillus flavus infection,
Medical Mycology, Vol.50, Issue 8, November 2012, p829–834
Douka
E.
1
Perlikos
F.
1
Malachias
S.
1
Gavrielatou
E.
1
Sarri
K.
1
Kougias
M.
1
Adamos
G.
1
Kostourou
S.
2
Zakinthinos
S.
1
1Icu, Evangelismos General Hospital, Athens, Greece
2Infection Control, Evangelismos General Hospital, Athens, Greece
P283. Influenza-associated invasive pulmonary aspergillosis in a 25-bed Greek ICU
during 2018-2019 influenza season.
Objectives: Invasive pulmonary aspergillosis (IPA) is an opportunistic fungal infection
that often affects severely immunocompromised hosts. It has been recently documented
as a co-infection in critically ill patients with severe viral influenza infection,
although the exact risk rate is less well determined. To study the prevalence of IPA
in critically ill patients with severe influenza in Evangelismos General Hospital,
Greece.
Methods: We retrospectively reviewed all the ICU records for the 2018-2019 influenza
season. Influenza was diagnosed using the polymerase chain reaction. Co-infection
had to be confirmed using standard bacteriological tests. 28-day ICU and hospital
mortality was calculated.
Results: of the 69 patients diagnosed with influenza (34 H1NH, 31 Influenza A, 1 H1N2,
1 H1N3, 1 H1N4) in our 1000-bed tertiary hospital, 9 patients where admitted to the
ICU (5 H1N1, 1 Influenza A, 1 H1N3, 1H1N4). Two patients had a co-infection (2,9%
of the hospitalized patients with influenza and 22% of influenza patients admitted
to the ICU). 3 ICU patients suffering influenza were severely immunocompromised but
none of them developed IPA. Underlying medical conditions included hematology malignancy
(3), diabetes mellitus (2), chronic renal failure (2). IPA was confirmed by histopathological
findings in one patient and in combination of clinical, microbiological and radiological
criteria in both. The galactomannan test in BAL was positive only in the patient with
endobronchial bulky disease. Microbiology BAL cultures were Aspergillus spp positive
both (A. Fumigatus and A. Niger respectively). Both patients were treated with isavuconazole
(6 and 12 weeks respectively) and were completely cured. The 28-days ICU mortality
was 22,2% in influenza ICU patients group. 90-day hospital mortality was 44,4%. None
of the 2 patients with IPA co-infection has died.
Conclusion: IPA co-infection in critically ill patients with severe influenza has
been increasingly reported worldwide in recent years. Infection with influenza may
increase the risk of invasive aspergillosis by breaking down the bronchial mucosa
facilitating Aspergillus invasion, inducing immunomodulatory effects. Aspergillus
infection should be considered as a possible cause of persistent or progressing respiratory
failure in immunocompetent critically ill patients suffering from influenza. The diagnosis
of IPA requires awareness in order to conduct timely and appropriate testing. Microbiology
laboratories also need to be aware of the suspicion of IPA. IPA is a rapidly progressive
disease with high mortality rates up to 90%. Mortality can be reduced with prompt
initiation of effective antifungal therapy. Isavuconazole has shown promising results
in our clinical setting, for the primary treatment of IPA, especially in patients
with endobronchial bulky disease and chronic renal failure.
El-Kholy
M.
1
Shawky
S.
2
Fayed
A.
3
Meis
J.
4
1Department of Microbiology and Biotechnology, College of Pharmacy, Arab Academy for
Science, Technology and Maritime Transport (AASTMT), Alexandria, Egypt
2Department of Microbiology, Medical Research Institute, University of Alexandria
(MRI), Alexandria, Egypt
3Department of Critical Care Medicine., Faculty of Medicine, University of Alexandria.,
Alexandria, Egypt
4Department of Medical Microbiology and Infectious Diseases, Excellence Center For
Medical Mycology (ecmm), Canisius Wilhelmina Hospital, Nijmegen, Netherlands
P284. Candida auris bloodstream infection in Egypt
Case Report:
Objectives:
Candida auris was first identified and described in 2009 in Japan. Genomic analysis
has shown that C. auris emerged in at least 3 different continents, including the
Middle-East, almost simultaneously. Here we report the first case of C. auris infection
in Egypt.
Methods: We present the first case of C. auris fungemia from a patient in Egypt.
Results: A 53-year-old male was admitted in December 2017 to a hospital in Cairo just
after returning travelling from Saudi Arabia. He suffered from vomiting, abdominal
pain, bone aches and muscle weakness. Further investigations showed a positive C-ANCA
and in a renal biopsy, small vessel vasculitis consistent with Wegeners granulomatosis.
He received steroids and endoxane after which he improved temporarily. However, he
became more ill and was transferred to ICU where he was intubated and received plasmapheresis.
Due to kidney failure he required hemodialysis during his ICU admission. On day 40
of hospitalization he was transferred to a tertiary care facility in Alexandria with
cardiovascular, respiratory and renal failure. He appeared septic with leukocytosis
and elevated C-reactive protein of 223. Peripheral blood cultures were obtained and
broad-spectrum empirical therapy with iv meropenem, colistin and caspofungin was started.
Bronchoalveolar cultures showed growth of multidrug resistant Pseudomonas aeruginosa
(only colistin and amikacin susceptible) and blood cultures showed the growth of yeasts
which were identified as C. auris with the VITEK2 with updated database (version 8.01)
and confirmed with MALDITOF. Despite the early proper empiric treatment with caspofungin
the patient passed away on day 4 after admission. Urine cultures were negative for
yeasts. After diagnosis, strict contact precautions were installed and no further
C. auris cultures were identified in our healthcare facility in the past year. CLSI
antifungal susceptibility testing showed resistance to fluconazole (64 mg/L), a higher
MIC of amphotericin B (1 mg/L) and low MICs of echinocandins (0.063 mg/L). AFLP and
MLVA genotyping showed that the isolate clustered with the south-Asian C. auris Clade.
Conclusion: We report the first case of C. auris (south-Asian clade) bloodstream infection
in Egypt. Since the patient resided shortly before in Saudi Arabia it is highly likely
that he was colonized while staying abroad and that his fast progressing illness predisposed
for this fatal outcome of C. auris fungemia. In the year after discovery no secondary
cases were detected in our healthcare facility.
Irfan
M.
1
Hussain
M.
1
Jabeen
K.
2
Awan
S.
3
Farooqi
J.
2
Nasir
N.
4
Hasan
Z.
5
1Medicine, Aga Khan University, Karachi, Karachi, Pakistan
2Pathology and Laboratory Medicine, Aga Khan University, Karachi, Pakistan
3Medicine, Aga Khan University, Karachi, Karachi, Pakistan, Pakistan
4Adult Infectious Diseases, Aga Khan University, Karachi-KHI, Pakistan
5Department of Pathology and Laboratory Medicine, Aga Khan University, Karachi, Pakistan
P285. Invasive pulmonary aspergillosis in patients admitted to the intensive care
unit with severe influenza pneumonia: An observational study from a developing country
Objectives: Invasive pulmonary aspergillosis (IPA) in patients with influenza is increasingly
being identified in recent years. Cases of influenza-associated IPA with high mortality
have been reported from several developed countries with a reported incidence between
19-28 % (1, 2). So far no significant data is available from developing countries.
The aim was to determine the incidence and outcome of IPA in patients with severe
influenza pneumonia admitted in an intensive care unit (ICU) of a developing country.
Methods: This was an observational study on confirmed influenza patients with respiratory
failure admitted to the Aga Khan University hospital (AKUH) ICU from November 2018-
March 2019. Patients older than 18 years, acute respiratory failure, pulmonary infiltrates
on imaging, and a confirmed influenza infection based on a positive influenza PCR
were included. The diagnostic criteria used for IPA was based on suggestive clinical
signs and symptoms, radiological findings and mycological data (2). Like other studies,
we have also not included the host factors as this criterion was largely created for
immunosuppressed hosts, and influenza-related aspergillosis may occur in previously
normal hosts (2). The clinical characteristics, radiology, laboratory data and outcome
were recorded on a predesigned performa.
Results: A total of 92 patients with confirmed influenza were admitted at AKUH during
study period. of these 16/92 (17.02%) were admitted in ICU due to respiratory failure.
Among these 16 patients, IPA was diagnosed in 5 patients, giving an incidence of 31.25%.
The mean age of IPA patients was 61.2 (±5.02) years (Range 53-65 years) and 60% were
female. Three (60%) patients had non H1N1 Influenza A and 2 (40%) had H1N1 Influenza
A infection. Three (60%) patients had underlying diseases (diabetes, hypertension
and ischemic heart disease) and one patient was immunosuppressed. Systemic steroids
were used after ICU admission in all patients. On admission 3 (60%) patients had acute
kidney injury and 2 (40%) had deranged liver enzymes. Invasive positive pressure ventilation
(IPPV) was required in 04 (80%) patients and one patient was managed on noninvasive
ventilation (NIV). The average duration of IPPV use was 10.8 (range: 5-24) days. All
5 patients had received voriconazole after diagnosis of IPA. The overall mortality
rate of influenza patients admitted in ICU was 50% and in patients with IPA was 60%.
The causes of death were ARDS and multisystem organ failure.
Conclusion: High incidence (31.25%) of IPA was found in influenza patients requiring
ICU admission and was associated with high (60%) mortality. High index of suspicion,
early diagnosis and appropriate treatment can improve outcome in these patient. Future
large multicenter studies are required to assess the risk factors and role of antifungal
prophylaxis to improve the outcome of influenza-associated aspergillosis. References:
Schauwvlieghe A, Rijnders BJA, Philips N, Dutch-Belgian Mycosis study group et al.
Invasive aspergillosis in patients admitted to the intensive care unit with severe
influenza: a retrospective cohort study. Lancet Respir Med 2018. Huang L, Zhang N,
Huang X, et al. Invasive pulmonary aspergillosis in patients with influenza infection:
A retrospective study and review of the literature. Clin Respir J. 2019 Apr;13(4):202-211.
Vrioni
G.
1
Tsilakis
D.
2
Grammelis
V.
2
Cheliotis
G.
3
Baka
V.
4
Christofidou
M.
5
Karahalios
S.
6
Kazila
P.
7
Mamali
V.
8
Maraki
S.
9
Maropoulos
G.
10
Panopoulou
M.
11
Perivolioti
E.
12
Petinaki
E.
13
Platsouka
E.
14
Themeli-Digalaki
K.
8
Tsiplakou
S.
15
Tsorlini
E.
16
Vagdatli
E.
17
Vogiatzakis
E.
18
Vourli
S.
19
Zerva
L.
19
Karahaliou
E.
1
Theodoridou
K.
1
Tsianos
C.
1
Skiadas
I.
2
Armaganidis
A.
20
Tsakris
A.
1
1Microbiology, Medical School, National and Kapodistrian University of Athens, Athens,
Greece
2Medical Affairs, Pfizer Hellas, Athens, Greece
3Biometrics Program Manager, Health Data Specialists, Athens, Greece
4Microbiology, Red Cross General Hospital, Athens, Greece
5Microbiology, Rio University Hospital, Patras, Greece
6Microbiology, Agioi Anargyroi Oncology Hospital, Athens, Greece
7Microbiology, Theageneio Oncology Hospital, Thessaloniki, Greece
8Microbiology, Tzaneio General Hospital, Athens, Greece
9Microbiology, University Hospital Crete, Herakleio, Greece
10Microbiology, Laiko General Hospital, Athens, Greece
11Microbiology, University Hospital of Alexandroupolis, Alexandroupolis, Greece
12Microbiology, Evangelismos General Hospital, Athens, Greece
13Microbiology, Larissa University Hospital, Larissa, Greece
14Microbiology, “Konstantopouleio-Patission” General Hospital of Neas Ionias, Athens,
Greece
15Microbiology, KAT General Hospital, Athens, Greece
16Microbiology, “G. Papanikolaou” General Hospital, Thessaloniki, Greece
17Microbiology, “Ippokrateio” General Hospital, Thessaloniki, Greece
18Microbiology, Sotiria General Hospital, Athens, Greece
19Microbiology, Attiko University Hospital, Athens, Greece
20Intensive Cate Unit, Attiko University Hospital, Athens, Greece
P286. Hellenic Invasive Candidiasis Study (HELICAS): Identification and Antifungal
Susceptibility of Candida species in Greek ICUs.
Objectives: HELICAS (HELlenic Invasive CAndidiasis Study) was a prospective non-interventional
study aiming to compare the cost of treatment of documented invasive candidiasis (IC)
by C. albicans and C. non-albicans species, in 18 Greek intensive care units (ICUs).
As part of this study, isolated Candida species were collected and analyzed. Additionally,
the epidemiology of IC by C. albicans and C. non-albicans, was evaluated by collecting
retrospective data from participating ICUs for an at least 3-month period.
Methods: A total of 100 adult subjects (49% C. albicans, 51% C. non-albicans) were
enrolled according to a pre-specified sample size, for both C. albicans and C. non-albicans
cases. Demographics, days of ICU stay, antifungal and concomitant medications, hospital
supplies, laboratory investigations and treatment patterns, as well as patient outcomes
and survival were documented. Eighty-two of the collected Candida isolates (39 C.
albicans, 43 C. non-albicans) were sent to the Department of Microbiology, Athens
Medical School, for further investigation. Candida isolates were identified by conventional,
biochemical tests (CHROMagar Candida Medium, Becton Dickinson; API 32C, BioMérieux),
supplemented with mass spectrometry (Vitek MS, BioMérieux), if the identification
score was < 95%. Antifungal susceptibility testing for amphotericin B, 5-flucytocine,
itraconazole, fluconazole, voriconazole, posaconazole, micafungin, caspofungin, and
anidulafungin was performed with the broth microdilution method (MICRONAUT-AM MHK
2, MERLIN Diagnostika GmbH), according to CLSI 2012 M27-A3 document. Revised CLSI
M27-S4 species–specific clinical breakpoints were applied.
Results: Eighty-two strains were studied, the majority isolated from candidemia cases;
39 (47.6%) C. albicans, 26 (31.7%) C. parapsilosis, 9 (11.0%) C. tropicalis, 6 (7.3%)
C. glabrata, 1 (1.2%) C. kefyr, and 1 (1.2%) C. lusitaniae. All strains showed low
MICs to amphotericin B, with MIC values ranging from 0.0625 to1 μg/ml. Sixteen isolates
were resistant to fluconazole: 15 C. parapsilosis (MIC range 8-32 μg/ml) and 1 C.
albicans (MIC ≥ 128 μg/ml), while 13 isolates exhibited dose-dependent susceptibility:
6 C. glabrata, 4 C. parapsilosis, 2 C. tropicalis and 1 C. albicans. Only 2 isolates,
i.e.1 C. albicans (MIC ≥ 128 μg/ml) and 1 C. parapsilosis, were resistant to voriconazole,
while 3 C. parapsilosis and 1 C. tropicalis exhibited dose-dependent susceptibility.
Resistance to echinocandins was not detected in any strain. Intermediate susceptibility
was demonstrated in 2 C. tropicalis to caspofungin and anidulafungin, and in 2 C.
glabrata to caspofungin. Based on the epidemiology data, extrapolated to one year,
the majority (64.3%) of IC cases in the participating ICUs were caused by C. non-albicans
spp. (C. parapsilosis: 45.7%), with the remaining 35.7% being due to C. albicans.
Conclusion: Epidemiological patterns of IC reveal a predominance of C. non-albicans
spp.in Greek ICUs, especially C. parapsilosis. Since 15/26 (57.7 %) of C. parapsilosis
isolates were resistant to fluconazole, this observation raises concerns, as fluconazole
has traditionally been considered the first-line choice for C. parapsilosis. In vitro
antifungal susceptibility testing should be performed to assist in the selection of
the most appropriate antifungal therapy, and in monitoring the development of resistance.
Cheikhrouhou
S.
1
2
Aloui
D.
1
2
Bouchekoua
M.
1
2
Messadi
A.
2
3
Thabet
L.
2
3
Trabelsi
S.
1
2
Khaled
S.
1
2
1Laboratory of Parasitology and Mycology, Charles Nicolle Teaching Hospital, Tunis,
Tunisia
2Faculty of Medicine of Tunis-Tunis El Manar University, Tunis, Tunisia
3Ben Arous Trauma and burn center, Tunis, Tunisia, Ben Arous, Tunisia
P287. Species distribution and antifungal susceptibility patterns of Candida isolated
from a Tunisian burn intensive care unit
Objectives: The objective of this study was to analyze the species distribution of
Candida isolates from a Tunisian burn intensive care unit and their antifungal susceptibility
patterns.
Methods: We performed a retrospective review of 35 severely burned patients admitted
to the burn intensive care unit of Ben Arous Trauma and Burn Center with one or more
positive culture sites for Candida, during the 28-month period from January 2017 to
April 2019. The susceptibility for 6 antifungal drugs (5-fluorocytosine, fluconazole,
ketoconazole, micronazole, itraconazole, amphotericin B) was determined using the
Fungitest® broth dilution method for patients with infected normally sterile body
sites or a Candida colonization index superior or equal to 0.4. Voriconazole and caspofungine
were tested when needed.
Results: A total of 74 Candida isolates were obtained. Positive urine cultures yielded
30% of Candida isolates. The rectal site represented 15% of colonized body sites,
followed by nasal and oral sites (13,5% each), axillary (9,4%), ocular (8%) and cutaneaous
and auricular sites (5,4% each). Candida albicans was the predominant species (43.2%),
followed by C. glabrata (31%), C. tropicalis (17,5%), C. parapsilosis (3%), C. colliculosa
(3%) and C. sake (1%). Among the strains whose antifungal susceptibility were determined,
the majority of Candida isolates were susceptible to fluconazole (64%), 20,5% of the
isolates were intermediate and 15,4% were resistant to this antifungal drug, mainly
C. tropicalis and C. glabrata. There was a high rate of strains with intermediate
susceptibility to itraconazole (46%), miconazole (50%) and ketoconazole (23%). Only
one strain was resistant to amphotericin B which was C. tropicalis.
Conclusion: Although the prevalence of non-albicans species has increased over recent
years, C. albicans remains the most frequent species in burn unit-acquired candidiasis.
Our antifungal susceptibility test results did not exhibit significant levels of resistance,
but non-albicans species must be taken into consideration when choosing antifungal
agents for calculated therapy.
Negro
G.M.B. Del
1
Naves
G.
2
Freitas
V.L.
3
Magri
M.
4
Junior
J. De Almeida
5
Abdala
E.
6
Sejas
O.N.
6
1Laboratório De Investigação Médica Lim53, Insituto de Medicina Tropical, São Paulo,
Brazil
2Laboratório De Investigações Médicas 53, Insituto de Medicina Tropical, São Paulo,
Brazil
3Laboratório De Investigação Médica Em Imunologia Lim48, Insituto de Medicina Tropical,
São Paulo, Brazil
4Clinica De Molestias Infecciosas, Hospital das Clinicas da FMUSP, São Paulo, Brazil
5Divisão De Laboratorio Central, Hospital das Clinicas da FMUSP, São Paulo, Brazil
6Instituto do Cancer do Estado de São Paulo, São Paulo, Brazil
P288. Performance of the (1,3)-β-DG-glucan assay for detection of candidemia in adult
ICU patients: a preliminary evaluation
Objectives: Invasive candidiasis (IC), including candidemia episodes, is a considerable
cause of morbidity and mortality in immunocompromised critically ill patients. Studies
have been reported that early diagnosis of IC is crucial for an appropriate treatment
and successful outcome of patients. Analyses using serum levels of the β-D-glucan
(BDG) biomarker have shown promising results for the early diagnosis of IC/candidemia.
This study aimed to evaluate the performance of the BDG assay as a diagnostic tool
for candidemia detection in ICU adult patients with risk factors for IC.
Methods: Thirty-nine patients admitted to five ICU from two tertiary hospitals were
enrolled, being 11 with proven candidemia (at least one blood culture positive for
Candida) and 28 patients with negative bloodstream cultures but clinical findings
consistent with candidemia (probable candidemia). Blood samples for cultures and BDG
testing were collected before administration of antifungal therapy. Candida spp. were
isolated from blood by using the Bactec™ system and identified by VITEK® MS which
uses MALDI-TOF technology. BDG serum concentrations were measured using the Fungitell®
assay following the standard operating procedures. All samples were tested in duplicate.
The analyses were performed using SPSS Statistics version 24.0.
Results: All patients with proven candidemia presented positive results with significantly
higher BDG values than those with probable candidemia (median 437.7 vs. 70.6 pg/ml,
p <0.001). Among the probable candidemia patients, four (14.3%) showed positive BDG
results (ranging from 85 to 276.5 pg/ml), two of them had proven bacteremia, and three
patients (10.7%) presented indeterminate values (≥ 60 and ≤ 80 pg/ml). Seven patients
of the probable candidemia group (negative bloodstream cultures) presented positive
cultures for Candida spp. in blood samples harvested from central venous catheter.
These seven patients showed significantly higher BDG values when compared to patients
with no positive cultures at all (median 151.4 vs. 51.7 pg/ml, p< 0.007).
Conclusion: We presented preliminary results from a prospective study for early laboratory
diagnosis of candidemia in ICU adult patients at high risk of this systemic infection.
Patients with proven candidemia had significantly higher BDG levels than those patients
in whom the bloodstream Candida infection could not be demonstrated. The BDG positive
values observed in patients with positive blood catheter cultures signal that these
patients might have true candidemia in spite of negativity of blood cultures. By the
other hand, the possibility of false-positive results in this group should be considered
and in this scenario another non-culture based methodology, such as real time PCR
technique would be required to firmly establish the diagnosis of candidemia.
Rudramurthy
S. M
1
Chakrabarti
A.
2
Shastri
P.
3
Patel
A.
4
Savio
J.
5
Karthik
R.
6
Kaur
H.
2
1Medical Microbiolgoy, Postgraduate Institute of Medical Education and Research, Chandigarh,
India
2Medical Microbiology, Postgraduate Institute of Medical Education and Research, Chandigarh,
India
3Critical Care, Sri Gangaram Hospital, Delhi, India
4Infectious Disease, Sterling Hospitals, Ahmedabad, India
5Microbiology, St. John’s Medical College, Bangalore, India
6Infectious Disease, Christian Medical College, Vellore, India
P289. Influenza associated invasive pulmonary aspergillosis in Indian intensive care
units
Objectives: Beyond the classical neutropenic hosts, invasive pulmonary aspergillosis
(IPA) is increasingly reported in non-neutropenic critically ill patients. Influenza
A H1NI infection is one among those emerging non-classical risk factors. In a multicentre
prospective observational study on invasive mould infections (IMIs)in Indian ICUs,
we identified 12 cases of IPA associated with influenza H1N1 [1]. Here, we analysed
those influenza associated aspergillosis (IAA) cases to evaluate its epidemiology
in Indian ICUs.
Methods: The study captured all cases of IMIs at 11 ICUs across India during April
2016 through September 2017. IMI was diagnosed as proven, probable and possible based
on the EORTC-MSG criteria in the classical immunosuppressed subjects (2), the criteria
proposed by Bulpa et al. in subjects with COPD (3), and Vandewoude et al. in non-COPD
subjects admitted to the ICU (4). Demographic, clinical presentation, laboratory investigations,
treatment and management of proven and probable IMIs were collected using predesigned
proforma (1). The present study is the subgroup analysis of clinical and laboratory
variables and management of 12 patients diagnosed as IAA.
Results: During the study period 135 IPA cases (proven and probable) were reported,
37 (27.5%) had classical risk factor and 98 (72.5%) non-classical risk factors. Among
non-classical risk factors 12 patients had IAA; one patient was proven and 11 cases
were probable IPA. Those patients were from four centres of India (north-1, south
-2, and west-1) and majority (58.3%) cases from a single centre at Delhi. 10/12 cases
occurred in summer. The mean age of those patients was 56.8 ± 12.34 years with equal
distribution of sex. The mean APACHE score was 18.41 ± 4.56. The diagnosis of IPA
was made at a mean of 3.83 ± 2.94 days after ICU admissions. The solid organ malignancies
were co-morbidities in two patients, eight patients were on ventilator. Aspergillus
species was isolated from ten cases (Aspergillus flavus-5, A. fumigatus -2, Aspergillus
spp. – 2 and A. flavus+A fumigatus -1). Serum and/or BAL galactomannan was positive
in eleven patients. Radiological evidence in the form of opacity (10 cases), cavity
(1 case), nodules (5 cases), infiltrates (2 cases), consolidation (7 cases) or pleural
effusion (3 cases) was noted in those patients. None of them had classical air crescent
or halo sign. The patients were treated with voriconazole (8 cases) or voriconazole
+ amphotericin B deoxycholate (1 case), voriconazole + caspofungin (1 case) and liposomal
amphotericin B (1 case). But, the 42-day or 84-day mortality remained at 58.3% despite
targeted antifungal therapy.
Conclusion: The study emphasizes that influenza H1N1 is an emerging risk factor for
IPA in Indian ICUs. Unlike other reports of A. fumigatus, A. flavus is the predominant
causative agent in Indian scenario. During influenza season, critically ill patients
should be thoroughly investigated for IPA. Early diagnosis and prompt therapy may
reduce high mortality rate of IAA cases. References Chakrabarti et al. (2019). J Crit
Care 51, 64–70. De Pauw B et al (2008). Clin Infect Dis 46:1813–21. Bulpa P(2007)
Eur. Respir. J. 30:782–800. Blot SI et al (2012) Am J Respir Crit Care Med: 186:56–64
Anitha
S.
1
Yamini
A.
1
Kindo
A.J.
2
1Microbiology, Sri Ramachandra Institute of Higher Education and Research Institute,
Chennai, India
2Microbiology, Sri Ramachandra Institute of Higher Education and Research, Chennai,
India
P290. rEarly detection of circulating candida antigen and antibodies in ICU patients
with candidemia
Objectives: Early detection of candida in blood using lateral flow assays and candida
mannan assay in ICU patients at risk of invasive candidiasis selected by candida scoring
and to compare the results with the Gold standard blood culture method.
Methods: Patients having an ICU stay of > 7 days were taken up. Candida scoring was
done and 33 patients who had scored ≥3 were considered for the study. Patients Age,
gender, clinical presentations at admission, severity scores including Acute Physiological
and Chronic Health Evaluation II (APACHE II), co- morbid medical conditions, surgical
and invasive procedures, exposure to antibiotics, corticosteroids, antifungals, secondary
bacterial infections, antifungal treatment instituted in the ICU and final outcome
were recorded. Patients were divided into 2 groups based on the presence or absence
of candida. The blood samples were collected at the onset of sepsis and assays were
performed. The samples were tested for the presence of IgM and IgG antibodies specific
for candida using lateral flow assay kits and mannan antigen using candida mannan
assay kit (Dynamiker Biotechnology (Tianjin) Co., Ltd) which would provide early detection
of candida in blood and thus facilitating in early start of suitable antifungal therapy
and a favorable outcome.
Results: A total of 33 patients admitted to the ICU from Jan 2019-April 2019 based
on candida scoring were considered for the study. of the 33 patients, six were proven
cases of candidemia and the remaining 27 were did not grow candida in blood culture.
The mean age of the patients who had candidemia ranged from 45-82yrs. Among the candidemia
patients the male female ratio was equally distributed. The duration of stay in ICU
was more for candidemia patients (median days 15). The APACHEII scoring was high among
candidemia patient (median 54.5) compared to non candidemia patients (median 36.5).
Also the candida scoring among candidemia patients was found to be high (median 4.5).
The analysis of the results for the presence of IgG, IgM antibodies specific for candida
and mannan antigen will be done and compared with gold standard blood culture method.
Conclusion: The three methods will be compared so that we don’t have to rely always
on culture to start the treatment. Development of diagnostic and predictive tests
will probably lead to increased use of preemptive therapy. The presence of biomarkers
is an indicator to assist in decision to start or stop antifungal treatment. In case
of positivity of fungal biomarker, it recommends to initiate preemptive therapy. In
case of negativity of fungal biomarker, it suggests to stop empiric therapy.
Helweg-Larsen
J.
1
Steensen
M.
2
Pedersen
F. Møller
3
Jensen
P. Bredahl
3
Bonde
J.
2
Perch
M.
4
Møller
K.
5
Olesen
B. Riis
6
Arendrup
M.C.
7
1Department of Infectious Medicine, Rigshospitalet, Copenhagen, Denmark
2Department of Intensive Care Medicine, Copenhagen University Hospital Rigshospitalet,
Copenhagen, Denmark
3Department of Thoracic Anaesthesiology, Copenhagen University Hospital Rigshospitalet,
Copenhagen, Denmark
4Department of Cardiology, Copenhagen University Hospital Rigshospitalet, Copenhagen,
Denmark
5Department of Neuroanaestesiology, Copenhagen University Hospital Rigshospitalet,
Copenhagen, Denmark
6Department of Economy, Copenhagen University Hospital Rigshospitalet, Copenhagen,
Denmark
7Rigshospitalet, Dept. of Clinical microbiology, Copenhagen, Denmark
P291. An ICU Antifungal Stewardship Programme based on T2Candida PCR and Candida Mannan
antigen.
Objectives: Management of invasive candidiasis (IC) in ICU patients is challenging
since traditional diagnostic methods and prediction scores have limited sensitivity
and specificity. Non-culture-based biomarkers could potentially improve diagnosis
and antifungal treatment (AFT), enabling safer discontinuation of unneeded therapy.
We evaluated a new antifungal stewardship programme (AFSP) including T2Candida and
Candida mannan antigen (MAg) screening of high-risk ICU patients.
Methods:
Patients in two ICU’s (abdominal/general and thoracic, respectively) at a tertiary
university hospital were eligible. The Figure and Table summarise inclusion criteria,
AFSP algorithm and IC classification criteria. The diagnostic value of T2Candida and
MAg in relation to traditional methods were evaluated. Consumption of antifungals
during the AFSP and the preceding 2 years was compared.
Results: 219 patients with 504 T2Candida/MAg samples were included. Median age was
63 years, median ICU stay was 19 days, 45% were receiving renal-replacement-therapy;
29% TPN, 22% underwent acute surgery and the overall in-hospital mortality was 42%.
At inclusion, 32% of patients had received AFT for >3 days. 30 patients had positive
T2Candida; 24 positive MAg and 18 had positive blood cultures (BC). IC was classified
as proven in 29, likely in seven and possible in 10 patients. Defining IC as proven/likely
versus unlikely, sensitivity/specificity/NPV values were 47%/100%/91% for BC; 56%/97%/90%
for T2Candida; and 39%/96%/88% for MAg. MAg identified five patients with IC, in whom
T2Candida or BC were negative. of these, one had proven C. lusitaniae IC and four
had possible IC (colonizing isolates without species-identification). The best diagnostic
performance was the combination of T2Candida&MAg taken ≤3 days after AFT: sensitivity/specificity/PPV/NPV
of 70%/90%/ 63%/93%, versus 61%/93%/63%/ 92% for all patients, respectively. Overall,
similar performance was observed if IC was defined as proven/likely/possible. The
use of T2Candida/MAg contributed to 15% of patients receiving early (<3 days) AFT
after a positive test, 25% of patients having early AFT discontinuation and 24% of
patients being managed without AFT. No reduction or overall change in use of echinocandin
or azole treatment was observed at the two ICU’s in the study period compared to the
two previous years.
Conclusion: Introduction of an AFSP based on T2Candida and MAg screening of high-risk
patients contributed to reducing unnecessary treatment but not overall AFT use. The
diagnostic performance of T2Candida for IC was higher than for BC and MAg, but lower
than initially reported. T2Candida detects cell-associated Candida DNA and thus depend
on circulating Candida cells. A significant proportion of our patients were already
on antifungal therapy at the time of sampling or suffered from BC-negative IC thus
likely representing patients with a low number of circulating Candida cells. Moreover,
a significant proportion of patients were only tested once or twice. Taken together,
our study suggests that the combination of T2Candida and MAg improved diagnostic performance
compared to T2Candida alone with a sensitivity of 70% and NPV of 93% in patients with
≤3 days AFT. This combination may contribute to improved management of IC in the setting
of an AFSP particularly if initiated before AF prescription and with strict adherence
to the AFSP.
Lupse
M.
1
Rus
M.A.
1
Herbel
L.
2
Flonta
M.
3
1Infectious Diseases, University of Medicine and Pharmacy Cluj-Napoca, Cluj-Napoca,
Romania
2Icu, University Hospital of Infectious Diseases, Cluj-Napoca, Romania
3Microbiology, University Hospital of Infectious Diseases, Cluj-Napoca, Romania
P292. Incidence, treatment and outcome of ICU-acquired Candidemia in 5 ICU from Cluj-Napoca,
Romania during a 6 years period
Objectives: To evaluate the incidence, etiology and outcome of candidemia in ICU from
our city
Methods: We retrospectively analyzed all cases of candidemia diagnosed based on patients
files, considering only the first episode/patient. We included non-neutropenic patients
from 5 ICU from our city (1pediatric ICU, 1 medical ICU and 3 surgical ICU) during
6 years (Jan 2007- Dec 2012). We evaluated: demographic data, etiology, antifungal
treatment and outcome.
Results: There were 107 patients, average age 44.5 (median age 55, min 1 day, max
85 years, median age for PICU patients was 1.4 months), 50 female (46.73%). The incidence
was 25.8/1000 admission in PICU and 2.8/1000 admission in SICU. The etiology was represented
by Candida albicans in 53 (48%) patients, Candida parapsilosis in 31 (29%), Candida
glabrata in 7 (6%) and other species in 15 patients. Candida parapsilosis was the
most frequent etiology of candidemia for children (45%). Crude mortality rate was
59.8%, higher in adults and in patients infected with Candida glabrata (5/6) and Candida
tropicalis (5/5). 55 patients were treated with fluconazole, 14 with caspofungin,
3 with voriconazole, 10 with ketoconazole and 25 patients did not receive any antifungals.
Resistance to fluconazole was 23.36% (24.48% for Candida albicans).
Conclusion: Candidemia was a frequent situation in ICU non-neutropenic patients. The
involvement of Candida albicans and Candida non-albicans was almost equal but significantly
different in adults and children. Fluconazole was the most used antifungals but the
resistance to Fluconazole was high. The mortality rate was very high, significantly
higher in adults than in children and in Candida sp. other than Candida parapsilosis.
Bulman
F.
1
2
Rosenberger
D.
2
3
Mueller
C. Von
2
3
Vylkova
S.
2
3
1Jena University Hospital, Jena, Germany
2Septomics Research Center, Friedrich Schiller University, Jena, Germany
3Leibniz Institute for Natural Product Research and Infection Biology, Hans Knöll
Institute, Jena, Germany
P293. A Native Human Burn Infection Model of Candidiasis
Objectives: A tenfold rise in fungal burn wound infections (BWI) has been observed
globally since the 1960s due to the introduction of broad spectrum antimicrobial agents
(Sarabahi et al, 2012). The fungal opportunistic pathogen Candida albicans is a common
isolate from BWI (Branski, Al-Mousawi et al. 2009). Systemic candidiasis originating
from burn wounds can result in up to 70% mortality rates, yet nothing is known about
the biology of infection. The objective of this study was to develop a reliable model
of infection. Current animal models such as murine, rat and porcine have major differences
in skin biology and inflammatory responses compared to human and are ethically questionable.
Therefore, we have developed a novel ex vivo human skin model of burn wound C. albicans
infection and examined the role of key fungal virulence determinants and host responses
throughout the progression of infection.
Methods: NativeSkin® human tissue (Genoskin, France) was burned with a 350°C soldering
iron for 15 sec, debrided and infection was initiated with 1x105 cells of C. albicans
wild-type SC5314 strain expressing luciferase. The progress of infection was measured
by bioluminescence at 1 and 6 days after burn induction, followed by sample fixation
for further histological analysis and immunofluorescence staining. Immune cell populations
were analysed by flow cytometry and media was measured for the presence of proinflammatory
cytokines and lactate dehydrogenase (LDH), a cytotoxicity marker. In some experiments,
we supplemented the media with 2x106 polymorphonuclear neutrophils (PMNs) to observe
tissue infiltration and effects on healing of the burn.
Results: The protocol proved to reliably cause third degree burn wounds in an operator
independent manner. Staining for high mobility group box 1 protein (HMGB1) showed
that while the unburned skin retained viability after 6 days, the burned skin samples
had marked necrosis. Histological analysis showed reduced depth of burn and area of
burn injury at day 6 compared to day 1, indicative of tissue regeneration. Flow cytometry
analysis of immune cell populations present in control (unburned) skin detected CD1a+
and CD207+ cells, indicating populations of viable langerhans and dendritic cells.
Further, staining for neutrophil elastase (NE) showed the presence of PMNs at the
edge of the burn. Levels of cytokines IL-1β, IL-6 and Il-8 were variable between skin
donors and experimental conditions, with the highest concentration found in burned
and infected samples. BWI with C. albicans resulted in prominent tissue penetration,
especially following debridement of the burn.
Conclusion: A reliable and ethical human skin ex vivo model for fungal BWI has been
established and various analyses methods were tested. Genoskin NativeSkin® samples
exhibit good healing potential, have viable immune cell populations and allow supplementation
with PMNs and other cells/factors, thus representing an excellent model for BWI candidiasis.
The outlook includes testing key virulence determinants for candidiasis, including
dual transcriptional profiling; mixed bacterial and fungal infections, and alterations
of approved treatment regimes. Such a model may shed light on early signs and symptoms
of candidiasis in the burn population, which could help to develop guidelines in patient
care.
Mansouri
R.
1
Touta
S.
1
Fetni
A.
1
Saadni
F.
1
Akil
S.
2
Grifi
F.
2
Atik
A.
2
1Pharmacy, faculté de medecine, annaba, Algeria
2Medecine, faculté de medecine, annaba, Algeria
P295. Laboratory tools applied in the diagnosis of invasive fungal infections at laboratory
of medical parasitology and mycology of the CHU of Annaba. Algeria.
Objectives: Invasive fungal infections play an important role as severe infection,
constantly rising in the services in charge of patients at risk. Diagnosis and treatment
are difficult because of the absence of pathognomonic signs and symptoms. The delay
in diagnosis leads to delayed treatment and thus worsened prognosis The objective
of this study is to identify the place of laboratory tools applied in the diagnosis
of deep candidiasis, Cryptococcosis, Pneumocystosis and invasive aspergillosis.
Methods: This is a descriptive prospective study spanning 07 years (2010-2016) and
having respective 998 samples from patients from several medical services: intensive
care, Internal Medicine, major burned, Onco-Hematology, infectious diseases, Cardiology,
Gastroenterology, Nephrology and Pediatrics. The seach of the mannan antigen for the
diagnosis of invasive candidiasis and galactomannan antigen for the diagnosis of invasive
aspergillosis occurs by Enzyme Linked ImmunoSorbent Assay. The search for the capsular
antigen of Cryptococcus held by slide agglutination and Pneumocystis spp. by DFA.
The search for antibody anti-Candida and anti-Aspergillus occurs by Enzyme Linked
ImmunoSorbent Assay. Blood cultures and device samples are also studied for these
patients.
Results: Among the 371 sera studied for suspected invasive candidiasis, 53 were positive
(14.28%) and among 286 sera investigated for suspected invasive aspergillosis, 22
were positive (7.69%). Four cases of Cryptococcosis were confirmed and three cases
of Pneumocystosis. Specie isolated from Blood cultures, device and peripheral samples
were 93 % C. albicans, 1,5 % were other species: C. parapsilosis, C.zeylanoides, C.
glabrata, C.krusei.
Conclusion: Invasive fungal infections remain under-diagnosed clinically and therefore
mycologically. The mycological diagnosis associated with sero-immunological diagnosis
is a decisive contribution in these conditions. A delay in diagnosis worsens the prognosis
of these violations where the interests of a multidisciplinary proper care.
Liu
J.
Ying
J.
Hua
P. Fu
The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China
P296. Non-HIV female cryptococcal meningitis with and without autoimmune diseases
Objectives: Cryptococcal meningitis (CM) is a rare infection in autoimmune diseases
(AIDs) patients. CM complicated with AIDs is more common in women. The aims of this
study were to describe the differences of female CM patients with and without AIDs.
Methods: We retrospectively compared the differences between 21 non-HIV female CM
with AIDs and 23 non-HIV female CM without AIDs from 2011 to 2018. Their demographic
data, clinical manifestations, cerebrospinal fluid (CSF) and MR features were analysed.
Results: We found that female CM with AIDs had lower CSF pressure, higher CPR and
ESR than female CM without AIDs when admitted to the hospital. There was no statistical
difference in clinical and MRI features between two groups. After antifungal therapy,
headache, fever, mRs scores and some CSF index were significantly improved in both
groups. In female CM with AIDs, mRs was positively correlated with India ink Cryptococcus
count and had no correlation with CSF pressure. But in female CM without AIDs, we
found that mRs was positively correlated with CSF pressure and had no correlation
with India ink Cryptococcus count.
Conclusion: Our data suggested that SLE was the most common in female CM with AIDs,
and revealed for the first time a difference between female CM with and without AIDs.
Although presenting symptoms of female CM patients with AIDs are usually unspecific.
But it is curable if timely make the diagnosis and treatment.
Shadrivova
O.
1
Leonova
O.
2
Bubnova
D.
3
Volkova
A.
4
Popova
M.
4
Bogomolova
T.
5
Ignatyeva
S.
5
Zubarovskaya
L.
4
Afanasyev
B.
4
Vasilyeva
N.
5
Klimko
N.
1
1Department of Clinical Mycology, Allergy and Immunology, North-Western State Medical
University named after I.I.Mechnikov, St. Petersburg, Russian Federation
2St. Petersburg Center for the Prevention and Control of AIDS and Infectious Diseases,
St. petersburg, Russian Federation
3North-Western State Medical University named after I.I.Mechnikov, St. Petersburg,
Russian Federation
4I.Pavlov First Saint Petersburg State Medical University, St. Petersburg, Russian
Federation
5Kashkin Research Institute of Medical Mycology, North-Western State Medical University
named after I.I.Mechnikov, St. Petersburg, Russian Federation
P297. Invasive aspergillosis in HIV-positive patients
Objectives: Analysis of underlying diseases, risk factors, etiology, clinical features,
treatment and survival rates in HIV-positive patients with IA.
Methods: Retrospective analysis of the register of patients with IAin 1998-2018 yy.
For diagnosis IA we used criteria EORTS/MSG, 2008.
Results: In group I we included 12 HIV-positive adult patients with IA from 25 to
52 years old, median – 34, males – 58%. The control group consisted of 545 adult patients
withhematological malignancies, from 18 to 78 years old, median - 47, males – 58%.
The study of risk factors showed significant differences between these groups. Lymphocytopenia
was detected predominantly in HIV-positive patients 75% vs 56%, duration 35 vs 14,5
days, (p = 0.006), while agranulocytosis in this group was observed less frequently
– 58% vs 81%, p = 0.008, duration 8 vs 13 days (p = 0.01). Among the HIV+ patients
there were no recipients of allogeneic stem cell transplants (0% vs 19%, p = 0.001)
and patients receiving immunosuppressive therapy (0% vs 29%, p = 0.02). The main sites
of infection were lungs - 100% vs 98%, however hemoptysis and dissemination of infection
more often registered in HIV+ patients – 17% vs 6% (p = 0.03)and 17% vs 8% (p = 0.04),
respectively.Galactomannan test in BAL was positive in 42% vs 75% cases. Aspergillus
spp. were isolated in 42% vs 44%, in all HIV+ patients the main etiological agent
of IA was A.fumigatus - 100% vs 45% (p = 0.001). Mixed fungal infection was detected
in 33% vs 11% (p = 0,001). «Proven» IA was diagnosed in 17% vs 7% (p = 0.02). Antifungal
therapy was used in 100% vs 99% of patients; the most commonly used drug was voriconazole
(58% vs 77%). Twelve weeks overall survival rate was 80% vs 81%.
Conclusion: The features of invasive aspergillosisin HIV-positive patientswere:prolonged
lymphocytopenia (75%),agranulocytosis was a rare risk factor (58%), more frequent
mixed infection - 33%, and high frequency of dissemination of aspergillosis – 17%.The
overall 12-week survival did not differ in the studied groups (82% vs 79%).
Shadrivova
O.
1
Pivovarova
V.
1
Chudinovskikh
Y.
2
Shneyder
T.
3
Uspenskaya
O.
3
Popova
M.
4
Volkova
A.
4
Desyatik
E.
5
Borzova
Y.
5
Ignatyeva
S.
5
Bogomolova
T.
5
Zubarovskaya
L.
4
Afanasyev
B.
4
Vasilyeva
N.
5
Klimko
N.
1
1Department of Clinical Mycology, Allergy and Immunology, North-Western State Medical
University named after I.I.Mechnikov, St. Petersburg, Russian Federation
2N.N. Petrov National Medical Research Centre of Oncology, Ministry of Health of Russian
Federation, St. Petersburg, Russian Federation
3Leningrad Regional Clinical Hospital, St. Petersburg, Russian Federation
4I.Pavlov First Saint Petersburg State Medical University, St. Petersburg, Russian
Federation
5Kashkin Research Institute of Medical Mycology, North-Western State Medical University
named after I.I.Mechnikov, St. Petersburg, Russian Federation
P298. Invasive aspergillosis in patients with multiple myeloma.
Objectives: Identification offeatures of invasiveaspergillosis (IA)in patients with
multiple myeloma (MM).
Methods: Retrospective analysis of 337 adult hematological patients with IA. In the
main group were included 39 patients with MM, median age – 56 years (41 - 79), females
- 59%. The control groupincluded 298 hematological patients (acute leukemia – 45%,
lymphoma – 36%; chronic leukemia – 13%, myelodysplastic syndrome – 5%; other – 1%),
median age – 53 years (40 - 78), females – 56%. The EORTS/MSG 2008 criteria were used
for IA diagnosis and assessment of response of therapy.
Results: The main risk factorsfor IA were steroid use (87.5% vs 59.5%, p = 0.03),
severe neutropenia (51% vs 76%, p = 0.03; median 14 vs 18 days), lymphocytopenia (33%
vs 53%, median 10 vs 12.5 days), auto-HSCT (28% vs 4%, p = 0.01), and allo-HSCT (8%
vs 13%). The main sites of infection were lungs (97,4% vs 97,3%), usually bilateral
(69% vs 77%). The main clinical symptoms were fever (80% vs 78%), cough (69% vs 61%),
chest pain (16% vs 5%, p = 0.03), and hemoptysis (0% vs 6.4%, p = 0.001). Aspergillus
spp. positive culture was received in 69% vs 46% patients. The etiology of IA in patients
with MM: A. niger– 45%, A. fumigatus– 35%, A.flavus– 10%, A. candidus– 5%, A. ochraceus–
5%.Antifungal therapy (voriconazole – 68,2% vs 64%) was used in 100% MM and 98% control
group patients. The overall 12-weeks survival rate was 96% vs 80%, p = 0.01.
Conclusion: The typical risk factors for invasive aspergillosis in multiple myeloma
patients were steroids use (87,5%), and auto-HSCT (28%). The main sites of infection
were lungs (97,4%). The main etiological agents were A. niger(45%) and A. fumigatus(35%).
In multiple myelomapatients the overall 12-weeks survival rate was significantly higher
compared to the control group (96%vs 80%, p = 0.01).
Shadrivova
O.
1
Bubnova
D.
2
Pogromskaia
M.
3
Klimko
N.
1
1Department of Clinical Mycology, Allergy and Immunology, North-Western State Medical
University named after I.I.Mechnikov, St. Petersburg, Russian Federation
2North-Western State Medical University named after I.I.Mechnikov, St. Petersburg,
Russian Federation
3Department of Infectious Diseases, North-Western State Medical University named after
I.I.Mechnikov, St. Petersburg, Russian Federation
P299. Analysis of invasive mycoses in hiv-infected patients by autopsy results in
Saint Petersburg, Russia
Objectives: Retrospective analysis of the autopsy data of HIV-infected patients with
invasive mycoses (IM).
Methods: In retrospective analysis were included 18 HIV-infected patients at the AIDS
stage, who were hospitalized in 2018 in the Saint-Petersburg Botkin’s hospital. The
median age of patients was 38 years (28 - 67), men – 83%.
Results: The duration of HIV infection before the IM development was > 10 years in
44% patients, 5-10 years - 17%, < 5 years – 11%. IM developed before the antiretroviral
therapy in 28% patients. Viral hepatitis was concomitant infection in 56% patients,
other viral infections (cytomegalovirus, Epstein-Barr and herpes zoster) – 61%, bacterial
diseases – 44% (pneumonia – 39%, sepsis – 5%), pulmonary tuberculosis – 11%, toxoplasmosis
– 11%. Chronic renal failure was comorbid pathology in 33% patients. The main risk
factors of the IM development were CD3+CD4+ <200 cells/mcl – 100%, intensive care
unit stay – 67%, and glucocorticosteroid therapy > 21 days (median - 29 days) – 17%.
Pneumocystis jirovecii pneumonia (PjP) was diagnosed in 67% patients, cryptococcal
meningitis – 28%, combination of PjP and cryptococcosis – 5%. PjP was diagnosed ante
mortem in 77% patients. Therapeutic doses of co-trimoxazole were used in 60% PjP patients,
low doses – 40%. Cryptococcosis was diagnosed ante mortem in 83% patients. Amphotericin
B 50 mg/d was used in 67% of these patients(the median duration – 3,5 days (1 - 9),
fluconazole 600–800 mg/d – 23%. The overall 14 days survival rate of patients with
IM was 39%, 30 days – 28%, 12 weeks – 5,5%.
Conclusion: The main risk factor of invasive mycoses (P. jirovecii pneumonia – 67%,
cryptococcosis – 28%, combination – 5%) in HIV-infected patients was a decrease of
CD3 + CD4 + < 200 cells/mcl (100%). The overall 12-weeks survival rate on patients
with invasive mycoses was 5,5%.
Rodríguez-Leguizamón
G.
1
Coque-Burgos
E.
2
Espitia-Castro
O.
1
Firacative
C.
3
1Hospital Universitario Mayor - Méderi, Bogota, Colombia
2Compensar, Bogota, Colombia
3School of Medicine and Health Sciences, Universidad del Rosario, Bogota, Colombia
P300. rFatal case of Cryptococcus albidus fungaemia in an elderly immunocompromised
woman presented with pleural effusion
Case Report: Cryptococcosis is an important opportunistic fungal infection that occurs
globally and in the vast majority of cases is caused by Cryptococcus neoformans, a
basidiomycetous encapsulated yeast. Although this mycosis presents most frequently
as meningitis or meningoencephalitis, pulmonary disease is an underdiagnosed clinical
presentation, mainly due to nonspecific symptoms and broadly categorized findings.
Similarly to C. neoformans, Cryptococcus albidus is a free-living, encapsulated yeast
that resides in the environment, but that has been rarely recognized as a human pathogen,
as is the case for other non-neoformans cryptococci, and whose clinical relevance
remains unknown. Here we describe a fatal case of C. albidus fungaemia in an 85-year
old woman from Colombia, who presented with pleural effusion. The patient had an underlying
condition of type 2 diabetes mellitus (T2DM) and arterial hypertension and was admitted
to the hospital for hyperglycemia, presenting fatigue, polyuria, polydipsia, and nocturia.
On day 4 after admission, she had an episode of atrial fibrillation. A computed tomography
(CT) scan of the head showed an old lacunar infarction on the left superior cerebellar
artery and chronic ischemic microangiopathy. On day 7, hypoxia (85%) and another episode
of atrial fibrillation occurred. Chest radiography showed opacification of the left
hemithorax, cardiac silhouette was not visible, and prominent pulmonary hilar vessels
with atelectasis on the apical right lobe were observed. A chest and an abdominal
CT scans confirmed left-sided pleural effusion and showed an abdominal mass, which
suggested a metastatic pleural neoplasm. The condition of the patient deteriorated
and she died after 21 days of admission. C. albidus was recovered from blood culture
post-mortem and identified with VITEK®2 YST ID card and confirmed by MALDI-TOF MS.
This is the first report to describe a fatal case of cryptococcemia caused by a non-neoformans
species, C. albidus, in a non-HIV, yet immunocompromised female patient, who presented
with pleural effusion. Not only are cryptococcal infections of the pleura unusual
amongst the pulmonary manifestations but, interestingly, such infections caused by
a non-neoformans species are even rarer. With an incremental rise in the number of
at-risk patients having predisposing factors such as T2DM, the incidence of cryptococcal
infection due to non-neoformans species has also increased, with C. albidus and C.
laurentii accounting for 80% of reported cases. Although bloodstream and central nervous
system are the most common sites of infection of these infrequent pathogens, cases
of pulmonary infection are also present and are mostly manifested as chronic, indolent
illnesses and pulmonary involvement that includes pneumonia, lung abscess and empyema,
which may appear as pulmonary nodules, masses or infiltrates, mediastinal lymphadenopathy
or, less frequently, pleural effusions. Among the few cases of cryptococcal pleural
effusion that have been reported, only one was caused by a non-neoformans species,
specifically C. laurentii. This case of lethal fungaemia in an elderly woman highlights
the importance of considering not only the advanced age and the underlying diseases
of the patient, but also the emergence of new pathogens, including fungal species,
in order to give an appropriate and prompt diagnostic.
Bose
P.
1
Mccue
D.
1
Wurster
S.
1
Wiederhold
N.
2
Kadia
T.
1
Borthakur
G.
1
Ravandi-Kashani
F.
Masarova
L.
1
Konopleva
M.
1
Estrov
Z.
1
Takahashi
K.
1
Yilmaz
M.
1
Rausch
C.
1
Marx
K.
1
Qiao
W.
1
Huang
X.
1
Bivins
C.
1
Pierce
S.
1
Kantarjian
H.
1
Kontoyiannis
D.
1
1Ut Md Anderson Cancer Center, 1515 Holcombe, Houston, United States of America
2Pharmacy, The University of Texas Health Science Center at San Antonio, San Antonio,
United States of America
P301. Isavuconazole (ISAV) as Primary Anti-Fungal Prophylaxis (PAP) in Patients (pts)
with Acute Myeloid Leukemia (AML) or Myelodysplastic Syndrome (MDS): An Open-Label,
Prospective Study
Objectives: Mold-active antifungal prophylaxis (ppx) is recommended in neutropenic
pts with newly diagnosed AML or MDS. ISAV is an extended spectrum triazole with superior
tolerability, reliability of absorption, fewer drug-drug interactions, lack of QTc
prolongation or need for therapeutic drug monitoring, approved for the treatment of
invasive aspergillosis (IA) and mucormycosis. NCT03019939 is an investigator-initiated,
phase 2 trial of PAP with ISAV in pts with AML/MDS.
Methods: Treatment-naïve adult pts with AML or MDS initiating remission-induction
chemotherapy (RIC) received ISAV per the dosing recommendations in the US label until
recovery from neutropenia (neutrophils (ANC) ≥ 0. 5 x 109/L) and attainment of complete
remission (CR), occurrence of proven or probable invasive fungal infection (IFI, EORTC/MSG
criteria), or for a maximum of 12 weeks. The primary endpoint was incidence of proven/probable
IFI during the study period (up to 30 days from the last dose of ISAV).
Results: 67 pts were enrolled (April 28, 2017 to February 14, 2019) and 60 pts were
eligible for assessment (median age 67 years, 57 pts with AML, median ANC on enrollment
was 660). Reasons for study completion were achievement of CR with ANC recovery (n
= 35), completion of 12 weeks of PAP (n = 9), possible IFI (n = 7), investigator decision
(n = 3), death (n = 2, 1 disease progression, 1 cardiac arrest), proven/probable IFI
(n = 3), and mild transaminitis, possibly ISAV-related (n = 2). The median durations
of neutropenia and ISAV ppx were 33 (7-86) and 31 (7-86) days, respectively. One microbiologically-proven
(gluteal abscess due to Candida glabrata) and 2 cases of probable breakthrough IFIs
(probable IA with positive galactomannan) occurred (IFI incidence 5%). ISAV trough
serum concentrations were available in 31 pts on both day 8 (median 3. 74 µg/mL, 2.
03-7. 65) and day 15 (median 4. 10 µg/mL, 2. 17-9. 25), and were not significantly
different.
Conclusion: ISAV is a safe and effective alternative for PAP in pts with newly diagnosed
AML/MDS undergoing RIC, with a breakthrough (proven/probable) IFI rate of 5%. ISAV
serum levels were adequate in pts with AML/MDS undergoing RIC. Pharmacological features
make ISAV attractive for PAP in the era of recently approved or emerging small-molecule
AML therapies.
Dinikina
Y.
1
Shadrivova
O.
2
Belogurova
M.
1
Ignatyeva
S.
2
Bogomolova
T.
2
Khostelidi
S.
2
Boichenko
E.
3
4
Klimko
N.
2
1Department of Chemotherapy and Bone Marrow Transplantation For Children, Almazov
National Research Centre, Saint-Petersburg, Russian Federation
2Department of Clinical Mycology, Allergy and Immunology, North-Western State Medical
University named after I.I.Mechnikov, St. Petersburg, Russian Federation
3Pediatric Hemathology, City Clinical Hospital №1, Saint-Petersburg, Russian Federation
4Department of Pediatric Oncohematology, City Clinical hospital №1, Saint-Petersburg,
Russian Federation
P302. Retrospective analysis of invasive aspergillosis in pediatric patients with
acute myeloid leukemia
Objectives: To examine the epidemiological, clinical, and therapeutic features of
invasive aspergillosis cases in children with acute myeloid leukemia. To underline
the necessity of early recognition and initiation of antifungal treatment for the
infection control and possibility to continue anticancer treatment.
Methods: Retrospective analysis of IA cases in pediatric patients with AML without
stem cell transplantation (SCT) registered from 1997 to January 2019. For the probable
and proven IA diagnosis EORTC / MSG, 2008 criteria were used.
Results: 46 pediatric patients with malignancies were included, median age - 8 years
(1 -18), males – 69,5%. Hematological malignancies were underlying diseases in 39
patients (84,7%), solid tumors – 7 (15,2%). Most frequent hematological diagnoses
were myeloid leukemia - 16 (34,7%) and acute lymphoblastic leukemia (34,7%). Median
age of pts with AML – 11 years, males – 62,5%. IA was diagnosed after cytostatic chemotherapy
in 13 patients (81,3%), prior to chemotherapy in 3 (18,7%) pts. Median number of chemotherapy
courses before IA manifestation was 3. Two pts were without neutropenia at AML and
IA presentation. Risk factors were: prolonged neutropenia – 81,3%, median - 43,9 days,
and lymphocytopenia – 81,3%, median – 27,4 days. Severe bacterial infections were
diagnosed in 37,5% and cytomegalovirus infection in 18,7%. Main signs of IA were fever
(93,7%), cough (75%), respiratory failure (33,4%). Main site of IA were lungs (93,7%).
Disseminated IA was diagnosed in 2 pts (12,5%), endophthalmitis - 1 case (6,25%).
Typical findings on chest CT scan included the halo sign – 37,5%. Bronchoscopy with
bronchoalveolar lavage was performed in 7 (37,5%) pts. Aspergillus spp. were isolated
in culture in 12,5%: Aspergillus fumigatus – 6,25%, A.niger – 6,25%. Galactomannan
test was positive in 92,8%. According received results “probable IA” was diagnosed
in 87,5% of pts and “proven” in 12,5%. Antifungal treatment received 100% of patients,
with voriconazole only – 50%. Surgical treatment (splenectomy) was performed in 1
patient with disseminated IA. Combined antifungal therapy received 33,3% of pts. Overall
12-week survival was 87,5%. In all cases of IA before chemotherapy after infection
control anticancer therapy was continued.
Conclusion: Among children with IA and malignancies, the AML patients without SCT
account for 34%. The main risk factors of IA were prolonged neutropenia (81,25%) and
lymphocytopenia (81,25%). The main sites of IA were lungs. 12-weeks overall survival
was 87,5%. There were no contraindications for anticancer therapy resumption after
infection control.
Sabadin
C.
1
Lopes
S.
1
Gompertz
O.
1
Rigo
L.
2
Melo
A.
1
Barbosa
D.
1
1Federal University of São Paulo, São Paulo, Brazil
2Meridional Faculty – IMED, Passo Fundo, Brazil
P303. Species distribution and antifungal susceptibility profile of Candida spp. recovered
from liver transplant patients with oral candidiasis
Objectives: To identify by molecular method Candida isolates recovered from liver
transplant patients with oral candidiasis and to determine the antifungal susceptibility
profile.
Methods: In a prospective cohort study, 97 liver transplant patients from a hospital
in southern Brazil were followed up from June 2016 to September 2018. On clinical
examination of oral cavity 15 of them presented oral candidiasis and were included
in this study. Two oral swabs were collected from these 15 patients with interval
of 6 months apart (collections 1 and 2) to detect the presence of infection/colonization
by Candida spp. Patients who had candidiasis were treated with topical nystatin for
30 days. The swabs were cultured on CHROMagar® Candida and the yeast colonies were
genotypically identified by sequencing the intergenic transcribed sequence (ITS region)
of ribossomal DNA. The susceptibility test was performed with the following antifungal
agents: Fluconazole, Amphotericin B and Micafungin using broth microdilution method
for yeasts recommended by Clinical and Laboratory Standards Institute (CLSI), document
M27-A3.
Results: Among fifteen patients included in this study, eight(53.4%) presented oral
candidiasis episodes in Collection 1 and seven (46.7%) in Collection 2. In fact, all
these patients had isolates recovered in both collections, isolates from patients
without clinical manifestation were classified as colonization. of those, eight patients
who presented oral candidiasis in the Collection 1, five were atrophic candidiasis
(AC) caused by Candida glabrata(n = 3), C. albicans(n = 1) and C. tropicalis(n = 1),
and threepseudomembranous candidiasis (PC) caused by C. albicans(n = 2) and C. tropicalis(n
= 1). In the Collection 2, seven patients presented oral candidiasis, five were AC,
caused by C. albicans(n = 4) and C. dubliniensis(n = 1); two patients had PC caused
by C. albicans. The yeasts classified as colonizers were C. albicans(n = 9), C. glabrata(n
= 3), C. dubliniensis(n = 2) and C. tropicalis(n = 1). None of the patient had infection
or colonization by more than one Candida species. Regarding fluconazole susceptibility,
10 of 15 Candida isolates recovered from patients with infection were susceptible
(S), 4 isolates were susceptible-dose dependent (SDD) and 1 resistant (R). of those
recovered from colonized patients 12 were S, 2 were SDD and 1 was R (Table 1). All
isolates studied were susceptible to amphotericin B and micafungin.
Conclusion: The main type of oral candidiasis identified in hepatic transplant patients
was AC. Candida albicans was the most prevalent species identified in this study.
The presence of Candida spp. was constant in the oral cavity regardless clinical manifestation.
Most isolates were susceptible to antifungal agents, however, one C. tropicalis isolate
and one C. albicans were resistant to fluconazole.
Serris
A.
1
Danion
F.
1
Benzakoun
J.
2
Sonneville
R.
3
Wolff
M.
3
Bretagne
S.
4
Sharshar
T.
2
Jouvion
G.
5
Herbrecht
R.
6
Naggara
O.
2
Lortholary
O.
1
Lanternier
F.
1
1Infectious Diseases, Necker hospital, Paris, France
2Neuroradiologie, Sainte Anne Hospital, Paris, France
3Icu, Bichat hospital, Paris, France
4Mycology, Saint Louis hospital, Paris, France
5Pasteur Institute, Paris, France
6Hematology, CHU de Strasbourg, Strasbourg, France
P304. Cerebral Aspergillosis Lesion Study (CEREALS): a multicenter retrospective French
study
Objectives: Central nervous system (CNS) aspergillosis is a rare form of invasive
aspergillosis. The objective of this study is to describe the clinical, mycological,
radiological features and outcomes of the patients with of CNS aspergillosis.
Methods: A multicenter, retrospective study was performed involving adults and children
with a diagnosis of proven or probable cerebral aspergillosis according to the European
Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG)
definitions of invasive fungal infections (IFI) published in 2008, modified by adding
diabetes to the host criteria, between January 2006 and September 2018.
Results: 119 patients with CNS aspergillosis (54 proven, 65 probable) were included
in 20 French hospitals. Sixty-six percent were men and the median age was 58 years
[48-66]. Hematologic malignancies (39.5% of the patients), solid organ transplantation
(28.6%), diabetes (8.4%), autoimmune diseases requiring steroid or immunosuppressive
treatment (7.6%), cirrhosis (2.5%) and a history of neuro or sinus surgery (2.5%)
were the predominant comorbid conditions. Eleven patients (9.2%) had no identified
risk factors of IFI. Fever was present in 40.3% of the patients and 88% of them had
an abnormal neurological examination. Only 23.5% of the patients had a history of
neutropenia in the month preceding the diagnostic. Infection of the CNS occurred by
hematogenous dissemination from an extracerebral site in 77 patients (64.7%) and direct
extension from the paranasal sinuses in 27 patients (22.7%). Fifteen patients had
no obvious primary organ involvement. Hematogenous dissemination was more frequent
in patients with hematologic malignancies (68% of the patients) and solid organ transplantation
(79%) whereas contiguous extension was more common in patients with diabetes (60%,
p = 0.01). Sensitivity of β-D-glucan, galactomannan and aspergillus PCR in sera were
83.3%, 63.5% and 55.1% respectively. Galactomannan was positive in 68.9% of the cerebrospinal
fluids tested. A fungal co-infection was diagnosed in 10.9% of the patients. Seventy-two
percent of the patients were treated with voriconazole alone or in dual therapy as
a first line treatment. Twenty-one patients underwent neurosurgical procedures, including
abscess resection or drainage of abscesses and ventricular shunt. Forty-three percent
of the patients presented a complication of the surgery such as edema, bleeding or
nosocomial infection. Overall survival was 48.3% at 3 months and 37.7% at 12 months.
Contiguous infection of the CNS was associated with a lower sensitivity of galactomannan
antigen, a higher frequency of vascular complication and a better outcome. Patients
with hematologic malignancy had a poorer survival (p = 0.04). Although a selection
bias should be considered, the sub-group of patients who had had neurosurgery had
a better survival (p = 0,003).
Conclusion: CNS aspergillosis should be considered in patients with hematologic malignancies
and solid organ transplantation with or without neutropenia but also in patients with
less (such as diabetes or cirrhosis) or no known immunosuppression. Mortality among
CNS aspergillosis patients remains high. When feasible, neurosurgical procedures may
improve prognosis although with significant morbidity.
Candoni
A.
1
Farina
F.
2
Perruccio
K.
3
Blasi
R. Di
4
Criscuolo
M.
4
Cattaneo
C.
5
Delia
M.
6
Lazzarotto
D.
1
Dubbini
M.V.
1
Dragonetti
G.
4
Fanci
R.
7
Martino
B.
8
Principe
M.I. Del
9
Potenza
L.
10
Vianelli
N.
11
Chierichini
A.
12
Garzia
M.
13
Nadali
G.
14
Verga
L.
15
Busca
A.
16
Pagano
L.
4
1University Hospital, Asuiud, Division of Hematology and Stem Cells Transplantation,
Udine, Italy
2IRCCS San Raffaele, Milan, Italy
3Division of Pediatric Hematology, University Hospital of Perugia, Perugia, Italy
4Division of Hematology, IRCCS A Gemelli-University CSR, Rome, Italy
5Division of Hematology, Spedali Civili, Brescia, Brescia, Italy
6Division of Hematology, University of Bari, Bari, Italy
7Division of Hematology, University of Firenze, Firenze, Italy
8Division of Hematology, Hospital B. Melacrino Morelli, Reggio Calabria, Reggio Calabria,
Italy
9Division of Hematology, University “Tor Vergata”, Rome, Rome, Italy
10Division of Hematology, University of Modena and Reggio Emilia, Modena, Italy
11Division of Hematology, University of Bologna, Bologna, Italy
12Division of Hematology, San Giovanni Addolorata Hospital, Rome, Rome, Italy
13Division of Hematology, San Camillo Hospital, Rome, Rome, Italy
14Division of Hematology, University of Verona, Verona, Italy
15Division of Hematology, San Gerardo Hospital, Monza, Monza, Italy
16Division of Hematology, AOU Citta della Salute e della Scienza, Torino, Turin, Italy
P305. Impact of Invasive Aspergillosis occuring during remission-induction therapy
on outcome of Acute Myeloid Leukemia (SEIFEM 2012B Study).
Objectives: Acute myeloid leukemia (AML) patients are at high risk of invasive aspergillosis
(IA) and required a mould active antifungal prophylaxis during induction chemotherapy
(CHT). Although IA risk factors have been identified, few data are available on impact
of IA, occurring during first remission induction phase, on overall AML outcome.
Methods: The primary endpoint of this prospective, multicentric, case-control study,
was to evaluate if IA, occurring during first remission-induction CHT, can affect
treatment schedule and, consequently, patient overall survival (OS). We identified
40 AML patients (cases) who developed IA during induction phase, 31 probable (67%)
and 9 proven (33%) IA. These cases were matched with a control group (80 AML) without
IA, balanced according to age, type of induction CHT, AML characteristics and cytogenetic-molecular
risk factors. The overall response rate to induction CHT was the same in the 2 groups.
Results: In the 40 cases with AI, the overall response rate (ORR) to antifungal treatment
was favorable (ORR 80%) but it was significantly affected by the achievement of leukemia
complete remission (CR) with induction CHT. In fact, in cases with AML responsive
to induction CHT, complete responses of IA to antifungal therapy were 96% compared
to 21% in cases of AML not responsive to induction CHT (p <0.0001). The adherence
to schedule and full doses of CHT was reported in 35% of cases (14/40) and in 76%
of controls (61/80) (p = 0.0001). After induction CHT a significant higher number
of cases (15/40; 37.5%) compared to controls (21/80; 26%) could not receive additional
cycles of CHT (p = 0.01). The IFI related mortality was 22.5%. Comparing OS of 40
cases with the OS of the 80 controls, the median OS of cases was significantly worse
with a difference of 12.3 months (12.1 vs 24.4 months, p = 0.04-Figure 1A). However,
the occurrence of IA during induction phase did not have a significant impact on the
OS of cases who achieved a CR with induction CHT which are able to proceed, despite
the IA, with their intensive therapeutic program, achieving the same OS as the control
group with an AML in CR (p = ns-Figure 1B).
Conclusion: These data showed that IAs during induction CHT can delay the subsequent
therapeutic program and has a significant impact on OS of AML, specifically in those
patients with IA occurring during induction phase who did not achieved a CR of AML
with the first course of CHT (Figure 1C).
Irshad
M.
1
Nasir
N.
1
Hashmi
U. Haider
2
Farooqi
J.
3
Mahmood
S.F.
1
1Adult Infectious Diseases, Aga Khan University, Karachi-KHI, Pakistan
2Haemonc, Aga Khan University, Karachi-KHI, Pakistan
3Microbiology, Aga Khan University, Karachi-KHI, Pakistan
P306. Invasive pulmonary infection by Syncephalastrum: two case reports
Case Report: Invasive pulmonary infection by Syncephalastrum: two case reports Memoona
Irshad; Nosheen Nasir; Urooj Haider Hashmi; Joveria Farooqi; Syed Faisal Mahmood ABSTRACT
Mucormycosis also known as Zygomycosis is a class of fungi including three orders.
The largest order is Mucorlaes. Most common species of this order are Rhizopus and
Mucor but there are certain rare species such as Syncephalastrum spp. These are often
responsible for opportunistic fungal infections in immunocompromised patients. Syncephalastrum
racemosum is the species of the genus Syncephalastrum and is potentially fatal to
human beings. Although usually results in cutaneous infections and onychomycosis,
there have been few cases reports of respiratory and CNS infections in immunocompromised
hosts. Since this is a rare entity, so hardly any data is available on treatment response
to different antifungal agents. However recent improvements in diagnostic strategies
result in early identification and treatment. Amphotericin B is the drug of choice
due to its low MIC. Objective: To describe the clinical manifestations of the rare
fungal species Syncephalastrum Methods: Two cases of this rare fungus presented with
respiratory complaints during 2019 Case presentation 1: A 55 years old gentleman recently
diagnosed with Acute Myeloid Leukemia and not on treatment, presented with severe
shortness of breath in emergency. Patient was provisionally diagnosed with hospital
acquired pneumonia in view of recent hospitalization and was started on empirical
treatment with intravenous piperacillin tazobactam and vancomycin. However his symptoms
progressively worsened. Sputum cultures of this patient showed Methicillin resistant
Staphylococcus aureus and Syncephalastrum spp in a good quality specimen. Patient
was continued on intravenous vancomycin and started on intravenous conventional amphotericin
B. Case presentation 2: 73 years old gentleman with known comorbid of chronic obstructive
pulmonary disease for which he was on long term systemic and inhaled steroids, presented
with shortness of breath in emergency room. Chest radiograph showed bilateral infiltrates
with left sided cavity formation. Patient was started on empirical treatment for hospital
acquired pneumonia in view of his recent hospitalization. Sputum cultures of this
patient showed methicillin resistant Staphylococcus aureus, Aspergillus flavus and
Syncephalastrum spp in a good quality specimen. Patient was started on intravenous
conventional amphotericin B along with vancomycin and voriconazole. However he developed
amphotericin induced nephrotoxicity and doses were renally adjusted Result: Both cases
were started on amphotericin B, case 1 continued to deteriorate rapidly and expired
after 12 days of therapy. However case 2 is under treatment, patient developed amphotericin
induced nephrotoxicity so he is currently on renal adjusted dose of amphotericin B
and is currently doing well so we had different outcomes in two different patients
on same treatment. Conclusion: Invasive pulmonary infection by Syncephalastrum species
is a rare entity. It is usually identified and reported as an environmental contaminant
but when identified as a pathogen it can have fatal outcome in immunocompromised patients.
Due to rarity of this infection its course and treatment response are not completely
understood.
Jitmuang
A.
1
Wongkamhla
T.
1
Chongtrakool
P.
2
1Medicine, Siriraj Hospital Faculty of Medicine, Mahidol University, Bangkok, Thailand
2Microbiology, Mahidol University, Bangkok, Thailand
P307. A case report of Talaromyces marneffei pharyngo-laryngitis: A rare manifestation
of talaromycosis
Case Report:
Talaromyces marneffei is a dimorphic fungus predominantly endemic in Southeast Asia.
After effective antiretroviral treatment, the incidence of T. marneffei infection
in HIV-infected individuals has been decreasing. However, the rate of T. marneffei
infection is rising among non-HIV immunodeficient persons, particulary patients with
anti-interferon-gamma autoantibodies. of these, T. marneffei usually causes invasive
and disseminated infection including fungemia. T. marneffei pharyngo-laryngitis is
a very rare manifestation of talaromycosis. Herein, we demonstrate a case of T. marneffei
pharyngo-laryngitis in a patient who had underlying anti-interferon-gamma autoantibodies.
Case report A 52-year-old Thai woman has been diagnosed anti-interferon-gamma (IFN-ɣ)
autoantibodies for 4 years. Last 4 years, she presented with prolonged fever, weight
loss 10 kilograms, and multiple cervical lymphadenopathies. Tissue biopsy from left
cervical lymph node grew Mycobacterium abscessus. Accordingly, disseminated M. abscessus
infection was diagnosed. She denied using illicit drugs, herbal medicines, or corticosteroids.
Immunological studies including anti-HIV testing were all negative, but anti-IFN-ɣ
autoantibodies was highly positive. She received intravenous imipenem 2 g/day and
amikacin 750 mg/day for a primary anti-mycobacterial therapy, later they were switched
to oral clarithromycin 1,000 mg/day and linezolid 600 mg/day for a maintenance therapy.
Following the treatment, she was in remission, and still has been receiving the oral
maintenance therapy. Before this admission, she had sore throat, particularly more
painful at right side of pharynx, and hoarseness for 3 weeks. She also had febrile
and lost her weight 5 kilograms. She received oral amoxicillin 1.5 g/day from a primary
physician, but her symptoms did not resolve. She denied foreign body sensation, and
had no dysphagia, stridor, or difficult breathing. Physical examination revealed marked
swelling and hyperemia of both sides of tonsils including uvula and palatal arches,
and a single left submandibular lymph node, sized 1 cm. was identified. Indirect laryngoscopy
demonstrated moderate swelling of epiglottis, arytenoid, and vocal cord with normal
airway opening. There were no skin nodules or hepatosplenomegaly found. Blood chemistries
including plain chest radiography were unremarkable. The patient was performed right
tonsillar biopsy at 1 day following the admission. The tissue biopsy Gram’s stain
including the pathological sections exhibited few oval and elongated yeast-like organisms
with some central transverse septum seen, and it subsequently grew few mold colonies
with diffusible red-colored pigment on fungal cultures suggestive of T. marneffei.
An amphotericin B deoxycholate 45 mg/day was commenced as a primary therapy for 1
weeks, followed by an oral itraconazole 400 mg/day. Four weeks after the treatment,
the swelling of pharynx and larynx were markedly reduced. She had continued itraconazole
for 4 months, later the dose of 200 mg/day has been using for secondary prophylaxis.
Her symptoms completely resolved without complication. Conclusion: In patients with
anti-IFN-ɣ autoantibodies, T. marneffei can rarely cause a local infection involving
pharynx and larynx. Fungal culture and pathological examination are warranted for
diagnosis T. marneffei pharyngo-laryngitis. This condition requires a long term antifungal
therapy.
Kalkanci
A.
1
Tug
E.
2
Fidan
I.
1
Tunccan
O. Guzel
3
Ozkurt
Z.N.
4
Yegin
Z.A.
4
Sahin
E.A.
1
Kuralay
Z.
1
1Department of Medical Microbiology, Gazi University School of Medicine, Ankara, Turkey
2Department of Medical Genetics, Gazi University School of Medicine, Ankara, Turkey
3Department of Infectious Disease and Clinical Microbiology, Gazi University School
of Medicine, Ankara, Turkey
4Department of Internal Medicine, Division of Hematology, Gazi University School of
Medicine, Ankara, Turkey
P308. Retrospective analysis of single nucleotide polymorphisms (SNPs) on TLR-4, PTX-3,
Dectin-1 genes and the association with the development of invasive fungal infections
among hematopoietic stem cell transplant recipients with oncohematological conditions
Objectives:
Candida and Aspergillus are the two most common causes of invasive fungal infections
after stem cell transplantation. The development of fungal infections can be associated
with several risk factors however, it is still difficult to predict why certain individuals
develop severe infections, while others, under similar conditions, do not. Genetic
polymorphisms are recognized as potential factors that could affect in the progress
of the infectious disease. Pattern recognition receptors (PRR) are germline encoded
receptors that recognise a variety of pathogen associated molecular patterns (PAMPs)
expressed by an invading microorganism. Single nucleotide polymorphisms (SNPs) on
coding genes of PRRs; Toll like receptor-4 (TLR-4), dendritic cell-associated C-type
lectin-1 (Dectin-1) as a C-type lectin receptor (CLR) and among signaling molecules;
Pentraxin-3 (PTX-3) in the complement activation cascade were analysed in order to
show associations with the development of invasive fungal infections (IFIs) among
hematopoietic stem cell transplant recipients with hematological conditions.
Methods: In this case control study, a cohort of 62 patients with hematological malignancies
were assigned by the EORTC-MSG criteria. There were 9 proven, 7 probable,13 possible,
total 29 IFI patients and 33 patients with no evidence of fungal disease included
in this study from 2008 to 2018 in Hospital of Gazi University. DNA and RNA were isolated
from deposited bone marrow samples of the patients, using DNA and RNA Isolation kit
(SNP Biotechnology, Turkey), respectively. Expression levels of TLR4, Dectin-1 and
PTX-3 genes were determined by normalized to the Actin gene using the One-run RT-PCR
kit (SNP Biotechnology, Turkey). The polymorphisms of TLR4 (rs4986790, rs4986791),
Dectin-1 (rs16910526, rs7309123) and PTX-3 (rs2305619, rs3816527) were determined
by RT-PCR. Continuous variables are reported as means ± standard error mean (SEM),
while categori cal variables are reported as frequencies and percentages (%). The
odds ratios (ORs) and cor responding 95% confidence intervals (CIs) were calculated
by unconditional logistic regres sion analysis. The homozygote for the most frequent
allele was regarded as the reference group. SPSS statistical package, version 11.0
(SPSS Inc., Chicago, IL, USA) for Windows was used for statistical analyses. All p
values was considered to be statistically significant when p< 0.05.
Results:
TLR-4, PTX-3, Dectin-1 genes were down-regulated in IFIs group when compared to patients
with no evidence of fungal disease in the group of hematological conditions. A higher
expression of TLR-4 was revealed in controls (0.0626±0.032) compared to IFI patients
(0.0077±0.014), significantly different (p = 0.026), as well as PTX-3 in controls
(0.5265±0.0043) compared to patients (0.0043±0.004), (p = 0.035). Dectin-1 expression
was down-regulated in IFI group (0.1887±0.072 & 0.0655±0.010), however, not statistically
significant (p>0.05). Conditional logistic regression analyses indicated that the
GT genotype of rs16910526 polymorphism in Dectin-1 gene was associated with lower
risk of IFIs. (Odds ratio = 3.635, 95% confidence interval = 0.690-3.138, p = 0.04
Conclusion: Our study continues to highlight the important role of the host’s underlying
genetic profile in determining susceptibility to IFIs. Selective genetic immunodeficiencies
stratify “at-risk” patients to specific pathogens. Dissecting the contribution that
host genetic variation has on the susceptibility to fungal infection will be principal
for personalizing medicine in infectious diseases.
Lecointe
K.
1
Coulon
P.
1
Cornu
M.
1
Sendid
B.
2
1Laboratory of Mycology and Parasitology, University Hospital of Lille, LILLE, France
2Laboratory of Mycology and Parasitology, University Hospital of Lille, Lille, France
P309. Cell wall composition of Mucorales during spore germination: towards better
understanding of host-pathogen interactions.
Objectives: The molecular composition and structural organization of the cell wall
of filamentous fungi underlie the ability of the host to sense them as pathogen (Gow
et al. 2017). Although the organization of the fungal cell wall, composed of 90% of
polysaccharides (PS), is similar from one fungi to another, their expression on the
cell wall may condition their ability to trigger pattern recognition receptors. Deciphering
cell wall polysaccharide composition of filamentous fungi of medical concern can lead
to a better understanding of mechanisms governing their pathogenicity. Those patterns
of PS could also serve as biomarkers for the diagnosis of invasive fungal infections.
Because the incidence of Mucormycosis, an emerging life-threatening infection due
to Mucorales, is rising worldwide (Prakash and Chakrabarti 2019), we intend in this
study to characterize the structure of cell wall of Lichtheimia corymbifera (one of
the most clinically important mucorales) and how this fungus is able to interact with
the host’s immune system.
Methods: Two strains of L. corymbifera were used, one reference strain - IHEM 21658
- and one strain isolated from skin lesions in burn patient. Spores and germ tubes
were obtained from cultures on SDA-amikacin and liquid medium with yeast extracts,
mannitol and glucose, respectively. Monosaccharides composition (MC) analysis of the
cell wall was conducted on spores and germ tubes, on alkali-insoluble and alkali-soluble
fractions through gas-chromatography using flame - ionisation detector. The same analysis
was also performed on secreted extracellular polysaccharides obtained after an 8-day
culture in YNB with glucose. Determination of the glycosidic linkages was performed
on soluble oligosaccharides isolated sequentially after zymolyase and chitinase digestion.
Confocal microscopy after immunofluorescence staining with two lectins (Con-A and
WGA, binding mannose and N-acetyl-glucosamine -GlcNAc-, respectively), and one anti-β-1,3-glucan
monoclonal antibody (1A5), was performed to determine the parietal remodeling during
germination. THP-1- derived macrophages stimulated by various fungal fractions were
used to assess fungi-host interaction in terms of Toll-like receptor and cytokine
expression.
Results: MC of both strains showed a large amount of glucose (69.9%) with smaller
amounts of mannose (16.5%) and GlcNAc (12.8%) and traces of glucuronic acid (0.9%)
in spores. Enrichment using alkalino-treatment showed the presence of glucuronic acid
and galactose in the alkali-soluble fraction. Fucose appeared in germ tubes. Analysis
of extracellular polysaccharides showed the presence of fucose, glucose, mannose,
galactose and GlcNAc. Tagging the spores with Con-A and WGA revealed the presence
of mannose around spores, whereas mannose was spread more evenly in germ tubes. Chitin
emerged along the germ tube and β-1,3-glucan was present in swollen conidia.
Conclusion: During the first hours of L. corymbifera germination, fungal cells synthetize
polysaccharides composed chiefly by glucose, GlcNAc and mannose, and to a lesser extent
by galactose, glucuronic acid and fucose. The role of these latter carbohydrates remains
uncertain (Bartnicki-Garcia 1968). Confocal microscopy suggests that the emergence
of these carbohydrates leads to a parietal reorganization of the cell wall. In the
near future, data from the in-vitro model could help us to understand the impact of
these changes on the primary immune response.
Menu
E.
1
Criscuolo
A.
2
Desnos-Ollivier
M.
3
Cassagne
C.
4
5
Ranque
S.
4
6
Berger
P.
7
Dromer
F.
3
1AP-HM La Timone - IHU Méditerranée Infection, Marseille, France
2Hub De Bioinformatique Et Biostatistique – Département Biologie Computationnelle,
Usr 3756 Cnrs, Institut Pasteur, Paris, France
3National Reference Center For Invasive Mycoses & Antifungals, Molecular Mycology
Unit, Cnrs Umr2000, Institut Pasteur, Paris, France
4IHU-Méditerranée Infection, Marseille, France
5Vitrome, Aix Marseille UnivIRD, AP-HM, SSA, Marseille, France
6Vitrome, Aix Marseille Univ, IRD, AP-HM, SSA, Marseille, France
7Institut Paoli-Calmettes, Marseille, France
P310. rSaprochaete clavata outbreak in a Hematology Unit in Marseille, France
Objectives: Objectives:
Saprochaete clavata is a rare pathogenic yeast that has been involved in invasive
fungal infection outbreaks in immunocompromised patients especially in patient with
haematological malignancies. From January 2016 to January 2018, infections due to
S. clavata were diagnosed in nine patients at a cancer center in Marseille, France
raising the question of a common source and of the mode of transmission.
Methods: Methods: We retrospectively reviewed patients’ files to assess risk factors,
hospitalization units and treatments, and carried out an environmental survey (59
samples collected). Whole genome sequencing (WGS) was performed for 17 clinical isolates
collected during (n = 15) or before (n = 2) the outbreak and 10 environmental isolates.
The type strain (CBS425.71) and representative clinical isolates related (clade A)
or unrelated (clade B) to the French national outbreak in 2012 (Vaux et al., 2014)
were used as reference.
Results: Results: All nine patients were hospitalized at the stem-cell transplant
or hematological intensive care units. The median age was 58 years (range: 38 to 68)
and six (67%) were male. The most common underlying disease was acute myeloid leukemia
(56%) and 67% of patients were treated with cytarabine. A total of 40 samples were
found positive for S. clavata: blood (n = 35, 1 to 9 /8 patients), bronchoalveolar
fluid or tracheal aspirates (n = 5), and fecal swab (n = 1). The case fatality rate
at day 60 was 44%; death occurred at a median of 9.5 days after diagnosis. Saprochaete
clavata was also cultured from 10 environmental samples including one from a surface
in a patient’s room, 5 from the dishwasher (water outlet, inside and door seal) made
available to patients and families, and 4 others from pitchers (lids and inside of
2 milk and coffee thermos). In order to control the outbreak, all the contaminated
materials were replaced and the dishwasher was discarded. No new case was reported
after the implementation of corrective actions. Phylogenetic analysis based on WGS
data showed that all clinical and environmental isolates from Marseille belong to
the same clade which was unrelated to clades A and B previously identified in France.
of note, attempt to grow S. clavata failed at temperatures above 48°C.
Conclusion: Conclusion: Our results place the dishwasher as the source of the transmission
of S. clavata and suggest that its heating system may have been deficient. Whether
the source of S. clavata was a diary product as in previously reported outbreaks remains
unclear. Vaux, S., Criscuolo, A., Desnos-Ollivier, M., et al. (2014). Multicenter
outbreak of infections by Saprochaete clavata, an unrecognized opportunistic fungal
pathogen. MBio, 5(6), e02309-14.
Rammaert
B.
1
2
Maunoury
C.
3
Rabeony
T.
4
Elie
C.
4
Bakouboula
P.
4
Alfandari
S.
5
Berger
P.
6
Rubio
M.-T.
7
Braun
T.
8
Correas
J.-M.
9
Montravers
F.
10
Lortholary
O.
2
1Current Address: Infectious Diseases, CHU Poitiers, Poitiers, France
2Infectious Diseases, APHP, Hopital Necker-Enfants malades, Paris, France
3Nuclear Medicine, Hopital europeen Georges Pompidou, Paris, France
4Urc Necker-cochin, APHP, Paris, France
5Infectious Diseases, Centre hospitalier Tourcoing, Tourcoing, France
6Institut Paoli-Calmettes, Marseille, France
7Hematology, CHU Nancy, Nancy, France
8Hematology, APHP, hopital Avicenne, Bobigny, France
9Adult Radiology, APHP, hopital necker-enfants malades, Paris, France
10Nuclear Medicine, APHP, hopital tenon, Paris, France
P311. 18F-FDG PET-computed tomography to assess chronic disseminated candidiasis:
results of a French pilot prospective multicentric study (CANHPARI)
Objectives: Chronic disseminated candidiasis (CDC) occurs after profound and prolonged
neutropenia, and consists in persistent fever and multiple abscesses in liver and/or
spleen. International guidelines recommend protracted course of antifungal therapy,
although there is some evidence that CDC belongs to spectrum of immune reconstitution
inflammatory syndromes. The aim of this study was to assess 18F-fluorodeoxyglucose
positron emission tomography/computed tomography (PET/CT) in the diagnosis and follow-up
of CDC.
Methods: A pilot prospective study was conducted in 38 French centers from November
2013 to March 2017 (NCT01916057). Hematological adult patients having experienced
febrile profound (neutrophils <100/mm3) and prolonged (≥10 days) neutropenia, and
suspect for CDC on imaging (<10 mm multiple nodules on liver and/or spleen) were included.
PET/CT and abdominal conventional imaging (CT or MRI) were performed initially and
at 3 months (M3). MSG/EORTC criteria were adapted to assess response to treatment.
At M3, clinical success was defined by resolution of fever attributable to CDC, complete
biological response was defined by normal liver enzymes and CRP ≤20 mg/L, and complete
radiological response by disappearance of lesions compared to initial imaging. The
primary endpoint was the global response to antifungal treatment at M3 defined by
clinical success and extinction of hepatosplenic metabolic uptake on PET/CT.
Results: A total of 52 cases were included; 6 were secondarily excluded for alternative
diagnosis and 2 for absence of PET/CT at diagnosis. Among the 44 remaining cases,
CDC was proven in 7 (6 candidemia, 1 positive culture of liver biopsy), probable in
13, possible in 24. Patients were mostly male (55%), with a median age of 46 y.o.
(IQR 32-62). Among 43 underlying hematological disease, there were 46% acute myeloid
leukemia, 30% acute lymphoid leukemia, 17% allogenic and 2% autologous stem cell transplantation,
and 5% lymphoma. One patient had a solid cancer. Corticosteroids were prescribed in
47% of patients ≤ 1 month before CDC diagnosis, growth factors in 68%, antifungal
prophylaxis in 64%. Patients were colonized with ≥1 Candida species in 75% of cases
before CDC diagnosis, mainly C. albicans. At diagnosis, fever and liver enzyme abnormalities
were present in 81% and 82% of cases, respectively. C-reactive protein was elevated
in 85%. All patients were treated with antifungal drugs, combined with corticosteroids
in 41%. At M3, 25% (8/32) of evaluable patients met the primary endpoint criteria.
However, among 35 patients who had clinical success, 77% (27/35) had complete biological
response, but 83% (29/35) failed to respond on conventional imaging. Among these patients,
extinction of hepatosplenic metabolic uptake on PET/CT was observed in 17% (5/29).
Conclusion: Clinical improvement of CDC occurs before radiological normalization.
In patients with clinical success at M3, PET/CT could be more helpful than conventional
imaging to guide antifungal duration.
Bellanger
A.-P.
1
Berceanu
A.
2
Scherer
E.
3
Desbrosses
Y.
2
Daguindeau
E.
2
Rocchi
S.
1
Millon
L.
1
1Mycology, University Hospital Jean Minjoz, Besancon, France
2Hematology, University Hospital Jean Minjoz, BESANCON, France
3Mycology - Umr6249 Cnrs Chrono-environnement, University Hospital Besançon, Besançon,
France
P312. Invasive fungal disease with isavuconazole treatment failure diagnosed using
a combination of fungal biomarkers.
Case Report: Isavuconazole is the most recent antifungal azole available to treat
invasive fungal disease (IFD) and has been approved as first line treatment for invasive
aspergillosis (IA) and as alternative treatment for mucormycosis. We present two cases
of IFD with isavuconazole treatment failure in acute myeloid leukemia (AML) patients
with prolonged neutropenia 100 days after hematopoietic stem cell transplantation
(SCT) and severe digestive graft-versus-host disease (GVHD). The first patient was
diagnosed with a probable invasive aspergillosis and a TR34/L98H azole resistant Aspergillus
fumigatus and the second patient was diagnosed with a mixed Aspergillus fumigatus-Rhizomucor
sp co-infection.The IFD were diagnosed using a combination of fungal biomarkers, applied
systematically and including the galactomannan antigen (GM) detection and fungal qPCRs,
targeting both A. fumigatus and Mucorales; this strategy proved to be optimal. These
cases highlight the interest of applying a close surveillance using fungal biomarkers
in very immunocompromised patients under antifungal treatment. Interestingly, the
fungal biomarkers were alternatively positive. Moreover, these cases also raise several
questions such as for example the influence of severe digestive GVHD on isavuconazole
levels.
Datcu
R.
1
Risum
M.
1
Helweg-Larsen
J.
2
Kampmann
P.
3
Overgaard
U.M.
3
Nielsen
O.J.
3
Munksgaard
L.
4
Rubek
N.
5
Hansen
L.V.
6
Hare
R.
1
Arendrup
M.C.
1
7
8
1Mycology Unit, Statens Serum Institute, Copenhagen, Denmark
2Department of Infectious Medicine, Rigshospitalet, Copenhagen, Denmark
3Department of Hematology, Rigshospitalet, Copenhagen, Denmark
4Department of Hematology, Roskilde Sygehus, Roskilde, Denmark
5Department of Otorhinolaryngology, Rigshospitalet, Copenhagen, Denmark
6Department of Orthopaedic Surgery, Rigshospitalet, Copenhagen, Denmark
7Rigshospitalet, Dept. of Clinical microbiology, Copenhagen, Denmark
8University of Copenhagen, Dept. of Clinical Medicine, Copenhagen, Denmark
P313. Six consecutive patients surviving invasive Mucormycosis: a result of improved
interdisciplinary management?
Objectives:
Mucorales infections are life-threatening, with mortality rates approaching 100% in
disseminated cases. ESCMID/ECMM guidelines strongly recommend surgical debridement,
liposomal amphotericin B, L-AmB (≥5 mg/kg/day), and reversal of predisposing conditions.
We introduced a multidisciplinary and aggressive management approach for invasive
mucormycosis. Here we present data on six patients managed by this approach, whom
all had a successful outcome.
Methods: Management principle: repeated surgical debridement until biopsies taken
from the resection margins were clean, defined by negative Blankophor microscopy,
Mucorales specific PCR (both reported within 24h) and cultures. Cultured isolates
underwent ITS sequencing for species identification and EUCAST E.Def 9.3.1 susceptibility
testing. Targeted antifungal therapy (mono/combination) combined with topical therapy
(when possible) was given according to the MIC (mg/L) and severity of infection. High
trough azole levels above MIC were ensured by therapeutic drug monitoring (TDM) (mg/L).
Fungal free survival was evaluated by case record review.
Results:
Demographic, microbiological, management and outcome data for the six patients are
summarised in the Table. All were neutropenic after chemotherapy except patient #5
at time of diagnosis. Patient #1 developed Lichtheimia ramosa/corymbifera infection
in the liver (PCR). Management included isavuconazole and surgery. Patient #2 developed
sino-orbital Rhizopus microsporus infection. The patient had smoked cannabis. Therapy
included surgery, L-AmB and isavuconazole. Isavuconazole MIC: 1 mg/L. Patient #3 developed
Rhizopus arrhizus infection extending extensively from the hard palate (picture).
The patient had smoked marihuana. Treatment consisted of unilateral hemimaxillectomy
followed by frequent supplementary debridements guided by PCR of resection margins.
The exposed bone was covered during palatal reconstruction with a free flap after
several mycosis negative debridements. Antifungal therapy included L-AmB and isavuconazole
(target TDM initially: >2 mg/L, then >6-8 mg/L until negative diagnostics followed
by maintenance >2 mg/L (isavuconazole MIC: 1 mg/L). Patient #4 developed Rhizopus
arrhizus sinuses infection during posaconazole prophylaxis. Management included L-AmB
and surgery, followed by isavuconazole (isavuconazole MIC: 2 mg/L). Patient #5 developed
disseminated Rhizomucor pusillus (lumbar spondylitis, a subdiaphragmatic and a lung
abscess). Surgical debridement including removal of vertebrae L4 in toto and surrounding
muscle and soft tissue. The spine was then stabilized with cage and pedicle screws.
PET-CT afterwards showed regression of the spondylitis. Target TDM for isavuconazole
>2 mg/L. Patient #6 developed Lichtheimia corymbifera sinus infection. Despite targeted
posaconazole oral solution aiming a target for TDM >2 mg/L (MIC: 0.25 mg/L), the infection
progressed to nasopharynx, orbit and brain. The patient recovered after surgical and
triple-compound systemic and amphotericin B intrathecal antifungal therapy1. Outcome:
At follow-up 6-70 months after diagnosis, patient #1-4 and #6 were cured of infection,
and patient #5 has had no sign of relapse (7 months follow-up).
Conclusion: Our data suggest improved outcome of Mucorales infections by a multidisciplinary
approach including: 1) Repeated (except patient #5) biopsy-controlled surgery until
resection boarders are negative, 2) Systemic and in most cases combination, TDM-adjusted
high dose systemic antifungal therapy, and 3) Topical therapy when possible to improve
antifungal exposure in tissue not sufficiently perfused due to the angioinvasive pathology
of the infection. 1Jensen, TSR et al. J Pediatr Hematol Oncol. 2017;39(4):211-215.
Cruz
S. Matos
1
Silva
L.
2
Catorze
G.
2
Sabino
R.
3
Verissimo
C.
3
Toscano
C.
1
1Microbiology Laboratory, Hospital Egas Moniz - CHLO, Lisbon, Portugal
2Dermatology Department, Hospital Egas Moniz - CHLO, Lisbon, Portugal
3Reference Unit For Parasitic and Fungal Infections, Infectious Diseases, National
Health Institute Dr. Ricardo Jorge, Lisbon, Portugal
P314. Majocchi’s Granuloma by Trichophytum rubrum in a kidney transplant patient -
A Case Report
Case Report:
Introduction: Trichophytum rubrum is a filamentous fungus, with worldwide distribution,
that usually causes superficial infections of skin and nails, namely tinea pedis,
tinea corporis, tinea cruris and onychomycosis. Rarely, severe dermatophytosis can
occur, presenting as deep dermatophytosis, Majocchi’s Granuloma or extensive dermatophytosis.
Objectives and Methods: Case report of Majocchi’s Granuloma in a kidney transplant
patient.
Results: A case of a 55-year-old woman who underwent a kidney transplant 7 months
before, under immunosuppressive therapy with tacrolimus and mycophenolate mofetil.
She attended a Dermatology consultation to clarify skin lesions that appeared 6 months
earlier. The skin exam revealed hard and painful plaque lesions on both legs, with
an ulcer on the left leg lesion, violaceous papular lesions on the dorsum of the left
foot and toes and a hard consistency nodule on the left leg. Some of the toe nails
presented dystrophy or onycholysis. The patient denied any previous trauma or contact
with plants or soil. Biopsies of lesions of the left leg and foot dorsum where sent
for histology and mycological culture and toe nails for mycological culture. The histological
examinations showed, in the reticular dermis and reaching the hypodermis, suppurative
granulomas with multinucleated giant cells and areas of necrosis. PAS (Periodic Acid-Schiff)
and GMS (Grocott’s Methenamine Silver) staining revealed multiple spores and septate
hypha within the granulomas but not in the stratum corneum. No remnants of hair follicles
where found. Culture of skin biopsies were positive for Tricophytum rubrum but nails´
culture was negative. Identification was further confirmed by sequencing of ITS region
of ribosomal DNA (GenBank accession number MK967277). Oral Itraconazole 100mg bid
and topic Sertoconazole where initiated. The patient was observed one month after
and reported general malaise, tiredness, exertional dyspnea, whitish stools and increased
abdominal volume. The physician chose to discontinue itraconazole and initiate oral
terbinafine 250mg id. After two months on oral terbinafine, there was regression of
the legs´ and left foot lesions with ulcer healing and disappearance of the left leg
nodule. Conclusion: Diagnosis of deeper dermatophytosis is difficult, in part because
there is no specific clinical presentation and, in many cases, it is even polymorphic.
However, especially in patients with immunodeficiency, this hypothesis should be weighed.
Confirmation is achieved by finding hyphae compatible with dermatophytes in the dermis
and a positive culture for a dermatophyte. Treatment should include systemic antifungal
agents, to which topical medication may be associated. Multiple therapeutic regimens
have been proposed, but randomized trials or large case series are lacking. Antifungal
therapy should be continued until the lesions are completely resolved. Surgical treatment
has been reported as an option for highly localized lesions.
Jenks
J.
1
Seidel
D.
2
Cornely
O.A.
3
Kauffman
C.
4
Miceli
M.
5
Slavin
M.
6
Chen
S.
7
Mehta
S.
1
Reed
S.
1
Hoenigl
M.
1
1Medicine, University of California San Diego, San Diego, United States of America
2Department I of Internal Medicine, Ecmm Excellence Centre of Medical Mycology, Cologne
Excellence Cluster On Cellular Stress Responses In Aging-associated Diseases (cecad),
University Hospital Cologne, Cologne, Germany
3Department I of Internal Medicine, Ecmm Excellence Centre of Medical Mycology, Cologne
Excellence Cluster On Cellular Stress Responses In Aging-associated Diseases (cecad),
German Centre For Infection Research, Partner Site Bonn-cologne, Clinical Trials C,
University Hospital Cologne, Cologne, Germany
4University of Michigan, Ann Arbor, United States of America
5Medicine, University of Michigan, Ann Arbor, United States of America
6Medicine, University of Melbourne, Parkville, Victoria, Australia
7Medicine, The University of Sydney, Camperdown, New South Wales, Australia
P315. Clinical Characteristics and Outcomes of Lomentospora prolificans Infections
in Fungiscope™ Registry
Objectives:
Lomentospora prolificans are filamentous fungi commonly found in soil and polluted
waters and are emerging opportunistic pathogens in immunocompromised individuals,
accounting for 2.4% and 11.1% of non-Aspergillus invasive fungal infections (IFIs)
in haematopoietic stem cell transplant (HSCT) and solid organ transplant (SOT) infections,
respectively. The objective of this study was to describe clinical manifestations,
treatment and outcomes of patients with L. prolificans infections causing IFI in cases
documented in Fungiscope™ - A Global Emerging Fungal Infection Registry, created in
2003.
Methods: We performed a retrospective review of medical records of all patients with
IFIs caused by L. prolificans in Fungiscope™ between 01/01/2008 - 12/31/2017 (10 year
period). Infections were determined to be disseminated if L. prolificans was isolated
from the blood or two non-contiguous anatomic sites. The study was approved by the
UCSD Human Research Protection Program (IRB #181119).
Results: A total of 37 cases with IFIs caused by L. prolificans, including 7 cases
from the University of California San Diego, were identified (Table 1). In all but
one case (36/37, 97.3%), a risk factor for IFI was found, including hematologic malignancy
(20/37, 54.1%), solid organ transplant (SOT) (3/37, 8.1%), ICU stay or trauma (6/37,
16.2%). The majority of infections were diagnosed based on positive culture (35/37,
94.6%) or histopathologic findings plus culture (5/37, 13.5) and most patients had
signs of IFI on CT scan (15/20, 75%), MRI (1/2, 50%), or CXR (1/2, 50%). At final
assessment, 19/37 (51.3%) were deceased, 8/37 (21.3%) had complete cure, and 8/37
(21.6%) and partial resolution or stable disease. of those who received antifungal
therapy following diagnosis and had an evaluable outcome (31/37, 84%), the majority
were treated with combination antifungal therapy (24/31, 77%; 54% survived). Most
antifungal regimens included voriconazole (VRC; 28/31, 90%; 54% survived) and/or terbinafine
(TRB; 19/31, 61%; 63% survived). Among those receiving a combination of both VRC and
TRB (+/- other antifungal agents) 11/17 (65%) survived, while 5/14 (36%) survived
among those receiving other treatments (p = 0.11). Overall, 7/37 patients (18.9%)
received surgery, including 5/7 enucleation or vitrectomy and two extensive debridement
(1 died, 5 survived, 1 with unknown outcome). Table 1.
Conclusion: In this study, the majority of patients had a significant underlying risk
factor for infection including hematologic malignancy, SOT, ICU admission, or preceding
trauma. Despite the use of combination antifungal therapy, the overall mortality rate
from invasive L. prolificans infection was 51%, with a trend towards lower infection-related
mortality (35%) in those receiving a combination treatment including both VRC and
TRB.
Ananda-Rajah
M.
1
2
Seah
J.
2
Liu
M.
3
Haffari
G.
3
Bergemeir
C.
3
Peel
T.
1
1Infectious Diseases, Alfred Health, Melbourne, Australia
2Alfred Health, Melbourne, Australia
3It, Monash University, Clayton, Australia
P316. Making continuous prospective surveillance of invasive mold diseases real-time
in hospitals using artificial intelligence
Objectives: Surveillance, audit and feedback of invasive mold diseases (IMD) is fundamental
to antifungal stewardship (AFS) but current methods are inefficient and restrictive.
Continuous prospective surveillance of IMD in all haematology patients has not been
possible because the majority of these infections present as a culture negative pneumonia.
Artificial intelligence (AI) targeting medical imaging provides an opportunity to
identify fungal pneumonia in real-time in order to present actionable data at the
point of care. Here we report preliminary results of real-world prospective validation
of deep learning based natural language processing (NLP) of chest imaging reports
for real-time detection of IMD in haematology patients that is part of a multicentre
clinical trial (Clin Trials.gov NCT03793231). Objective: To validate NLP of chest
computed tomography reports for prospective surveillance of IMD in all hospitalised
haematology patients. As a validation study, NLP alerts are not being fedback to clinicians
but rather the machine is being validated against human surveillance. Presented here
are preliminary results from one activated site, Alfred Health, a major quaternary
Australian transplant centre.
Methods: Prospective active manual and electronic surveillance of invasive fungal
diseases (IFD) among hospitalised patients under the haematology service commenced
on 1 December 2018. Hospitalised patients with IFD were identified by research coordinators
through active case finding every week. All suspected IFD cases were reviewed again
at 30 days and data collected to determine if they met internationally accepted criteria
for IFD diagnosis (EORTC/MSG 2008 definitions). Chest CT reports were prospectively
processed by NLP hosted on the hospital server and predictions were compared to IFD
cases detected by active surveillance.
Results: From 1 December 2018 to 31 April 2018, there were 8 patients with suspected
IFD identified. of these, one case was excluded because it did not meet internationally
accepted criteria for IFD, resulting in 7 patients with confirmed IFD associated with
the following characteristics: all had IMD; all had pulmonary site of infection; 2
cases were probable/proven; 2 possible cases had a positive Aspergillus PCR on bronchoalveolar
lavage. There were 6 cases of breakthrough IMD on antifungal prophylaxis involving
posaconazole in 4, liposomal amphotericin in 1 and fluconazole in 1 case. of 90 chest
CT reports screened by fungalAi-NLP over the study period, 6 of the 7 IFD patients
were correctly flagged. The case missed by NLP was an intubated patient with probable
pulmonary invasive Aspergillosis associated with isolation of A. terreus. The NLP
output in this case had probabilities of 37.8% and 41.0% on serial reports which was
below the 50% threshold for triggering a positive alert. The radiologist’s conclusion
was not definitive in either report. There were 10 (11%) false positive reports. NLP
is tuned for fungal pneumonia so may miss extra-pulmonary sites. False positives could
be minimised with multimodal analysis combining other sources of data.
Conclusion: Real-time, embedded, NLP of chest imaging reports facilitates surveillance
of diagnostically challenging IMD for an entire haematology population. NLP utilises
diagnostic imaging readily available in hospitals rather than the electronic medical
record. Generalisability of NLP will be enhanced by our multicentre clinical trial.
Melekhina
J.
1
Borzova
Y.
2
Ignatyeva
S.
2
Desyatik
E.
3
Bogomolova
T.
4
Ziuzgin
I.
5
Vasilyeva
N.
2
Klimko
N.
1
1Department of Clinical Mycology, Allergology and Immunology, North-Western State
Medical University named after I.I. Mechnikov, Saint-Petersburg, Russian Federation
2Kaschkin Research Institute of Medical Mycology, Department of Medical Microbiology,
North-Western State Medical University named after I.I. Mechnikov, Saint-Petersburg,
Russian Federation
3Kashkin Research Institute of Medical Mycology, North-Western State Medical University
named after I.I.Mechnikov, St. Petersburg, Russian Federation
4North-Western State Medical University named after I.I. Mechnikov, St. Petersburg,
Russian Federation
5FSBI «Petrov Research Institute of Oncology» of the Ministry of Healthcare of the
Russian Federation, Saint-Petersburg, Russian Federation
P317. The case of successful treatment of disseminated cryptococcosis in a patient
with Non-Hodgkin’s lymphoma
Case Report: Objectives. Clinical case of successful treatment of disseminated cryptococcosis
in patient with Non-Hodgkin’s lymphoma. Methods. The EORTC/MSG 2008 diagnostic criteria
were used. Results. Patient B., 32-year-old, was hospitalized in a city hospital in
June 2016. Non-Hodgkin’s lymphoma was diagnosed. He received 8 cycles of R-CHOP therapy,
two cycles of R- DHAP with peripheral blood stem cell transplantation, one cycle of
GDP protocol and after achieving partial remission, the patient was consolidated with
peripheral blood stem cell transplantation using the BEAM conditioning regime. In
September 2017, hematological remission was achieved. In October 2018 an infiltrate
17x12 mm in the right lung S4 was revealed on computed tomography (CT) scan. Surgical
removal the lung lesion was performed. Histological examination revealed granulomatous
inflammation, and culture was positive with Cryptococcus neoformans. Positive “Crypto
+” test in blood serum and cerebrospinal fluid was detected and diagnosis disseminated
cryptococcosis was established. The patient was treated with amphotericin B 50 mg/day
for 14 days, followed by fluconazole 800 mg/day with positive effect. In February
2109 the decrease of infiltration and the reduction of the cavity were detected on
chest CT scan. Cryptococcal antigen test was negative in the blood serum and in the
cerebrospinal fluid. The total duration of antimycotic therapy was six months. Conclusion.
Disseminated cryptococcosis is possible in patients with Non-Hodgkin’s lymphoma.
Kandemir
O.
1
Tombak
A.
2
Koyuncu
M. Bakır
3
Şahinoğlu
S.
3
Türker
A.
3
Horasan
E. Sahin
4
1Infection Dieseases, Mersin University, mersin, Turkey
2Hematology, mersin university, MERSIN, Turkey
3Mersin University, MERSIN, Turkey
4Infection Diseases, mersin university, mersin, Turkey
P318. Comparison of fluconazole and posaconazole based antifungal prophylaxis in patients
with newly diagnosed acute myeloid leukemia
Objectives: Antifungal prophylaxis with posaconazole during remission induction chemotherapy
of patients with newly diagnosed acute myeloid leukemia has been shown to decrease
invasive fungal infections (IFI) and increase overall survival. However, real-life
studies about this issue is not much.
Methods: This was a retsopective cohort study incluiding a total of 58 patients with
acute myeloid leukemia who were received intensive remission induction therapy. 27
patients received fluconazole prophylaxis between January 2013 and January 2015, 31
patients received posaconazole prohylaxis between February 2015 and February 2017.
Results:
Demographic characteristics of the patients were similar in both groups (Table 1).
At day 100, patients receiving posaconazole had a significantly lower incidence of
possible IFIs (45.2% vs 74.1%, p = 0,026). There was no significant difference in
100-day overall mortality (32.3% in the posaconazole group vs. 25.9 % in the fluconazole
group, p = 0,597), breakthrough IFIs (31.3% in the posaconazole group vs. 29.6 % in
the fluconazole group, p = 0,829) and death during active treatment (44.4% in the
posaconazole group vs. 32.3% in the fluconazole group, p = 0,340) (Table 2).
Conclusion: Posaconazole prophylaxis decreased the incidence of IFIs but did not improve
short-term overall survival.
Pereira
A.
1
Santos
D. Langanke Dos
2
Alvares-Saraiva
A.
2
Hurtado
E.
2
Alvarez
J.
2
Lallo
M.
2
1Biomedicina, Centro Universitário São Camilo, São Paulo, Brazil
2Post-graduation Program In Environmental and Experimental Pathology, Paulista University,
São Paulo, Brazil
P319. B-1 cell deficiency decreases the population and function of dendritic cells
in murine encephalitozoonosis
Objectives: Microsporidia are obligate intracellular pathogens belonging to the kingdom
Fungi, relatively common enteric microorganisms that usually result in self-limited
or asymptomatic infections in humans. Although protection against microsporidia is
predominantly dependent on the onset of a strong T cell response, dendritic cells
(DCs) play a critical role in stimulating T cell response through pattern recognition
receptors. Previous studies have suggested that DCs can regulate B cell activity and
Encephalitozoon intestinalis infection blocks the differentiation of DCs, thus compromising
the immune response against the pathogen, characterizing an evasion mechanism. The
present study aimed to evaluate the relation between B-1 cells and DCs of Peyer’s
patches (PPs) in mice infected with Encephalitozoon cuniculi, a species of microsporidia.
Methods: BALB/c mice, BALB/c Xid mice (deficient in B-1 cells) and Xid+B-1 (B-1 cells
adoptive transfer to mice) were infected or not with E. cuniculi orally and the same
immunosuppressed with cyclophosphamide (Cy) because it is an opportunistic pathogen.
After 21 days of the infection, the DCs of PPs were quantified by flow cytometry.
Results: Infected mice had a lower population of DCs on PPs than uninfected animals
(Figure 1). This result suggests the ability to block DC differentiation for the pathogen
E. cuniculi. The BALB/c mice, with higher resistance to E. cuniculi infection, had
the highest DCs populations when compared to the other groups (Figure 1). Xid animals
showed the smallest populations of DCs, with the exception of the Xid animals that
received B-1 cell transfer. MHC II expression decreased in Xid animals. Cy induced
an increase in the DCs population in PPs from uninfected BALB/c mice in relation to
the other uninfected groups, indicating an immunomodulatory effect of the drug.
Figure 1. Evaluation of dendritic cells present in the Peyer’s Patches from BALB/c,
BALB/c XID and XID+B-1 mice inoculated (+) or not (-) with E. cuniculi. A) Percentage
of CD11c+ dendritic cells in non-treated mice. B) Geometric mean of the fluorescence
intensity of the MHC II molecule in dendritic cells CD11c+. C) Percentage of CD11c+
dendritic cells. Two-way ANOVA analysis with Tukey post-test revealed # statistically
significant differences and p <0.05*, versus BALB XID and versus XID+B-1, respectively
(A). Data were transformed for statistical analysis, analysis of two-way ANOVA with
Tukey test post revealed p <0.05* versus BALB c.
Conclusion: These data suggest that B-1 cell deficiency compromises the population
and the DCs response of PPs in E. cuniculi infection.
Hegarty
F.
Microbiology, Mater Misericordiae University Hopsital, Dublin, Ireland
P320. rEvaluation of the Wako™ β-D-Glucan assay in Patient Serum for the Diagnosis
of Invasive Aspergillosis.
Objectives: Invasive Aspergillosis is a fungal disease that can carry a high morbidity
and mortality, especially in those patients who are immunosuppressed. Early diagnosis
of Invasive Aspergillosis (IA) is key to implementing early treatment and improve
patient prognosis. Currently in MMUH, serum β-D-Glucan (BDG) is available as an external
referral test only (average TAT 109.5 days). Expanding our current repertoire of tests
to include BDG, would greatly aid in the early diagnosis of IA. Delay or misdiagnosis
of IA can result in drug toxicity due to inappropriate treatment, high medical expenses
and mortality. Given the complexity of invasive fungal diseases a number of tests
are required for correct diagnosis. Therefore implementation of another test that
can quickly and accurately aid in the diagnosis of IA would have great benefits for
overall patient care, whilst limiting unnecessary use of antifungal therapy within
the hospital. Aim: To evaluate the Wako™ β-D-Glucan assay in the laboratory and its’
implications for clinical practice. To assess the contribution this test can make
to patient care
Methods: Fifty-one random patient samples (serum) were used to complete this pilot
study. Samples included ICU, lung transplant and haematology patients. Positive and
negative controls were included weekly. The Wako™ β-D-Glucan was evaluated as per
the manufacturer’s guidelines
Results: The results obtained from the BDG were compared to external referral results.
They were also analysed in conjunction with previous microbiological cultures, galactomannan,
OLM™ lateral flow device calcofluor/KOH results, histological specimens, radiological
imaging and clinical details where applicable. EORTC criteria were used to categorise
patients into proven, probable or possible IA. BDG results demonstrated 83% sensitivity
and 100% specificity as well as a positive predictive value of 100% and a negative
predictive value of 88%.
Conclusion: Based on results of this study, Wako™ β-D-Glucan will be introduced in
MMUH, for patients with suspected IA, or those severely immune-suppressed.
Hegarty
F.
Microbiology, Mater Misericordiae University Hopsital, Dublin, Ireland
P320. rEvaluation of the Wako™ β-D-Glucan assay in Patient Serum for the Diagnosis
of Invasive Aspergillosis.
Objectives: Invasive Aspergillosis is a fungal disease that can carry a high morbidity
and mortality, especially in those patients who are immunosuppressed. Early diagnosis
of Invasive Aspergillosis (IA) is key to implementing early treatment and improve
patient prognosis. Currently in MMUH, serum β-D-Glucan (BDG) is available as an external
referral test only (average TAT 109.5 days). Expanding our current repertoire of tests
to include BDG, would greatly aid in the early diagnosis of IA. Delay or misdiagnosis
of IA can result in drug toxicity due to inappropriate treatment, high medical expenses
and mortality. Given the complexity of invasive fungal diseases a number of tests
are required for correct diagnosis. Therefore implementation of another test that
can quickly and accurately aid in the diagnosis of IA would have great benefits for
overall patient care, whilst limiting unnecessary use of antifungal therapy within
the hospital. Aim: To evaluate the Wako™ β-D-Glucan assay in the laboratory and its’
implications for clinical practice. To assess the contribution this test can make
to patient care
Methods: Fifty-one random patient samples (serum) were used to complete this pilot
study. Samples included ICU, lung transplant and haematology patients. Positive and
negative controls were included weekly. The Wako™ β-D-Glucan was evaluated as per
the manufacturer’s guidelines
Results: The results obtained from the BDG were compared to external referral results.
They were also analysed in conjunction with previous microbiological cultures, galactomannan,
OLM™ lateral flow device calcofluor/KOH results, histological specimens, radiological
imaging and clinical details where applicable. EORTC criteria were used to categorise
patients into proven, probable or possible IA. BDG results demonstrated 83% sensitivity
and 100% specificity as well as a positive predictive value of 100% and a negative
predictive value of 88%.
Conclusion: Based on results of this study, Wako™ β-D-Glucan will be introduced in
MMUH, for patients with suspected IA, or those severely immune-suppressed.
Hegarty
F.
Microbiology, Mater Misericordiae University Hopsital, Dublin, Ireland
P321. Evaluation of OLM™ Lateral Flow Device (LFD) in Bronchoalveolar Lavage for the
Diagnosis of Invasive Aspergillosis
Objectives: Invasive Aspergillosis is a fungal disease that can carry a high morbidity
and mortality, especially in those patients who are immunosuppressed. Early diagnosis
of Invasive Aspergillosis (IA) is key to implementing early treatment and improve
patient prognosis. While respiratory samples may culture Aspergillus, it is essential
to differentiate between active infection verses colonisation of the lung. This differentiation
can often be difficult as well as time consuming. This potential for delay in treatment
commencement could contribute to a poor outcome, or if treated incorrectly may expose
the patient to unnecessary antifungal therapy. Therefore utilisation of a test that
can reliably differentiate between colonisation and active infection would have benefits
for patient care. Aim: To evaluate the OLM™ LFD in the laboratory and implications
for clinical practice. To assess the contribution this test can make to patient care
Methods: Twenty random patient samples (bronchoalveolar lavage) were used to complete
this pilot study. Nine samples were Aspergillus culture positive, fifteen samples
were Aspergillus culture negative and two samples NEQAS controls; Aspergillus sp.
and Trichophyton sp. culture positive. The OLM™ LFD was evaluated as per the manufacturer’s
guidelines, which included the pre-treatment steps as required
Results: The results obtained from the LFD analysed in conjunction with previous microbiological
cultures, calcofluor/KOH results, histological specimens, radiological imaging and
clinical details where applicable. LFD results demonstrated 100% sensitivity and 85%
specificity as well as a positive predictive value of 71% and a negative predictive
value of 100%. The LFD detected the presence of Aspergillus in three samples which
was missed in the previously carried out culture and calcofluor/KOH. In this instance,
Aspergillus infection was suspected from patient scans, which nicely correlated with
the LFD result.
Conclusion: Based on results of this study, OLM™ LFD will be introduced in MMUH, for
patients with suspected IA, or those severely immune-suppressed.
Shagdileeva
E.
1
Shadrivova
O.
1
Chudinovsky
J.
2
Motalkina
M.
2
Zyuzgin
I.
2
Bogomolova
T.
3
Vybornova
I.
3
Ryabinin
I.
3
Hohlova
A.
2
Artemeva
A.
2
Klimko
N.
1
1North-West State Medical University named after I.I. Mechnikov, Saint-Petersburg,
Russian Federation
2N.N. Petrov National Medical Research Centre of Oncology, Saint Petersburg, Russian
Federation
3Kashkin Research Institute of Medical Mycology, North-Western State Medical University
named after I.I.Mechnikov, St. Petersburg, Russian Federation
P322. The first case of invasive candidiasis caused by two pathogens (Candida albicans
and Candida glabrata) in a patient with Hodgkin’s lymphoma
Case Report: Woman with HL, 32 years old, in severe condition was admitted to the
Petrov Oncology National Medical Medical Research Centre for cytotoxic chemotherapy
on 23.01.2019. Risk factors for IC were central venous catheter (CVC), broad-spectrum
antibacterial drugs therapy, prolonged neutropenia, and glucocorticosteroids treatment.
The patient didn’t receive antifungal prophylaxis. Clinical manifestation of IC was
body temperature > 38 °C, refractory to antibiotic use. Diagnosis of invasive candidiasis
was confirmed with blood culture – C.albicans with susceptibility to fluconazole and
voriconazole in vitro. Pathogen identification was determined by MALDI-TOF mass spectrometry.
In the first 24 hours after diagnosis antifungal therapy (caspofungin) was used, and
CVC was removed. Anaphylactic reaction with angioedema developed after caspofungin
administration, and liposomal amphotericin B (7 days), then fluconazole (7 days) and
amphotericin B deoxyholate (6 days) were used. Death was caused by septic shock after
20 days of antifungal therapy. On the autopsy IC with lungs, liver, spleen, and skin
involvement was found. Culture from the autopsy materials was identified as C.glabrata
with dose-dependent susceptibility to fluconazole in vitro.
Conclusion. In the treatment of invasive candidiasis in immunocompromised patients,
the possibility of several pathogens should be considered.
Antonets
A.
1
Kit
O.
2
Kutsevalova
O.
3
Panova
N.
3
Miroshnichenko
D.
4
Popovyan
E.
5
Homenko
O.
6
1Laboratory of Molecular Oncology, Rostov Research Institute of Oncology, Rostov-on-Don,
Russian Federation
2Rostov Research Institute of Oncology, Rostov-on-Don, Russian Federation
3Laboratory of Clinical Microbiology, Rostov Research Institute of Oncology, Rostov-on-Don,
Russian Federation
4Clinical Pharmacology Department, Rostov Rostov Regional Clinical Hospital №2, Rostov-on-Don,
Russian Federation
5Pulmonology Department, Rostov Rostov Regional Clinical Hospital №2, Rostov-on-Don,
Russian Federation
6Hepathology Department, Central City Hospital №1 named N.A. Semashko, Rostov-on-Don,
Russian Federation
P323. Pulmonary aspergillosis in patient with autoimmune hepatitis: the report of
unusual case
Case Report: Objectives: To present the case of immunocompromised patient who was
diagnosed with pulmonary aspergillosis in a short period of time after established
diagnosis of autoimmune hepatitis. Methods: We examined 69-year-old man who had spitting
blood, cough, signs of abscess pneumonia in x-ray examination after 5 weeks of immunosuppressive
therapy with decreasing dose of glucocorticoids that was prescribed after the second
episode of bilirubinemia when diagnosis of autoimmune hepatitis was established after
scrutinizing diagnostic search. Sputum was analyzed with standard and fluorescent
microscopy with white calcofluor and cultural method. Standard methods were applied
to exclude tuberculosis and aspergillosis. Galactomannan (GM) detection was performed
with immunoenzymatic assays XEMA GaIMAg EIA kit (Russia). Positive level of GM accounted
for ≥0.59 OD (optical density). Results: Cultural methods revealed the presence in
sputum E.coli (107) and A.flavus (105) (pic.1).
GM level initially was positive in blood (0.73 OD). Antifungal voriconazol therapy
was started immediately. One week after starting treatment the second cultural testing
of nose crust showed A.flavus (104) (pic.2).
GM was negative in blood and urine (0.41 OD and 0.14 OD respectively), and positive
in bronchoalveolar lavage fluide (BALF) accounting for 2.34 OD. Futher cultural testing
did not detect A.flavus. GM level was positive only in BALF. Spiral computed tomography
revealed several aspergillomas, the size of the largest one was 70x50 mm. The patient
did not have leukopenia at any time from the first episode of hepatitis, however slight
lymphopenia was observed. One month after diagnosis of pulmonary aspergillosis pneumothorax
and secondary bacterial complications occurred. Despite this the patient has demonstrated
positive clinical effect continuing antifungal and antibiotic therapy (in accordance
with the sensitivity of microorganisms) with accompanying therapy by the third month
since the diagnosis of aspergilllosis. The remission of autoimmune hepatitis has been
observed. Conclusion. It should be taken into consideration that immunocompromised
patients even without leukopenia/agranulocytosis and short period of immunosuppressive
therapy are at risk of development fungal infection. GM testing demonstrates its usefulness
for early diagnosis Aspergillus spp. A comprehensive approach to diagnosis and alertness
of doctors for fungal infection can improve the detection of cases of aspergillosis.
Ito
R.
1
Ibrahim
K.
1
Bonazzi
P.
1
Junior
J. De Almeida
2
Santiago
M.
1
Silva
A.C. Da
1
Vieira
M.
1
Freire
M.
1
Silva
D.
3
Martins
M.
3
Szeszs
M.
3
Bonfietti
L.
3
Pereira
J.
1
Rocha
V.
2
Diz
M.D.P.
1
Hoff
P.
1
Melhem
M.
3
Abdala
E.
1
1Instituto do Cancer do Estado de Sao Paulo, Sao Paulo, Brazil
2Hospital da Clinicas da Universidade de Sao Paulo, Sao Paulo, Brazil
3Instituto Adolfo Lutz, Sao Paulo, Brazil
P324. Challenges in the management of fusariosis in oncology patients: a single-centre
series of 27 cases
Objectives: This study aims to describe clinical, epidemiological and laboratorial
aspects of fusariosis in cancer patients.
Methods: We retrospectively identified patients from an oncology hospital with proven
fusariosis, according to the EORTC/MSG modified criteria, since January 2011 to January
2018. Demographic, clinical and laboratorial data from patients were obtained. Mortality
rates at 30 and 90 days were evaluated.
Results: Twenty-seven cases were identified. Median age was 55 years and 15 were female.
Most patients had haematological malignancies (81.5%) and neutropenia (88.9%). All
patients received chemotherapy and/or corticosteroids in the last 3 months. Eighteen
had disseminated disease, 7 had fungaemia alone and 2 had localized skin infection.
Out of 18 patients with disseminated fusariosis, 13 had skin involvement, 8 had pulmonary
disease and 5 had sinusitis. Fourteen patients received a lipid formulation of amphotericin
B and voriconazole, 10 amphotericin B alone and 3 died before treatment. Species identification
was performed in 10 isolates, and all of them were from F. solani species complex.
Six isolates had susceptibility profile to antifungals and all had MIC ≥8 mg/L to
azoles; two isolates had MIC ≥2 mg/L to amphotericin. Mortality rates at 30 and 90
days were 51.9% and 63.0%, respectively. At 90 days, no patients with solid tumor
or fungaemia had survived
Conclusion: Haematological malignancies, neutropenia, and the use of immunosuppressive
therapies were the conditions most associated to fusariosis in cancer patients. Disseminated
disease was the most common clinical presentation. Most patients received combined
therapy, once the best therapeutic option has not yet been defined, and due to identification
of isolates with high MICs to azoles and amphotericin. Mortality rates were higher
in solid tumor patients and those with fungaemia alone.
Dunbar
A.
1
Schauwvlieghe
A.
2
Algoe
S.
1
Hellemond
J. Van
3
Reynders
M.
4
Vandecasteele
S.
5
Boelens
J.
6
Depuydt
P.
7
Rijnders
B.
1
1Department of Infectious Diseases, Erasmus University Medical Center Rotterdam, Rotterdam,
Netherlands
2Department of Hematology, Erasmus Medical Center, Rotterdam, Netherlands
3Medical Microbiology, Erasmus Medical Center, Rotterdam, Netherlands
4Clinical Microbiology, AZ St-Jan Brugge-Oostende Hospital, Bruges, Belgium
5Nephrology and Infectious Diseases, AZ St-Jan Brugge-Oostende Hospital, Bruges, Belgium
6Laboratory Medicine, Ghent University Hospital, Ghent, Belgium
7Intensive Care, Ghent University Hospital, Ghent, Belgium
P325. Epidemiology of Pneumocystis jirovecii pneumonia and (non-)use of prophylaxis
in three tertiary care hospitals.
Objectives: Pneumocystis jirovecii pneumonia (PCP) is caused by the fungus Pneumocystis
jirovecii (PJ) and is almost exclusively diagnosed in severely immunocompromised patients.
While in the recent past the vast majority of PCP-cases were diagnosed in patients
with HIV, this is no longer the case in resource-rich countries with unrestricted
access to HIV care. The benefit of prophylaxis against PJ has been convincingly demonstrated
for patients with HIV and a CD4 T-cell count <200 cells/mm but the benefit of prophylaxis
is less well established for other patient groups. This study aimed to describe the
epidemiology of PJP in recent years and assess how many patients with PCP did or did
not receive appropriate prophylaxis in the months preceding the infection.
Methods: A multicenter retrospective study was performed in 3 Belgian and Dutch tertiary
care centers: Erasmus MC, University Medical Center in Rotterdam, AZ Sint-Jan in Bruges
and UZ Gent in Ghent. A list of patients that underwent broncho-alveolar lavage sampling
for PCP testing with an in-house PJ PCR was retrieved from the 3 microbiology labs.
An in-house PJ PCR was used in each of the centers with a cycle threshold (Ct) value
of <29 (Rotterdam and Bruges) or <31 (Ghent) considered a
probable
PCP. For patients with a positive PJ PCR but above this threshold a predefined case
definition was used to classify patients as follows: a
possible
case of PCP had to fulfill all 3 of the following criteria; 1. PCR Ct value <35 and
2. Clinico-radiological diagnosis compatible with PCP and 3. Patient died or patient
received PCP therapy and survived. Patient files from all PCR+ patients were reviewed
to determine whether the patient fulfilled the PCP case definition and if antimicrobial
prophylaxis had been provided prior to PCP. Disease specific guidelines (NIH AIDS,
ECIL, ASOT, local hospital guidelines) were used to evaluate if prophylaxis was indicated.
Fisher’s exact test, t-test or Mann-Whitney U tests were used as appropriate.
Results: From 2012 to 2018, 482 patients with positive PCR PJ of respiratory samples
were reviewed. 278 were excluded mostly because CT value was>35 (n = 247). Other reasons
were age<18, insufficient clinical data available, other respiratory sample than BAL.
of the remaining 204, 153 had a probable (n = 90) or possible (n = 63) PCP. The remaining
51 were considered colonized (Table 1). PCP patients were HIV negative in 74%. Only
11 (7%) of PCP cases had received prophylaxis, despite that 133 (87%) had an indication
for prophylaxis according to guidelines.
Conclusion: The vast majority of patients diagnosed with PCP had not received prophylaxis
and therefore the infection could be considered at least partially preventable. In
regions where HIV testing and treatment is available without restrictions, PCP is
mainly diagnosed in non-HIV immunocompromised patients. Strategies to improve awareness
of and compliance with antimicrobial prophylaxis guidelines in immunocompromised patients
are urgently needed.
Millon
L.
1
Caillot
D.
2
Berceanu
A.
3
Gbaguidi-Haore
H.
4
Letscher-Bru
V.
5
Dalle
F.
6
Denis
B.
7
Bretagne
S.
8
Alanio
A.
9
Boutoille
D.
10
Morio
F.
11
Lanternier
F.
12
Bougnoux
M.-E.
13
Botterel
F.
14
Chouaki
T.
15
Charbonnier
A.
16
Ader
F.
17
Dupont
D.
18
Rocchi
S.
19
Scherer
E.
19
Herbrecht
R.
20
1Parasitology - Mycology - Umr6249 Cnrs Chrono-environnement, University Hospital
Besançon, Besançon, France
2Clinical Haematology, University Hospital of Dijon, Dijon, France
3Hematology, University Hospital Jean Minjoz, BESANCON, France
4Infectious Risk Control, University Hospital Jean Minjoz, BESANCON, France
5Parasitology-mycology, University Hospital of Strasbourg, Strasbourg, France
6Parasitology-mycology, University Hospital Dijon, Dijon, France
7Tropical and Infectious Diseases Department, Lariboisière Saint-Louis Fernand Widal
Hospital, Assistance Publique-Hôpitaux de Paris (AP-HP), PARIS, France
8Mycology, Saint Louis hospital, Paris, France
9Paris-diderot, Sorbonne Paris Cité University, Institut Pasteur, Molecular Mycology
Unit, Cnrs Cmr2000, Parasitology-Mycology Laboratory, Lariboisière Saint-Louis Fernand
Widal Hospitals, Assistance Publique-Hôpitaux de Paris, Paris, France
10Tropical Infectious Diseases, University Hospital of Nantes, Nantes, France
11Ea1155 Iicimed, Nantes University Hospital, NANTES, France
12Infectious Diseases, Necker hospital, Paris, France
13Parasitology Mycology, APHP Hôpital Necker-Enfants-Malades, Paris, France
14Parasitologie-mycologie, CHU Henri MONDOR, Créteil, France
15Parasitology-mycology, University Hospital of Amiens, Amiens, France
16Haematology, University Hospital of Amiens, Amiens, France
17Infectious Diseases, University hopital of Lyon, Lyon, France
18Parasitology and Medical Mycology / Umr 5292, Université Lyon 1 / Hospices Civils
de Lyon, Lyon, France
19Mycology - Umr6249 Cnrs Chrono-environnement, University Hospital Besançon, Besançon,
France
20Hematology, CHU de Strasbourg, Strasbourg, France
P326. Prospective evaluation of Mucorales DNA qPCR detection in serum for early diagnosis
of mucormycosis: First results from the ModiMucor study
Objectives: Early initiation of specific treatment is essential to improve prognosis
of mucormycosis. Several retrospective studies have reported that qPCR detection of
Mucorales DNA in serum could anticipate the diagnosis of mucormycosis by an average
of 8 days in hematological patients and in critically ill burn patients1-4. Modimucor
is a French prospective multicentre study (9 university hospitals) that aims to assess
the performance (sensitivity, specificity, positive and negative predictive values
and likelihood ratios) of the circulating DNA detection test for the diagnosis of
mucormycosis in comparison to CT scan imaging, histopathology, microscopy and culture.
Methods: Patients with risk factors for invasive mold infection (IMI) such as patients
with hematological diseases, burns diabetes, solid organ transplantation, trauma,
or diabetes, and patients diagnosed with mucormycosis were enrolled prospectively.
Serum samples were collected twice-a-week, and Mucorales PCR were performed at the
time of serum sampling, in each center, as previously described1. Clinical data and
biological data were collected up to 6 months after enrollment. An interlaboratory
quality control was done to ensure uniformity of the assays. Data analysis was performed
using STATA 14 software.
Results: Were included 250 patients with suspicion of invasive fungal infection from
January 2015 to June 2017 (Strasbourg n = 156; Dijon n = 39; Besançon n = 21; APHP
Saint Louis n = 10; APHP Necker n = 9; Nantes n = 9; APHP Mondor n = 3; Amiens n =
2; Lyon n = 1). Classification of patients according to EORTC/MSG criteria was as
follows: 47 probable (n = 13) or proven (n = 34) mucormycoses, 64 probable or proven
aspergilloses, 5 other probable or proven IMI (fusariosis n = 2; scedosporiosis n
= 2; histoplasmosis n = 1). A very low interlaboratory variation of the Mucorales
PCR results was observed (SD = 1,8 cycles [range 1.3; 2.9]). At least one serum with
positive Mucorales PCR was found for 35/47 patients with proven or probable mucormycosis.
Among the 12 PCR-negative patients, 4 were given liposomal amphotericin B for more
than 10 days at the date of serum sampling, and 3 patients had only a positive direct
examination, without positive culture. Twenty-two patients with possible IMI had at
least one positive Mucorales PCR. Sensitivity, specificity, positive and negative
predictive values were 74.5% (35/47), 89.2% (181/203), 61.4% (35/57) and 93.8% (181/193),
respectively. In addition, positive and negative likelihood ratios were 6.8 and 0.28,
respectively. The first PCR-positive sample was observed in average 6.5 days [range
0-22; median = 4] before the sampling date of the first mycological positive specimen,
and 7 days [range 0-44; median = 4] before the first imaging criteria.
Conclusion: This prospective study confirms the good performance of serum Mucorales
PCR for early diagnosis of mucormycosis. This assay with an acceptable time frame
and a reasonable cost helps to anticipate the diagnosis of mucormycosis and is becoming
essential in the clinical diagnosis of mucormycosis. 1.Millon L. Clin Infect Dis,
2013; 2.Millon L. Clin Microbiol Infect 2016; 3.Caillot D. Open Forum Infect Dis,
2016; 4.Legrand M. Clin Infect Dis 2016.
Adebiyi
I.
1
2
Ogunleye
V.
2
1Department of Medical Microbiology, University College Hospital, Ibadan, Ibadan,
Nigeria
2Department of Medical Microbiology, University College Hospital, Ibadan, Nigeria
P327. Cryptococcal Infections among Human Immunodeficiency Virus /Acqiiref Immune
Deficiency Syndrome (HIV/AIDS) Patients Accessing HAART Treatment in Ibadan
Objectives: Cryptococcosis occurs in man and animals in almost all the regions of
the world. People with AIDS are particularly vulnerable to this infection. In particular,
a higher mortality due to the infection is said to be associated with a CD4+ count
less than 200cells/μL. Although Nigeria has the second highest number of people living
with HIV globally, there is paucity of data on the prevalence of cryptococcal infections
among this people. This study aimed to determine the burden of cryptococcal infections
among HIV/AIDS patients accessing highly active antiretroviral therapy (HAART) treatment
in Ibadan as well as determine the CD4+ count associated with cryptococcal infections
in this locality.
Methods: Adult male and female HIV/AIDS patients accessing HAART treatment at the
President’s Emergency Plan for AIDS Relief (PEPFAR) clinic in Ibadan with CD4+ count
less than 200cells/μL were enrolled into the study. Other HIV/AIDS patients with CD4+
count higher than 200cells/μL but who had a high index of cryptococcal meningitis
were screened at the attending physician’s request. A cryptococcal antigen test was
performed on blood samples from patients with CD4+ count less than 200cells/μL and
CSF samples from patients referred by the attending physician. The CSF samples were
also cultured on Sabouraud Dextrose Agar culture media to isolate the organism.
Results: 200 HIV/AIDS patients were enrolled in the study. The mean CD4+ count was
145.2cells/μL and 49% of participants were newly diagnosed and had not commenced HAART.
The overall prevalence of cryptococcosis was 2%. All positive samples were from patients
referred by the attending physician. All positive samples were from patients with
CD4+ higher than 200cells/μL. 100% of patients with cryptococcosis were already receiving
HAART.
Conclusion: Cryptococcosis was found in HIV/AIDS patients with CD4+ count higher than
200cells/μL. Hence timely and appropriate cryptococcal screening in HIV/AIDS patients
could help reduce cases of and mortality due to cryptococcal infection and also guide
treatment needs. Cryptococcal screening should always be implemented for HIV/AIDS
patients with low CD4+ counts regardless of symptoms or receipt of HAART to identify
asymptomatic infected patients. It is also important to promptly screen HIV/AIDS patients
with CD4+ count higher than 200cells/μL with a high index of cryptococcal meningitis.
Mhamdi
G.
1
Kilani
M.
1
Abdelmalek
R.
1
Berriche
A.
1
Kilani
B.
1
Ammari
L.
1
Benaissa
H. Tiouiri
1
Kallel
A.
2
Kallel
K.
2
Chelly
I.
3
Haouet
S.
3
Boukriba
S.
4
Mizouni
H.
4
1Department of Infectious Diseases, The Rabta hospital, Tunis, Tunisia
2Department of Parasitology-mycology, The Rabta hospital, Tunis, Tunisia
3Department of Anatomopathology, The Rabta hospital, Tunis, Tunisia
4Department of Radiology, The Rabta hospital, Tunis, Tunisia
P328. Disseminated phaeohyphomycosis associated to Tuberculosis in a pregnant woman
Case Report: Introduction: The phaeohyphomycosis are superficial or deep mycosis that
occur due to pigmented frequently opportunistic fungus. We are reporting a case of
co-infection, deep pheaohyphomycosis and tuberculosis in a 7 months pregnant patient
unknowingly immunosupressed. Observation: The patient is Mrs Y.S. 24 years old, admitted
for convulsions. The diagnosis of cerebral and lymph node tuberculosis was held because
of right hemi-body convulsions, a positive quantiferon, an evocative lymph node aspiration
cytology and the presence of tuberculomas on the cerebral MRI. The patient received
a quadruple antitubercular treatment. In two months, there was recurrence of the seizures
and persistance of a left abscessed cerebral lesion with an important edema contrasting
with the disappearance of a big number of tuberculomas in MRI control. The examination
found a discreet right mowing, no meningeal syndrome and a firm cervical lymph node
of 1,5 cm. The HIV serology was negative. We have maintained the antitubercular treatment
and associated a corticosteroid therapy. A complement of surgical exeresis was realised
for treatment and diagnosis purposes. She developed two cervical renitent lymph nodes
and new seizures. The CT Scan identified a numerous parenchymal pulmonary nodes some
of which are excavated, many necrotic cervical lymph nodes and the reappearance of
the cerebral lesion at surgical site. The lymph node puncture aspirated greyish pus
containing mycelian filaments. The molecular biology identified Arthrocladium. The
anatomopathology study of cerebral abscess confirmed both tubercular and mycosic co-infection.
We have associated Amphotericin B and Itraconazole. The evolution was slowly favorable.
Conclusion: phaeohyphomycosis is a rare pathology with a difficult diagnosis. The
prognosis is dark for the systematic forms. The treatment is medico-surgical.
Armstrong-James
D.
1
Bercusson
A.
1
Gonzales
L. Herta
1
Warris
A.
2
1Nhli, Imperial College London, London, United Kingdom
2Mrc Centre For Medical Mycology, University of Aberdeen, Aberdeen, United Kingdom
P329. Mechanisms of Ibrutinib-dependent susceptibility to invasive aspergillosis
Objectives: Patients receiving Ibrutinib, a small molecule inhibitor of Bruton’s tyrosine
kinase (Btk), are at increased risk of invasive and disseminated aspergillosis. Given
the increasing use of this drug as front-line treatment for haematological malignancies,
better understanding of the mechanistic basis for this risk is urgently required.
Whilst Btk is recognised as an important tyrosine kinase in B cell receptor signalling
pathways, we and others have shown an important role for Btk in macrophage-based phagocytic
responses to Aspergillus fumigatus and Candida albicans. Furthermore, Ibrutinib is
also known to inhibit other TEC kinases such as TEC, which is important for macrophage
responses to Candida albicans. Our objective was to characterise the impact of Ibrutinib
on macrophage behaviour during A. fumigatus exposure.
Methods: Human monocyte-derived and alveolar macrophages, or murine J774A.1 macrophages,
[AB1] were infected with A. fumigatus after treatment with Ibrutinib and the impact
on phagocytosis, cell signalling, fungal killing and cell death responses assayed
using a combination of imaging, western blotting and cell death kinetic assays. Selected
findings were confirmed to be Btk-specific by siRNA.
Results: We show that Ibrutinib is a potent inhibitor of both NFAT and NFκB responses
in human macrophages during infection with A. fumigatus. We show that A. fumigatus
induces human macrophage Btk phosphorylation and that Btk depletion impairs NFAT and
NFκB responses in human macrophages. Furthermore, Ibrutinib strongly inhibited macrophage
necrosis in response to infection. Our findings in a human in vitro model suggest
Btk involvement in a TLR9-dependent endosomally driven pathway in accordance with
previous findings in our murine model. How this mediates fungal dissemination is currently
unknown.
Conclusion: These observations suggest that defects in macrophage Btk signalling contribute
to susceptibility to pulmonary aspergillosis. We are currently developing in vivo
models to further study the effects of ibrutinib on outcomes from pulmonary aspergillosis.
Marini
C.
Chorão
P.
Bergantim
R.
Aguiar
E. Vale
Pires
J. Cancela
Trigo
F.
Centro Hospitalar Sao Joao, Porto, Portugal
P331. Ten year experience with fungal Infections in Acute Myeloid Leukemia in the
Posaconazole Era
Objectives: Posaconazole prophylaxis in patients with acute myeloid leukaemia (AML)
changed the paradigm of fungal infections (FI). AIMS: Description of FI and analysis
of factors that might predispose to this type of infection in non-allotransplant AML
patients in the posaconazole era.
Methods: Retrospective analysis of 217 consecutive non-M3 AML patients diagnosed between
June 2008 and June 2018 in a tertiary center and treated with intensive chemotherapy
regimens, including autologous transplantation when performed in these patients. Posaconazole
prophylaxis was introduced since January 2009. Multivariate regression analysis was
performed to identify characteristics related to FI.
Results: Median age at diagnosis was 51 years [19;71], 52% were females and all patients
had an age-adjusted Charlson score ≤3. Genetic risk group according to the European
Leukemia Net 2017 classification was favourable in 24%, intermediate in 43% and adverse
in 22%. Twelve percent of patients (n = 26) had secondary AML. Patients were included
from completed remission-induction intensive therapy up to transplant referral, the
latter which occurred in 48% of patients (n = 104), with a global median follow-up
of 7 months [1;132]. Autologous transplantation was performed in 15% of patients (n
= 34). Fluconazole prophylaxis was administered in 7 patients, all others received
posaconazole. There were 20 patients that had episodes of prophylaxis interruption,
14 during auto-transplantation, 3 due to toxicity and 3 out of our protocol. During
treatment, patients with no relapse presented a global median time of neutropenia
of 69 days [26;421] whereas relapsed patients had a median of 104 days [30;356]. Ten
percent of patients had >2 episodes of prolonged neutropenia (defined as ≥28 consecutive
days of <500 neutrophils/microliter). Fungal infections were identified in 41 patients
(19%), 3 of which were in the non-posaconazole group, and classified according to
EORTC criteria, with 13 proven, 17 probable and 10 possible infections. In 32% of
patients FI occurred during remission-induction (n = 13) of which 3 were diagnosed
in the first week of neutropenia, 19% during consolidation treatment in first remission
and 49% during relapse treatment (n = 20). of the confirmed infections, 5 were aspergillosis,
3 candidemia, 2 by Fusarium and 1 mucormycosis. Probable infections had pulmonary
origin with no microbiology isolate or serologic positivity but identified by typical
radiologic evidence; of these, broncho-alveolar lavage and/or pulmonary biopsy was
performed in 6 patients (35%) with negative results. For possible infections, antifungal
therapy was initiated in a pre-emptive manner for high risk patients with persistent
fever despite large spectrum antibiotics. In a multivariate analysis for the posaconazole
group, patients with FI had a higher odds of having relapsed AML, refractory AML at
first approach and >2 episodes of prolonged neutropenia during treatment (p<0.01,
p = 0.01 and p = 0.012, respectively). There was no mortality related to FI in this
cohort of patients.
Conclusion: Despite posaconazole prophylaxis, FI is still prevalent in AML patients.
Infection during salvage treatment for refractory or relapsed disease and >2 previous
periods of neutropenia exceeding 4 weeks should raise awareness for an increased risk
of FI.
Mhamdi
G.
1
Bachrouch
S.
1
Berriche
A.
1
Ammari
L.
1
Abdelmalek
R.
1
Kilani
B.
1
Benaissa
H. Tiouiri
1
Kallel
A.
2
Kallel
K.
2
1Department of Infectious Diseases, The Rabta hospital, Tunis, Tunisia
2Department of Parasitology-mycology, The Rabta hospital, Tunis, Tunisia
P332. Neuromeningeal cryptococcosis: experience of a department of infectious diseases
Objectives: Introduction: Neuromeningeal cryptococcosis (NMC) is a serious infection.
Frequently described during HIV infection, it can nevertheless occur on any field
of cellular immunosuppression and even in the immunocompetent. Aim: describe the clinical,
para-clinical, therapeutic and evolutionary aspects of NMC cases.
Methods: A descriptive retrospective study carried out at the infectious diseases’
department of La Rabta Hospital over 25 years, between January 1993 and December 2018,
including all patients hospitalized for NMC.
Results: We collected 30 cases of (NMC). The median age is 34 years old [18-73] with
a sex ratio of 1.7. 76 % of patients were HIV-positive. The average CD4 count was
58.8 cells / μl. Among HIV-negative patients, 4 patients were followed for lymphoma,
one patient was diabetic, one had Histiocytosis X and only 1 was immunocompetent.
The median time of treatment was 6 days [1-75]. The main symptoms were fever (73%)
and headache (93%). Seizures and signs of localization were present in 1/3 of the
cases. A Physical meningeal syndrome was noted in 63% of the cases. Cerebrospinal
fluid (CSF) analysis revealed a highly variable cytology [0-3000], hypoglycorrhachia
(69%) and hyperproteinorachia (83%). The dosage of cryptococcal antigen in the CSF
ranged between 1/64 and 1/25000. The treatment of attack was based primarily on Amphotericin
B. The mortality was 56%.
Conclusion: NMC is a life-threatening fungal infection. Only a high level of clinical
suspicion, early diagnosis and appropriate medical care can improve the prognosis.
Bertin
C.
1
Sitterlé
E.
1
Scemla
A.
2
Fraitag
S.
3
Dellière
S.
1
Guegan
S.
4
Leclerc-Mercier
S.
5
Rouzaud
C.
4
Lanternier
F.
6
Bougnoux
M.-E.
1
7
1Parasitology Mycology, APHP Hôpital Necker-Enfants-Malades, Paris, France
2Transplantation, Hopital Necker, Paris, France
3Anatomopathology, Hopital Necker, Paris, France
4Infectious Diseases, Hopital Necker, Paris, France
5Hopital Necker, Paris, France
6Infectious Diseases, Necker hospital, Paris, France
7Laboratoire De Parasitologie Mycologie, APHP Hôpital Necker-Enfants-Malades, Paris,
France
P333. Deep cutaneous mycoses in renal transplant patients: diagnostic and therapeutic
challenges
Objectives: Deep cutaneous mycoses are infections extending throughout the dermis
and hypodermis, often occurring after inoculation of pathogen fungi. In kidney transplant,
deep cutaneous mycoses count for up to 5% of all documented infections. The aim of
this study was to describe the clinical features, histopathological findings and mycological
characteristics of deep cutaneous fungal infections in renal transplant patients,
as well their management and outcome.
Methods: We performed a retrospective review of cases of proven deep cutaneous mycosis
(histologically extending to dermis and/or hypodermis with mycological documentation)
occurring among kidney transplant recipients in our institution since 2011. We studied
the type of transplant, immunosuppression, histopathology, treatment with prospective
follow-up of cutaneous lesions
Results: Nineteen kidney transplant patients were included; the average age was 41
(range, 28 to 91). The immunosuppressive regimens at the time of diagnosis included
corticosteroids (n = 19) and anti calcineurin (tacrolimus (n = 18), cyclosporin (n
= 1)). In 10 and 6 patients a treatment by mycophenolate mofetil and azathioprine
was associated, respectively. Co-morbidities were frequent: diabetes mellitus (n =
12), cancer (n = 2), HIV (n = 1). In 70% of cases a skin cancer was initially suspected
(epidermoid carcinoma n = 5, kaposi sarcoma n = 6). The skin lesions were solitary
in ten patients, mainly nodular (84%) and affected the extremities (95%), without
fever or fatigue in the majority of cases (63%). Histopathologic examination of skin
biopsies showed pseudohyphae and/or irregularly branched hyphae (n = 16/16, 100%),
a granulomatous dermatitis (n = 6/16, 37%), and an inflammatory infiltrate (n = 8/16,
50%). Mycological examination of the biopsy showed the presence of hyphae on direct
examination in 84% of the cases (n = 16/19). Identification was obtained by culture
and / or molecular biology. Eleven different fungal species were observed, including
dematiaceous molds (n = 13), hyphomycetes (n = 4), dermatophyte (n = 1), and Mucorale
(n = 1). No dissemination to other organs was observed. The detection of the fungal
(1-3) beta-D-glucan antigen in serum was positive in 100% of the cases (n = 17/17).
Fifteen patients received antifungal treatment with azoles (posaconazole n = 10, voriconazole
n = 3), or liposomal amphotercin B (n = 2) for a mean duration of 3 months, and surgery
was associated in 11 patients. Side effects were common (64%) with mainly tacrolimus
overdosage (21%) or acute renal failure (50%).
Conclusion: This work highlights the great diversity of fungal species responsible
for deep cutaneous mycoses in renal transplant patients. The mycological documentation
is necessary to adapt the antifungal treatment according to the sensitivity to antifungal
agents of the isolated species. Drug interactions between antifungals and immunosuppressive
treatment require appropriate monitoring.
Mansour
M.
Dept of Medicine, Division of Infectious Diseases, Massachusetts General Hospital,
Boston, United States of America
P336. Neutrophil profiling in response to the human fungal pathogen, Candida albicans,
in transplant recipients
Objectives: Neutrophils are innate immune cells and the first line of defense against
a variety of invading pathogens including yeast and mold species. While low circulating
neutrophils numbers (neutropenia) is a clear risk factor for developing invasive fungal
infections (IFI), there is lacking evidence if neutrophil dysfunction contributes
to infectious risk. In this study, we profiled circulating neutrophil anti-Candida
fungicidal activity from high-risk patients at our tertiary care hospital, specifically
transplant recipients compared to healthy controls.
Methods: Solid-organ (SOT) and stem cell transplant (SCT) patients were identified
and consented from September 2018 until April 2019. Healthy control patients (HC)
were identified at primary care clinics. EDTA-anticoagulated peripheral blood was
obtained from healthy and transplant patients 2-4 months post-transplant. Neutrophils
were isolated by negative selection. C. albicans was co-incubated for 2 hours with
and without human neutrophils at a multiplicity of infection (MOI) of 1, 5 and 10.
Following neutrophil cell lysis, percent remaining live Candida was measured using
a viability dye. In addition, growth inhibition of yeast by neutrophil swarming to
C. albicans spotted onto glass slide arrays was also assessed by live-cell imaging.
Results: 22 SOT (15 kidneys, 7 livers), 20 SCT (allograft) and 22 HC were enrolled.
Neutrophils from SCT and SOT had lower C. albicans killing percentages compared to
HC at MOI 10 (HC: 47%, SOT: 29%, SCT 24% p = 0.0041); MOI 5 (HC: 72%, SOT: 35%, SCT
38% p < 0.0001) and MOI 1 (HC: 91%, SOT: 48%, SCT: 45% p < 0.0001). Neutrophil swarming
and fungal control of C. albicans spots were significantly inhibited by neutrophils
from SCT when compared to SOT and controls (p <0.0001). Analysis of medications, including
tyrosine kinase inhibitor (TKI) use, did not demonstrate significant differences in
a specific drug class when patient groups are compared (SCT versus SOT).
Conclusion: This study indicates that neutrophils from patients with SOT and SCT have
a decreased ability to kill C. albicans, despite normal circulating numbers. There
were no medications or laboratory values that predicted a functional neutrophil outcome.
These data strongly support the use of functional neutrophil profiling to risk stratify
those individuals at higher risk for invasive fungal infections, although additional
studies are required to determine if PMN dysfunction results in increased clinical
infection. These findings will inform us of potential therapeutic interventions for
restoring immune function and preventing invasive fungal infections in high-risk transplant
patients.
Shagdileeva
E.
1
Faizullina
R.
2
Belova
O.
3
Voronovi_H
S.
3
Kuznetsova
T.
4
Rubin
G.
3
Vorobeva
S.
4
Bogomolova
T.
2
Vybornova
I.
2
Klimko
N.
1
1North-West State Medical University named after I.I. Mechnikov, Saint-Petersburg,
Russian Federation
2North-Western State Medical University named after I.I. Mechnikov, St. Petersburg,
Russian Federation
3City Children’s Hospital № 17, St. Petersburg, Russian Federation
4City Children’s Hospital № 1, St. Petersburg, Russian Federation
P340. rInvasive candidiasis in neonatal patients in Saint-Petersburg, Russia
Objectives: To investigate the etiology, risk factors, clinical manifestations, clinical
features, and results of treatment of invasive candidiasis (IC) in neonatal patients
in St. Petersburg, Russia.
Methods: A prospective study was conducted from January 2015 to February 2019. We
evaluated neonates with clinical manifestations of infection and a confirmed diagnosis
of IC. The EORTC/MSG 2008 criteria were used for IC diagnosis and evaluation of the
effectiveness of therapy.
Results: “Proven” IC was detected in 29 neonates patients, girls – 62%. Premature
neonates were 86%, twins – 21%. Median birth weight was 1280 g (560 – 4000 g), median
gestational age at birth – 30 weeks (23 – 40). IC developed on the 5 – 88 days of
hospitalization (median – 20). The main underlying conditions: premature neonates
– 86%, intraamniotic infections – 72%, HIV – 7%. Broad-spectrum antibacterial drugs
were prescribed for 100% patients, mechanical ventilation – 97%, central venous catheter
(CVC) use – 93% (median time – 9,5 days, 4 -25 days), parenteral nutrition – 69 %.
Antifungal prophylaxis with fluconazole received 93% patients. The main clinical variants
of IC were candidemia – 97%, chorioretinitis – 6%, meningitis – 3%, and hepatitis
– 3%. Candida spp. were isolated from CVC in 86% of patients, blood – 41%, cerebrospinal
fluid – 3%. The pathogens of IC were C.albicans – 38%, C.parapsilosis – 32%, C.famata
– 14%, C.pelliculosa – 7%, C.tropicalis – 3%, C.guiliermondii – 3%, Candida sp. –
3%. In vitro to voriconazole were resistant 3% Candida spp., fluconazole – 3%, dose-dependent
susceptibility to fluconazole – 7%. In the first 24 hours after diagnosis antifungal
treatment was used in 100% patients: fluconazole (100%), micafungin (38%), amphotericin
B deoxycholate (14%), and voriconazole (14%). The median duration of treatment was
20 days (1 - 54). The overall 30-day survival rate was 86%.
Conclusion: IC developed in premature neonates (86%), the median gestational age at
birth was 30 weeks, the median birth body weight - 1280 g. Risk factors for IC were
broad-spectrum antibacterial drugs therapy (100%), mechanical ventilation (97%), and
central venous catheter (93%). Main etiology agents were C. albicans – 38% and C.
parapsilosis (32%). The main clinical variant of IC was candidemia (97%). Antifungal
treatment was used in 100% patients, fluconazole – 100%, micafungin – 38%. The overall
30-day survival rate was 86%.
Kozlova
O.
1
Isaeva
N.
1
Frolova
E.
1
Bogomolova
T.
1
Suspitsin
E.
2
Gabrusskaia
T.
3
Klimko
N.
1
1Department of Clinical Mycology, Allergology and Immunology, North-Western State
Medical University n.a. I.I. Mechnikov, Saint-Petersburg, Russian Federation
2St. Petersburg State Pediatric Medical University, Saint-Petersburg, Russian Federation
3Saint-Petersburg State Pediatric Medical University, Saint-Petersburg, Russian Federation
P341. AIRE and STAT1 gene mutations in Children with Chronic Mucocutaneous Candidiasis
in Saint-Petersburg, Russia
Objectives: Publications on AIRE and STAT1 gene mutations in children with chronic
mucocutaneous candidiasis (CMC) are limited.
Methods: Molecular genetic studies were performed with multigenic targeted sequencing
(MiSeq, Illumina, USA).
Results: In prospective single center study included 11 patients with CMC. Median
age was – 7.5 y, range - 6 to 13 y, males - 92%. Molecular genetic study has shown
heterozygous mutation in the STAT1 gene in 4 patients and mutations in autoimmune
regulator (AIRE) in 7 patients. CMC started between 2 months - 4 years (median – 2
y). Sites of CMC were oral mucosa – 100 %, skin – 64%, esophagus - 36%, and nails
– 36%. Candida albicans was isolated in all patients. Recurring CMC was diagnosed
more than 4 times per year in all patients. Endocrine disorders were present in patients
with AIRE syndrome: primary adrenal insufficiency – 71%, hypoparathyroidism - 71%,
autoimmune thyroiditis with hypothyroidism - 29%; autoimmune disorders: autoimmune
gastritis – 71%, alopecia – 29%, and hemolytic anemia – 14%. Patients with impaired
STAT1 did not have endocrine disorders and autoimmune gastritis was in one patient
(29%).
Conclusion: In 100% patients CMC with mutations in autoimmune regulator AIRE endocrine
system disorders and autoimmune diseases. STAT1 gene autoimmune diseases diagnosed
in 29% patients. This work was supported by Russian Scientific Fund grant 18-15-00256
Kozlova
O.
1
Mironuk
E.
2
Kapilov
V.
2
Ustinova
A.
2
Muratov
P.
2
Serikova
E.
2
Pinevskaia
M.
2
Ignatyeva
S.
1
Klimko
N.
1
1Department of Clinical Mycology, Allergology and Immunology, North-Western State
Medical University n.a. I.I. Mechnikov, Saint-Petersburg, Russian Federation
2Children’s City Multidisciplinary Clinical Center for High Medical Technologies.
K.A. Rauffusa, Saint-Petersburg, Russian Federation
P342. Pneumocystis jirovecii pneumonia associated with severe viral infection in children
Objectives: As an opportunistic infection, Pneumocystis jirovecii pneumonia (PjP)
is a severe and life-threatening complication in immunocompromised patients. Publications
on PjP associated with severe viral infection in children are limited.
Methods: We report the 2 cases of PJP associated with severe viral infection in children.
Results: A 13-year-old girl admitted in children’s hospital with interstitial pneumonia,
sepsis, and respiratory and cardiovascular failure. 7 days before, she presented with
the history of fever and dry cough. The patient is diagnosed with influenza. She was
in acute respiratory distress and required immediate intubation and transfer to the
intensive care unit. On examination, she was hypoxic with SaO2 70-82 % despite 100%
flow oxygen. Despite empiric intravenous therapy with ceftriaxone, amikacin, meropenem
and vancomycin, she remained hypoxic. Lymphopenia (1,0 × 109/l) was in blood analysis.
HIV testing was negative. The chest CT scan showed bilateral interstitial infiltrates.
MONOFLUO™ KIT P. jirovecii test and PCR test for P. jirovecii were positive in BAL.
Intravenous trimethoprim–sulfamethoxazole (TMP–SMX) (12 mg/kg TMP) and prednisone
0.5 mg/kg twice daily were initiated, which resulted in improvement in respiratory
status and weaning of respiratory support over 15 days (SatO2 - 88-92%, PO2 - 46-52%).
A 16-year-old boy was diagnosed with chronic bronchiolitis, secondary arterial hypertension,
and secondary asymmetric hypertrophic cardiomyopathy. 3 month before, he presented
with the history of high fever and maculopapular rashes, spots Koplika and cough.
Vaccination details were not available. The patient received several courses of antibiotic
therapy and prednisone for a long time at a dose of 2.5-7.5 mg/kg without positive
dynamics. Due to the development of severe respiratory failure, the patient was hospitalized.
The patient had dyspnea of mixed character (SpO2 - 70-80%). The patient was immunocompetent
(CD4 = 2,130*109/l) HIV testing was negative. The serology for measles was positive.
The CT showed bilateral interstitial infiltrates. MONOFLUO™ KIT P. jirovecii test
was positive in BAL. Intravenous TMP–SMX (12 mg/kg TMP), which resulted in improvement
in respiratory status and weaning of respiratory support over 7 days.
Conclusion: Severe viral infection associated with several weeks of immune suppression
with the consequence may be the cause of secondary infections.
Shagdileeva
E.
1
Vorobeva
S.
2
Kotina
N.
2
Bogomolova
T.
3
Vybornova
I.
3
Klimko
N.
1
1North-West State Medical University named after I.I. Mechnikov, Saint-Petersburg,
Russian Federation
2City Children’s Hospital № 1, St. Petersburg, Russian Federation
3Kashkin Research Institute of Medical Mycology, North-Western State Medical University
named after I.I.Mechnikov, St. Petersburg, Russian Federation
P343. The first case of successful treatment of invasive mycosis caused by Candida
guiliermondii and Exophiala dermatitidis in a neonatal patient
Case Report: Newborn girl on the 2nd day of life was admitted to the City Children’s
Hospital № 1 on 25.01.2019. Birth weight was 3050 g, gestational age at birth – 40
weeks. Mother 43 years old: pregnancy - 8, childbirth - 6, HIV since 2014. The underlying
condition was early hemorrhagic disease of the newborn, gastric bleeding. Result of
HIV-1 DNA PCR test was negative, and antiretroviral prophylaxis (zidovudine) was used.
Risk factors for IM were prolonged central venous catheter (CVC) use and broad-spectrum
antibacterial therapy. Clinical manifestation of IM was body temperature > 38 °C,
refractory to broad-spectrum antibacterial therapy. A patient was treated empiricaly
with fluconazole. Diagnosis of IM was confirmed with positive blood culture: resistant
to fluconazole in vitro C. guiliermondii and E.dermatitidis. Antifungal treatment
was changed to micafungin. Clinical condition was improved and results of multiple
blood cultures were negative. The total duration of micafungin treatment was 30 days.
Conclusion. In the treatment of invasive mycosis in neonatal patients, the possibility
of several pathogens should be considered.
Tragiannidis
A.
1
Giantsidi
A.
2
Kotsos
D.
2
Tsotridou
E.
2
Saranti
A.
2
Antari
V.
2
Hatzipantelis
E.
2
Vyzantiadis
T.-A.
3
1Hematology Oncology Unit, 2nd Pediatric Deparment, Aristotle University of Thessaloniki,
AHEPA Hospital, Thessaloniki, Greece
2Hematology Oncology Unit, 2nd Pediatric Deparment, Aristotle University of Thessaloniki,
AHEPA Hospital, Thessaloniki, Greece
3First Department of Microbiology, Medical School, Aristotle University of Thessaloniki,
Thessaloniki, Greece
P344. Invasive Fungal Infections in a Paediatric Haematological-Oncological centre:
a retrospective study of 15 years.
Objectives: Despite the major progress made in the last few years regarding the prevention,
diagnosis and treatment of invasive fungal infections (IFIs), they still represent
an important cause of morbidity and mortality in paediatric patients with malignancies
and those undergoing HSCT. The aim of this retrospective study was to collect data
of the incidence and outcome of IFIs in children with haematological malignancies
and solid tumors in a Paediatric Hematology Oncology Unit. Additionally, specific
data on diagnostics, predisposing factors and treatment was also collected
Methods: Data was collected retrospectively from patients with an IFI (proven, probable,
possible), who were diagnosed between January 1, 2003 and December 31, 2017 with haematological
malignancies and solid tumors in the Haematological-Oncological Unit of the 2nd Department
of Paediatrics, AHEPA University Hospital. By reviewing the patients’ records, information
regarding gender, age at the time of the diagnosis of the IFI, underlying disease,
treatment phase, the presence of neutropenia and its duration (> or < 10 days) as
well as severity (< or > 500/mm3) and the site of the fungal infection was obtained.
Last follow-up was performed after 12 months after the end of chemotherapy and included
the status of disease, cause of death and in case of the occurrence of an IFI, the
outcome of the infection.
Results: Eleven out of the twenty-six children enrolled in the study were males. The
underlying disease was ALL for 19 patients (73%) and AML for 6 (23%). Twenty of them
were under parenteral nutrition during the manifestation of the IFIs and 20 of the
patients were presented with neutropenia (ANC<500/mm3). The most common location of
the IFIs were the lungs and the most common pathogen was Aspergillus spp. According
to the revised criteria of the European Organization for Research and Treatment of
Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of
Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) for the diagnosis
of IFSs, 5 of the diagnoses were classified as proven, of which two patients developed
zygomycosis, one chronic disseminated candidiasis, one cryptococcosis and one systematic
aspergillosis. Out of the rest 21 patients, 12 were classified as being affected by
probable and 9 as being affected by possible IFIs. Eleven patients received a combination
of antifungal drugs, whereas the rest of them were treated with monotherapy. Twenty
received liposomal amphotericin B (LAMB), eleven voricanazole, two caspofungin, two
micafungin and one fluconazole. IFI was lethal in 9 out of the 26 patients (34%).
Patients with zygomycosis were successfully treated with surgical excision of the
lesion combined with high dose of LAMB.
Conclusion: The results of this study suggest that, despite the progress regarding
the diagnosis and treatment of IFIs with use of new antifungal drugs, mortality remains
high. Therefore, it becomes obvious that early suspicion as well as prophylactic and
empirical therapy are significant factors contributing in the improvement of the outcome
of IFIs.
Kim
C.S.
Department of Pediatrics, Keimyung University School of Medicine, Daegu, Republic
of Korea
P345. Candida sepsis successfully treated with micafungin add-on therapy in extremely
preterm infants: report of two cases.
Case Report: Objectives: Candida sepsis is a common hospital-acquired infection in
the neonatal intensive care unit and occurs usually in extremely preterm infants.
Echinocandine is a new drug with a different mechanism of action from existing antifungal
agents which induces fungal cell wall damage, and is mainly used in adults, and neonatal
applications are extremely rare. Case 1: A female infant was born by an emergent Cesarean
section with fetal distress at the gestational age 27 weeks. The birth weight was
720 g. After birth, the baby received intensive care for respiratory distress and
feeding difficulties, including mechanical ventilation, central venous catheterization,
and parenteral nutrition. On the 16th day of admission, the patient was suspected
of sepsis and Candida parapsilosis was detected in the culture of both blood and central
venous catheter specimens. Initially, amphotericin-B (initial dose: 0.5 mg/kg/day,
maintenance dose: 1-1.5 mg/kg/day) was administered but fungemia persisted after 10
days of treatment. Thereafter, micafungin (therapeutic dose: 15 mg/kg/day) was added
and administered concurrently. After 4 days of the combination therapy, CRP was decreased,
and candida was no longer detected in blood samples. The patient was treated with
amphotericin-B for 19 days and micafungin for 8 days, and there was no recurrence
of fungal infection. There were no adverse effects of antifungal agents in laboratory
findings at the end of treatment. Case 2: A female infant was born with vaginal delivery
at the gestational age 26 weeks and 2 days. The birth weight was 750 g. After birth,
the baby received neonatal intensive care, including mechanical ventilation and parenteral
nutrition. On the 13th 15th day of hospitalization, C. parapsilosis was consecutively
detected in blood samples of the patient. Initially, amphotericin-B was administered,
but fungemia and elevated titers of CRP were persisted after 10 days of treatment.
Thereafter, micafungin was added. Candida was no longer detected in the blood samples
after 7 days of the combined therapy. The patient was treated with amphotericin-B
for 25 days and micafungin for 14 days, and there was no recurrence of fungal infection.
In addition, there were no adverse effects of antifungal agents in laboratory findings
at the end of treatment. Conclusion: I report two cases of extremely preterm infants
with Candida sepsis successfully treated with micafungin add-on therapy that did not
resolve with amphotericin-B alone.
Tragiannidis
A.
1
Saranti
A.
2
Giantsidi
A.
2
Kotsos
D.
2
Tsotridou
E.
2
Papambougiouki
M.
2
Hatzipantelis
E.
2
Vyzantiadis
T.-A.
3
1Hematology Oncology Unit, 2nd Pediatric Deparment, Aristotle University of Thessaloniki,
AHEPA Hospital, Thessaloniki, Greece
2Hematology Oncology Unit, 2nd Pediatric Deparment, Aristotle University of Thessaloniki,
AHEPA Hospital, Thessaloniki, Greece
3First Department of Microbiology, Medical School, Aristotle University of Thessaloniki,
Thessaloniki, Greece
P346. Invasive Aspergillosis in a Paediatric Haematological-Oncological center: a
retrospective study of 15 years
Objectives: Invasive aspergillosis (IA) represents an important cause of morbidity
and mortality in patients with primary and secondary immunodeficiencies and them undergoing
Hematopoietic Stem Cell Transplantation (HSCT). In particular, Aspergillus spp. constitute
the second most frequent cause of fungal infections in immunosuppresed patients, while
IA seems to be responsible for higher mortality rates in comparison with candidiasis.
Methods: Retrospective case study of IA managed and treated in the Hematological-Oncological
Unit between 2001 and 2016. The study included in total 16 children with possible,
probable and proven IA according to the European Organization for Research and Treatment
of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute
of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group
criteria.
Results: Among the 16 children included in the study, there were 6 boys. The underlying
condition was acute lymphoblastic leukaemia (ALL) in 10 patients (62.5%) and acute
myeloid leukaemia (AML) in 6 (37.5%). Out of the 16 patients, 13 were neutropenic
with an absolute neutrophilic count lower than 500/mm3 at the time of diagnosis. According
to the revised criteria of the EORTC/MSG for the diagnosis of IFIs came up that in
1 patient the diagnosis was proven, the diagnosis of 9 patients was classified as
probable and finally in 6 as possible. Combined antifungal treatment was administered
in 4 patients, while 12 patients received a single antifungal agent. Liposomal amphotericin
B (LAMB) was administered in 10/16 patients, voriconazole in 7/16, caspofungin in
1/16 (as combined treatment) and micafungin in 1/16 patients (as combined treatment).
Six out of the 16 patients (37.5%) died due to the IFI.
Conclusion: According to the above findings there was concluded that despite the progress
that was made regarding the diagnosis and treatment of IA with the use of newer formulations
of antifungal agents, morbidity is still high. High suspicion and prompt initiation
of empiric and prophylactic antifungal treatment in selected cases constitute significant
factors for the improvement of the outcome of IA.
Kozlova
O.
1
Suslova
I.
2
Avdeenko
Y.
2
Borzova
Y.
2
Klimko
N.
3
1Department of Clinical Mycology, Allergology and Immunology, North-Western State
Medical University n.a. I.I. Mechnikov, Saint-Petersburg, Russian Federation
2North-Western State Medical University n.a. I.I. Mechnikov, Saint-Petersburg, Russian
Federation
3Department of Clinical Mycology, Allergy and Immunology, North-Western State Medical
University named after I.I.Mechnikov, St. Petersburg, Russian Federation
P347. Thoracic actinomycosis mimicking tumor in a children
Objectives: Thoracic actinomycosis is rare and publications are limited.
Methods: Prospective, single-center study of 186 patients with different clinical
forms of actinomycosis (2005 – 2017 yy.). Pulmonary actinomycosis was in 9% (n = 17)
cases of them there were two children. We present two cases successful treatment of
thoracic actinomycosis in children without risk factors whose initial diagnosis was
malignancy.
Results: 14-year-old boy, previously healthy, was admitted with a history of continuous
chest pain during the past three weekes, fever, dry cough and hemoptysis. He was referred
to the oncology center with the hypothesis of malignant disease. Weight and height
of the patient was appropriate the age and sex. Respiratory sounds were decreased
in the right lower field. Laboratory findings demonstrated slight anemia (hemoglobin
of 110 g/l) and elevated inflammatory markers, such as white blood cell count (leukocyte
count of 16 * 109/l) and C-reactive protein (25.5mg/dL). Tuberculin test was negative.
A chest CT scan revealed a consolidation (70*40 mm) with spiculated margins and areas
of ground-glass attenuation in the right lower lung. A percutaneous thoracoscopic
biopsy of right lower lobe was performed. Histological examination revealed the presence
filamentous organisms and sulfur granules, without evidence of malignancy. The thoracic
actinomycosis was diagnosed. The patient received penicillin 15000000 units/day) during
three weeks, followed by 5 months of amoxicillin (40mg/kg/day). During the first month
of treatment, he showed clinical and radiograph improvement. He was followed up every
2 months until completing oral antibiotic therapy and remained asymptomatic year after
the end of the treatment. Previously healthy 14-year-old girl was admitted with a
history of hemoptysis during the past month, continuous chest pain, and fever. Respiratory
sounds were decreased in the right lower lobe, the patient’s oxygen saturation was
96% while breathing ambient air. CT showed dense consolidation in the right lower
lobe. The disease was initially interpreted as the malignant disease. A lobectomy
was performed. Histological evaluation of surgical specimens with hematoxylin and
eosin staining shows classic sulfur granules consistent with actinomycosis. She was
started on intravenous sodium penicillin 8 000 000 units/day during two weeks, followed
by 3 months of amoxicillin (40mg/kg/day), resulting in a rapid clinical response with
the disappearance of all symptoms. General state of health was satisfactory, indicators
of clinical and diagnostic studies within the normal range. CT imaging was normal.
Conclusion: Actinomycosis is an uncommon disease in children. Thoracic actinomycosis
should be considered in the differential diagnosis of neoplasia.
Lora-Téllez
V.
Lara-Hernández
M.F.D.C.
Bonifaz-Trujillo
A.
Rodríguez-Coello
G.
Mycology Laboratory, Hospital para el Niño Poblano, Puebla, Mexico
P348. Coccidioidomycosis disseminated disease in pediatric patient
Case Report: Objective. To describe the clinical manifestations of the disease and
laboratory diagnosis. Methods. Clinical characteristics, symptoms, direct examination
of the pathological material, culture and biology molecular methods. Results. 11 years
old female patient with malnutrition, who lives in semiarid region in the south-east
of Mexico. 3 months of clinical evolution Fever 38°C Multiple lesions such as subcutaneous
nodes tissue with localization in back, ilium region, knee, and feet. Without pleuritic,
aching chest pain and cough. Subcutaneous abscesses at computer assisted tomography
suggests with sarcoma diagnosis. Pathological material biopsy for laboratory diagnosis
by Hematoxylin Eosin, Periodic Acid-Schiff stains, KOH 10% solution, culture on fungal
media. Direct microscopic observation of the histopathological sections demonstrated
the inflammatory response contained granulomatous elements, collections of neutrophils,
areas of caseous necrosis and mature, ruptured spherules. The mature spherules measure
30 to 60 µm, with double walls up to 2 µm in thickness. Inside of the mature spherules
multiple endospores measure 2 to 5 µm containing. The growth of colonies white and
floccose at first 3 days was evident by culture methods. Coccidioidomycosis for diagnosis
of the disease by the direct examination of the pathological material was enough.
The fungal therapy with amphotericin B was successful. The molecular biology method
by polymerase chain reaction in real time technique (PCR-rt) match with Coccidioides
posadasii by the examination of the in vitro fungal growth. Conclusion. Coccidioidomycosis
is usually acquired through inhalation of arthroconidia and the disease is known to
be endemic to the United States, Mexico restricted to the semiarid and desert regions.
It is also found in a lesser degree in Central and South America. At present, the
etiological agents of coccidioidomycosis in man are Coccidioides immitis and Coccidioides
posadasii. Although the both etiological agents have equal phenotypic characteristics
and produced the same disease, there are differences between them, such as the geographic
distribution and genetic characteristics. Coccidioides immitis is located in the north
region of Mexico, whereas Coccidioides posadasii is located in the south region of
the country. It is important to known the specific etiological agent for epidemiological
reasons. Lesions of skin and subcutaneous tissue are among the most common manifestations
of hematogenous dissemination. The usual dissemination as a complication of primary
infection it was because malnutrition and the endemic region habitat.
Lora-Téllez
V.
Pérez-Ricardez
M.L.
Lara-Hernández
M.F.D.C.
López-Hernández
G.
Mycology Laboratory, Hospital para el Niño Poblano, Puebla, Mexico
P349. Zygomycosis in pediatric patients and laboratory diagnosis.
Case Report: Objective. To describe the distribution of the clinical manifestations
and the etiological agents of zygomycosis identification in pediatric patients. Methods.
Various clinical forms description in four pediatric patients with underlying condition.
Etiological agent identification by direct examination and culture on Sabouraud Dextrose
Agar of the pathological material. Results. Female 7 years old, acute lymphocytic
leukemia as underlying condition. Sites of the infection were paranasal sinuses, eye´s
orbital section, palate and face. Paranasal sinuses biopsy was selected as pathological
material for direct examination and fungal culture. Aseptate hyphae branching at angle
of 90 degrees approximately was observed by hematoxylin eosin and periodic acid-Schiff
stains by direct examination. The etiological agent was Lichthiemia spp by fungal
culture. Female 7 years old, acute lymphocytic leukemia as underlying condition. Site
of the infection was paranasal sinuses. In the paranasal sinuses biopsy was observed
aseptate hyphae 10 µm measure. Fungal culture on Sabouraud Dextrose Agar at 25°C,
after 4 days a woolly colony growth was observed. Microscopic morphology of the colony
was identified Mucor spp as etiological agent. Male 8 years old, burn trauma as underlying
condition. Sites of the infection were legs, arms and hands. Tissue debridement was
observed by direct examination with potassium hydroxide KOH 40% solution, and cultured
on fugal media. A white-gray woolly colony growth was observed after 2 days on Sabouraud
Dextrose Agar at 25°C, after 4 days incubation, the surface of the colony was covered
with dark spots like a “salt – and- pepper” appearance. At microscope examination,
the differential characteristics of the Rhizopus were observed, such as a single umbrached
sporangiophore, a globose sporangia, sporangiospores and rhizoids. Microscopic morphology
of the colony was identified Rhizopus spp. Female 1 month old, gastric perforation
as underlying condition. At computer assisted tomography examination a mass like a
tumor was observed in the gastric cavity. Gastric mucosa biopsy by direct examination
multiple aseptate and very broad hyphae were observed. Histopathological examination
of the gastric mucosa biopsy demonstrated the vascular invasion by hyphae, leading
to thrombosis and infarction of tissue. Rhizopus spp as etiological agent by fungal
culture examination. Conclusion. Zygomycosis is characterized by vascular invasion
with hyphae, infarction, and necrosis of tissue and by an acute or subacute course.
Zygomycosis caused by Rhizopus, Lichtheimia, Rhizomucor and Mucor has a wide geographic
distribution. The distribution of the clinical manifestations was usual sites of infection
because underlying condition such as acute lymphocytic leukemia with neutrocytopenia,
and burn trauma. But gastric cavity was uncommon as site of infection due was introduced
by oral-gastric catheter. Because the hallmark of Zygomycosis is vascular invasion
by hyphae, leading to thrombosis and infarction of tissue, the antifungal therapy
with Amphotericin B was successful while is delimited the original site of the infection,
such as paranasal sinuses, before it presents in contiguous structures of the orbit,
palate, face, nose, or brain. The early diagnosis as much as the site of infection
contributed for a good clinical evolution.
Farooqi
J.
1
Memon
S.
2
Laiq
S.
1
Naqvi
F.
1
Iqbal
K.
1
Tariq
S.
1
Zafar
A.
3
Jabeen
K.
4
1Pathology and Laboratory Medicine, Aga Khan University, Karachi-KHI, Pakistan
2Microbiology, Karachi University, Karachi, Pakistan
3Pathology and Laboratory Medicine, Aga Khan University, Karachi, Pakistan
4Pathology and Laboratory Medicine, Aga Khan University, Karachi, Pakistan, Pakistan
P350. rSpectrum of invasive yeast infections in children in Pakistan over the last
five years: 2015-2019
Objectives: We aim to describe here the changing spectrum of invasive yeast infections
in children in the last 5 years in Pakistan, using retrospective laboratory data from
2015-May 2019
Methods: Records of archived yeast isolates from Jan 2015-May 2019 kept at the Aga
Khan University Clinical Laboratories, section of Microbiology in Karachi, were retrieved
for analysis. A total of 1119 non-duplicate isolates were identified, of which 992
were invasive specimens from blood (833), CSF (51), wounds (47), intraabdominal collections
(36), Central venous lines (17) and other sterile fluids (8). The patients were divided
into neonates (259), paediatric age group (253) and adults (477). Frequencies of different
Candida and non-Candida species isolated over the last 5 years were computed and compared
for age groups using chi-square test. Hypothesis of a change in the spectrum over
the years among each age group was also tested.
Results:
Candida species made up 87.4% and 86.6% of all invasive yeasts in neonates and children,
respectively. The most common group in neonates was a diverse group of rare Candida
species (including C. lusitaniae, C. guilliermondii and C. pelliculosa to name a few)
at 45.4%, followed by C. tropicalis (24%) and C. parapsilosis (15.7%); Ustilago species
were the most common non-Candida yeasts at 9.2%. In children, the most common species
was C. tropicalis (32%), followed by rare Candida species as described above (22.8%)
and C. albicans (15.1%). Ustilago was again the most common non-Candida yeast at 11.1%.
There was a statistically significant difference in the distribution of invasive yeasts
between neonatal and paediatric age groups (p<0.001). The change in spectrum of invasive
yeast over the years was significant for infants and children (p = 0.035) and adults
(p = 0.029); however, we could not demonstrate a significant difference in neonatal
(p = 0.096) and elderly (p = 0.270) spectrum of invasive yeast infections over the
years. Fluconazole resistance in yeasts isolated from neonates was found to be 8.2%
while it was 22.8% in infants and children, possibly due to an emergence of C. auris
infections in children (12.8%) versus neonates (1.3%).
Conclusion: We conclude that it is essential to monitor the spectrum of yeast infections
in neonates and children as they are the population where there is greatest diversity
of invasive species, not limited to Candida species. Emerging pathogens are most likely
to arise from this population, including resistant strains, hence, it is also important
to monitor resistance trends.
Pleško
S.
1
Novak
M.
2
Benjak
V.
2
Hadžić
D.
3
Kardum
D.
4
Tomulić
K. Lah
5
Polić
B.
6
Bucat
M.
7
Mišanović
V.
8
Kondža
K.
9
Mikšić
M.
10
Bakoš
M.
2
Ćaleta
T.
2
1Depatment For Clinical and Molecular Microbiology, University Hospital Centre Zagreb,
Zagreb, Croatia
2Department of Pediatrics, University Hospital Center Zagreb, Zagreb, Croatia
3Department of Intensive Therapy and Care Pediatrics Clinic, University Clinical Center
Tuzla, Tuzla, Bosnia and Herzegovina
4Department of Pediatrics, Neonatal Intensive Care Unit, University Hospital Osijek,
Osijek, Croatia
5Depatrtment of Pediatric, University Hospital centre Rijeka, Rijeka, Croatia
6Department of Pediatrics, University Hospital Centre Split, Split, Croatia
7Department of Pediatrics, Nicu, University Hospital Centre Split, Split, Croatia
8Department of Pediatrics, University clinical Center Sarajevo, Sarajevo, Bosnia and
Herzegovina
9Department of Pediatrics, Childrens Hospital Zagreb, Zagreb, Croatia
10Department of Pediatrics, university Hospital Centre Maribor, Maribor, Slovenia
P351. Changing epidemiology of candida infection in NICU and PICU – multicenter study
Objectives: Invasive Candida infections are considered as very important cause of
morbidity and mortality in neonatal intensive care unit (NICU) and pediatric intensive
care unit (PICU). Although C.albicans is the most commonly isolated species from invasive
candida infections the frequency of non-albicans candida has increased. Geographical
differences in epidemiology, and even more important changes in susceptibility patterns
have been described. It is very important to know the epidemiology in order to recognize
patients in risk and to start the appropriate empirical therapy and improve patient
outcome. The aim of this study was to determine changes in Candida infections epidemiology
in NICU and PICU from three countries.
Methods: We included the data from three neighboring countries: Croatia, Bosnia and
Herzegovina and Slovenia (10 centers altogether), during the two year period. We collected
data about species of Candida causing infection, site of infection and antifungal
susceptibility of strains. In first year, overall 65 patients with invasive fungal
infection (IFI) (25 from NICU and 40 from PICU), and in second year 107 patients with
IFI (59 from NICU and 48 from PICU) were enrolled in the study.
Results: During the first year of surveillance in NICU, Candida albicans was the most
frequent isolate in IFI patients in 4 centers and Candida parapsilosis in one center.
According to collected data, Candida tropicalis, Candida krusei and Saccharomyces
cerevisiae were observed as rare and sporadic causes of IFI. During the second year
of surveillance we observed the shift of species as leading cause of IFI between centers:
C. parapsilosis was most frequent isolate and C albicans was less frequent. In some
centers C. famata, C. glabrata, C krusei, C. guillermondii was reported, sporadically.
In PICUs, during first year C. albicans was most common in four centers followed by
C. parapsilosis except in one center where C. lusitaniae and C. krusei were recorded.
C. glabrata and C. guillermondii were recorded, sporadically. In second year, we also
noted the shift of species as leading cause of IFI: the number of C. albicans decreased
and number of C. parapsilosis increased in 4 centers. It is important to emphasize
that 60% of C. parapsilosis in second year were susceptible only to amphotericin B
(resistant to fluconazole ad intermediate to echinocandins according to the EUCAST
interpretation breakpoints). C. glabrata, C. tropicalis, C. famata, Crytococcus laurentii
and C. lusitaniae were reported, sporadically.
Conclusion: The results of this observational study indicate the changing epidemiology
of IFI pointing to differences in fungal isolates as leading causes of IFI between
centers and in the same centers, during observational period of two years. In addition,
this study noted the increase of MICs for some antifungals and shift in susceptibility
of fungi, addressing the importance of monitoring and optimization of therapeutic
guidelines of IFI in NICU and PICU.
Slarve
M.
1
Holznecht
N.
2
Adler-Moore
J.
2
Ho
S.
3
Fuji
G.
3
1Biology, Cal Poly Pomona, Riverside, United States of America
2Biology, Cal Poly Pomona, Pomona, United States of America
3Molecular Express, Inc, San Dominguez, United States of America
P355. Liposomal Vaccine Containing Aspergillus Proteins Protects Immunosuppressed
Male and Female Mice Against Pulmonary Aspergillosis Caused by Azole Resistant or
Sensitive Strains of Aspergillus fumigatus
Objectives: This study tested the efficacy of an immunotherapeutic liposomal Aspergillus
vaccine (VesiVax® LAsV), with or without additional antifungal drug treatment. The
vaccine contained Aspergillus proteins Aspf3 and Aspf9, and the adjuvant lipidated
tucaresol (LT1). LAsV was tested in female mice challenged with two azole resistant
strains of Aspergillus fumigatus (V29 and V80), and in male mice with an azole sensitive
strain of A. fumigatus (ATCC#13073).
Methods: Six week old Swiss Webster mice were vaccinated with LAsV (Molecular Express,
Inc.) at 7.5 µg Aspf3/dose,7.5 µg Aspf9/dose and 5 µg LT1/dose, subcutaneously on
d0, with intranasal boosts on d21 and d42. Control mice were given phosphate buffered
saline (PBS). Prior to challenge, spleens and blood were collected (n = 5/group) for
ELISpot cytokine analysis and anti-spore antibody agglutination assays. On d53, d55,
and d57, vaccinated mice were immunosuppressed intraperitoneally with 28 mg/kg triamcinolone
acetonide. On d56, mice were intranasally challenged with A.fumigatus conidia (2-2.8
X 10ex7/mouse depending on fungal strain). Some vaccinated groups were also given
a short course of 7.5 mg/kg liposomal amphotericin B (AmBisome®, AmBi, Gilead Sciences
Inc.) intravenously at 12h, 36h, and 60h post-challenge. Lungs were collected (n =
7/group) 3 days post challenge for fungal burden, and remaining mice (n = 9/group)
were monitored for survival to d77.
Results: LAsV vaccinated male and female mice had reduced lung fungal burdens compared
to PBS control mice regardless of the strain of A. fumigatus challenge. Female mice
vaccinated with LAsV, challenged with strain V29 or V80, and then given subsequent
AmBi treatment, had higher survival than PBS control mice (V29 = 79% vs 21%; V80 =
57% vs 25%) and the vaccine alone also yielded some protection when mice were challenged
with V29 (55% survival). Non-vaccinated female mice given AmBi alone had only 37%
survival following V29 challenge, and 22% survival with V80 challenge. Results in
male mice vaccinated with LAsV and given AmBi after challenge, were similar to the
female mice, yielding 55% survival following A fumigatus challenge (#13073) versus
22% survival for mice given AmBi alone or PBS. ELISpot cytokine analysis showed that
LAsV vaccinated female and male mice had a significantly higher number of IL-4 than
IFN-γ secreting splenocytes following incubation with Aspf3 and Aspf9 (p = 0.0178
for males, p = 0.0290 for females), which is characteristic of a Th2 response. Anti-spore
antibody agglutination titers demonstrated that LAsV vaccinated mice also had increased
anti-A.fumigatus spore antibodies in the serum than PBS control mice (p = 0.0009 for
males, p<0.0001 for females).
Conclusion: Prophylactic LAsV vaccination of immunocompetent female or male mice,
which were then immunosuppressed prior to Aspergillus challenge, followed by a short
course of AmBi treatment, provided more protection against pulmonary aspergillosis
than AmBi treatment alone whether the infection was caused by azole resistant or sensitive
strains of A.fumigatus. Elevated levels of anti-A.fumigatus spore antibody titers
correlated with protection, serving as a possible biomarker to indicate protective
immune responsiveness to the vaccine. The LAsV has the potential to be used as an
immunomodulatory treatment to further enhance the efficacy of antifungal drugs.
Cao
C.
Dermatology, The first affiliated hospital of Guangx medical university, Nanning,
China
P356. Anti–interferon-γ autoantibodies underlie disseminated Talaromyces marneffei
infection
Objectives: Anti–interferon-γ autoantibodies underlie disseminated Talaromyces marneffei
infection Obsjects: T. marneffei causes life-threatening opportunistic infections,
mostly in Southeast Asia and South China. Most T. marneffei infections occur in patients
infected with human immunodeficiency virus (HIV), but this infection can, nevertheless,
occur in HIV-negative individuals in the absence of immunosuppression. We investigated
the role of anti-interferon (IFN)-γ autoantibodies in severe T. marneffei infection
in HIV-negative patients.
Methods: Anti–interferon-γ autoantibodies underlie disseminated Talaromyces marneffei
infection Obsjects: T. marneffei causes life-threatening opportunistic infections,
mostly in Southeast Asia and South China. Most T. marneffei infections occur in patients
infected with human immunodeficiency virus (HIV), but this infection can, nevertheless,
occur in HIV-negative individuals in the absence of immunosuppression. We investigated
the role of anti-interferon (IFN)-γ autoantibodies in severe T. marneffei infection
in HIV-negative patients. Method: We enrolled 58 HIV-negative adults who developed
T. marneffei severe infections but were otherwise healthy in this study. We evaluated
the presence of anti-IFN-γ autoantibodies and their neutralizing activity.
Results: The prevalence of neutralizing anti–IFN-γ autoantibodies was high (94.8%)
in these previously healthy adults suffering from severe T. marneffei infections.
The presence of anti–IFN-γ autoantibodies was strongly associated with HLA-DRB1*16:02
and DQB1*05:02 in these patients.
Conclusion: Adult-onset acquired immunodeficiency due to autoantibodies against IFN-γ
is the major cause of severe T. marneffei infection in HIV-negative patients in the
regions in which this fungus is endemic. The high prevalence of pathogenic HLA class
II DRB1*16:02 and DQB1*05:02 alleles, which are strongly associated with anti-IFN-γ
autoantibodies, may account for the restriction of severe T.marneffei infection to
Southeast Asia. Our findings clarify the pathogenesis of T. marneffei infection and
pave the way for the development of novel treatments.
Neves
J.
1
Konno
F.
1
Lallo
M.
1
Alvares-Saraiva
A.
2
Hurtado
E.
1
Alvarez
J.
1
Coutinho
S.D.A.
1
1Post-graduation Program In Environmental and Experimental Pathology, Paulista University,
São Paulo, Brazil
2Institute of Physical Activity and Sports Sciences, Cruzeiro do Sul University, São
Paulo, Brazil
P358. Phagocytic activity and cytokines production by murine RAW-264.7 macrophages
in response to Microsporum canis
Objectives: Dermatophytes are important fungi for public health, as they are transmitted
between animals and humans causing zoonoses. The innate immune system plays an important
role in the establishment of dermatophytic infection; however, there are few studies
on the relationship between innate immune response and Microsporum canis. Thus, the
aim of this work was to evaluate the response in vitro of macrophages challenged with
Microsporum canis microconidia. We evaluated the ability of macrophages stimulated
or not with LPS, to phagocytize, to produce nitric oxide and cytokines, and the cell
viability.
Methods: Murine Raw-264.7 macrophages were cultivated overnight on glass coverslips
in a 24 well plates. Macrophages were infected with M. canis (ATCC-36299) in the ratio
of 0.25:1 (microconidia/macrophages) and incubated at 37˚C with 5% CO2 for 30 min,
1 h, 3 h and 6 h. After incubation intervals, glass coverslips were collected and
submitted to Giemsa stain. Two hundred cells were counted and calculated the percentage
of macrophages that internalized at least one spore. LPS (1mcg/mL) was added to the
macrophages 24 h prior M. canis infection in one group. Supernatants were collected
for detection of nitric oxide and cytokine levels, and to check the cell viability.
Results: The phagocytic index showed an increase in phagocytosis over time (Figures
1 and 2), reaching the maximum at 6 h. The percentage of non-LPS stimulated macrophages
that phagocytosed M. canis microconidia was 28.0%, 33.5%, 62.5% and 71.5%, respectively
in 30 min, 1 h, 3 h and 6 h (Figure 1). In macrophages stimulated with LPS the phagocytic
index was 34.5%, 47.5%, 67.0%, and 74.0%, respectively in 30 min, 1 h, 3 h and 6 h
(Figure 1). It was observed similar nitric oxide production in both, macrophages challenged
and not with M. canis. Levels of TNF-α have increased over time in macrophages challenged
with M. canis stimulated or not with LPS when compared with macrophages non-infected
(statistically significant). Higher levels of IL-6 were produced by macrophages stimulated
with LPS and infected with M. canis at 1 h (statistical difference). Cell viability
decreased over time, with the peak of macrophages killing at 6 h. Microbicity test
showed that, even after phagocytosis, spores of M. canis remained viable inside macrophages.
Conclusion: Raw-264.7 macrophages showed high phagocytic activity against M. canis,
which increased when macrophages were previously stimulated with LPS. LPS stimulation
also influenced the production of cytokines, which suggests greater modulation in
the response to infection. Increased production of the pro-inflammatory cytokines
TNF-α and IL-6, which are important in the recruitment of new phagocytic cells and
in the resolution of the disease, have been found in macrophages challenged with M.
canis. The results suggest that, although macrophages present phagocytic activity
and cytokine production when challenged with M. canis, their viability and microbicidal
activity are impaired.
Kenno
S.
Harpf
V.
Rambach
G.
Speth
C.
Würzner
R.
Div Hygiene & Med. Microbiology, Med. Univ. Innsbruck, Innsbruck, Austria
P359. Importance of using media with physiologic glucose concentration for studying
host defence against C. albicans in vitro
Objectives:
Candida albicans is a clinically important opportunistic yeast and has evolved different
immune evasion molecules on its cell surface. In the study on the role of one of these,
the transmembrane protein “High affinity glucose transporter 1” (Hgt1p), involved
in glucose metabolism, but also high-jacking the complement regulator factor H (FH)
from host serum, we have used different media, one of them the commonly used YPD medium
(yeast extract 1%, peptone 2% and dextrose 2%) to study the role of this evasion molecule
in immune defence.
Methods:
C. albicans strains (SC5314 und others) were opsonized with human serum and incubated
with anti-FH and anti-complement C3b/iC3b antibodies; their deposition on the cell
wall surface was assessed by fluorescence-activated cell sorter (FACS). In parallel,
C. albicans strains were co-cultured with fresh human polymorphonuclear neutrophils
(PMNs). All these experiments were done at different glucose concentrations, mainly
because a glucose transporter was involved.
Results:
Candida wild type strain SC5314 showed a clear FH deposition on its cell wall at the
physiological glucose concentration of 0.1%, but this was completely higher and thus
overestimated when using the two high glucose (0.3%, 2%) containing media. Consequently,
the resulting low C3b/iC3b deposition at 0.1% was even lower at the unphysiologically
high glucose levels. Also the thus impaired phagocytosis, observable at 0.1%, appeared
much more dramatic at glucose levels which are not achievable in vivo.
Conclusion: The main events in immune defence, such as opsonisation by C3b/iC3b and
phagocytosis by PMNs and the counteracting evasion strategy by Candida to high-jack
FH are all highly dependent on the glucose concentration and all experimental data
are thus completely misleading, if media with unphysiological glucose concentration
are employed, such as the commonly used YPD.
Hidifira
A. Miyuki
1
2
Pereira
A.
1
2
Alvares-Saraiva
A.
1
Hurtado
E.
1
Konno
F.
1
Alvarez
J.
1
Lallo
M.
1
2
1Post-graduation Program In Environmental and Experimental Pathology, Paulista University,
São Paulo, Brazil
2Biomedicina, Centro Universitário São Camilo, São Paulo, Brazil
P360. rImmune response in C57BL mice in Encephalitozoon cuniculi infection
Objectives:
Encephalitozoon cuniculi is a microorganism belonging to the phylum Microsporidia
and to the kingdom Fungi, considered an opportunistic and obligatory intracellular
pathogen. The first line of defense against E. cuniculi is the innate immune response
through the action of phagocytic cells and production of cytokines that are responsible
for the activation of T lymphocytes. The immune response against E. cuniculi has not
been fully elucidated and further studies are needed to understand the involvement
of cell types such as macrophages in the role of microsporidiosis. This study evaluated
the populations of macrophages and lymphocytic cells in E. cuniculi infection in C57BL/6
mice.
Methods: SPF C57BL/6 mice were used and divided in 4 groups: uninfected, infected,
uninfected-immunosuppressed and infected-immunosuppressed. Immunosuppression was made
by intraperitoneal injection of cyclophosphamide and euthanasia was performed by anesthetic
deepening to collect the peritoneal lavage and remove the spleen. Lymphocytes and
macrophages (F4/80) were identified by cell surface markers using flow cytometry.
Results: The results of the evaluation of spleen and peritoneal cells indicate that
in the peritoneum there was an increase in CD8+ T cells in infected group in relation
to the uninfected and infected-immunosuppressed groups and a decreased in CD4+ T population
in infected-immunosuppressed group. The B cells populations were decreased in both
groups infected (immunosuppressed and non-immunosuppressed). In the spleen, there
was an increase of CD8+ T cells and B cells in infected-immunosuppressed mice and
a decrease in CD4+ T in the same groups evaluated. Macrophage markers analysis revealed
expression of F4/80+ CD80/86high MCHIIhigh in both infected animals (immunosuppressed
and non-immunosuppressed) in the peritoneal lavage and in the spleen these cells were
increased only in infected-immunosuppressed group.
Conclusion: The results of this study help to understand the involvement of cells
as macrophages in the E. cuniculi infection in C57BL/6 mice.
Hemanth
V.
1
Kindo
A.J.
2
Subramanian
A.
3
Krishnan
A.
3
1Microbiology, Sri Ramachandra Medical college and Research Institute, chennai, India
2Microbiology, Sri Ramachandra Medical College and Research Institute, SRIHER, Chennai,
India
3Dermatology, Sri Ramachandra Medical College and Research Institute, SRIHER, Chennai,
India
P361. Level of Mannose binding Lectin protein in dermatophytic patients and its correlation
with MIC values
Objectives: 1.Speciation of dermatophytes from the clinical isolates 2.To study the
antimicrobial pattern of frequently used antifungal agents 3.To determine the serum
MBL levels in patients with dermatophytosis 4.To compare MIC levels with serum MBL
levels
Methods: METHODS: Clinically diagnosed dermatophytic patients were sampled for microscopy
and culture, grown dermatophytes was subjected to phenotypic identification by conventional
method. Antifungal susceptibility testing was done by broth microdilution method according
to CLSI M38-A2 document guidelines. Trichophyton mentagrophytes ATCC 4439 was included
in each run of susceptibility testing as a quality control. Final drug concentrations
in the microdilution plates ranged from 64 to 0.25 µg/ml for fluconazole and AmphotericinB
and from 16 to 0.25 µg/ml for Itraconazole, Voriconazole, Terbinafine and Griesofulvin.
This is an ELISA based assay performed in microwells coated with a monoclonal antibody
against the MBL carbohydrate- binding domain. Bound MBL is detected with the same
antibody that has been labeled with biotin, followed by development with horseradish
peroxidase (HRP)-conjugatedstrepavidin and incubation with a chromogenic substrate.
Reading was taken at 620 nm.
Results: Out of the 100 clinical isolates tested, 58 of them were identified as Trichophyton
mentagrophytes, 40 were Trichophyton rubrum. and the remaining two isolates were identified
as Epidermophyton Floccosum and Microsoprum nanum Antifungal susceptibility testing
was carried out for 100 isolates of Trichophyton species against 6 antifungal agents.
All isolates showed detectable growth after 4-5days of incubation. MICS for Trichophyton
mentagrophytes ATCC 4439 were within the established range. Mannose Binding Lectin
ELISA: Results awaited for ELISA Assay.
Conclusion: In this study Trichophyton mentagrophytes was the most common cause of
dermatophytosis. The findings of this study showed that the patients with dermatophytosis
have higher MBL serum levels, the acute phase protein plays a role in dermatophyte
infections. It is well known that mutations in MBL genes are associated with reduced
serum levels, which is a risk factor for infection. In this study, the high level
of MBL levels could be used as prognostic marker for dermatophytic infections. Further
studies with larger samples have to be under taken to come to definite conclusion.
Danion
F.
1
Aimanianda
V.
2
Bayry
J.
3
Duréault
A.
1
Bougnoux
M.-E.
4
Tcherakian
C.
5
Alyanakian
M.-A.
6
Guegan
H.
7
Puel
A.
8
Picard
C.
9
Lortholary
O.
1
Lanternier
F.
1
Latgé
J.-P.
2
1Infectious Diseases, Necker hospital, Paris, France
2Institut Pasteur, Paris, France
3Equipe-immunopathologie Et Immunointervention Thérapeutique, INSERM, Paris, France
4Parasitology Mycology, APHP Hôpital Necker-Enfants-Malades, Paris, France
5Pneumology, Hopital Foch, Paris, France
6Immunology, AP-HP Necker Hospital, Paris, France
7Laboratoire De Parasitologie-mycologie, CHU de Rennes, Rennes, France
8Inserm U1163, Institut Imagine, Paris, France
9Centre D’étude Des Déficits Immunitaires (cedi), AP-HP Necker Hospital, Paris, France
P362. Aspergillus infection in STAT3-deficient patients is associated with defective
interferon-gamma and Th17 responses
Objectives: Signal transducer and activator of transcription 3 (STAT3)-deficient patients
are highly susceptible to many bacterial and fungal infections including aspergillosis,
although its pathogenesis remains largely unknown. Objective: To investigate the immune
responses of STAT3-deficient patients against Aspergillus fumigatus infection
Methods: Phagocytic and killing efficiencies as well as cytokine responses of mononuclear
cells and neutrophils of STAT3-deficient patients were investigated against A. fumigatus.
Results: We compared results among three groups, STAT3 deficient patients with or
without aspergillosis and healthy controls. STAT3-deficient patients with aspergillosis
showed elevated titers of A. fumigatus specific IgE and IgG compared to STAT3-deficient
patients without aspergillosis as well as healthy controls. A. fumigatus conidial
phagocytic and killing efficiencies of monocytes and neutrophils from STAT3-deficient
patients with or without aspergillosis were similar to those from healthy controls.
When stimulated with A. fumigatus conidia, peripheral blood mononuclear cells from
patients with STAT3-mutation showed reduced levels of secreted (pg/mL) and intracellular
(%) cytokines compared to healthy controls (IFN-γ: 1836 vs 3248 pg/mL and 3.7% vs
11.4%; IL-17: 16 vs 80 pg/mL and 2.6 vs 7.0%; IL-22: 64 vs 170 pg/mL). In addition,
there were significantly lower levels of IFN-γ and TNF-α in STAT3-deficient patients
with aspergillosis compared to those without aspergillosis (IFN-γ: 683 vs 3420 pg/mL;
TNF-α: 779 vs 4743 pg/mL). In the STAT3-deficient cohort, three patients with aspergillosis
were treated with IFN-γ in addition to antifungals; two of them showed favorable outcome.
Conclusion: STAT3-deficiency leads to defects in adaptive immune responses against
A. fumigatus, particularly with a low IFN-γ response in those with aspergillosis,
suggesting potential therapeutic benefit of recombinant IFN-γ in these patients.
Blaize
M.
1
Blez
D.
1
Lebray
P.
2
Meghraoui-Keddar
A.
1
Combadière
C.
1
Boissonnas
A.
1
Rudler
M.
2
Fekkar
A.
1
1Sorbonne Université, Inserm, CNRS, Centre d’Immunologie et des Maladies Infectieuses,
Cimi-Paris, F-75013, PARIS, France
2AP-HP, Groupe Hospitalier Pitié-Salpêtrière, Service d’Hépato-Gastro-Entérologie,
F-75013, Paris, France
P363. Functionality of neutrophils against Aspergillus fumigatus in patients with
alcoholic cirrhosis
Objectives: Patients with liver cirrhosis are at risk of developing bacterial or fungal
infections. The incidence and severity of these infections depend on the stage of
the disease. Severe alcoholic hepatitis, the most serious stage of liver failure in
patients with alcohol abuse and chronic liver disease, has been associated with the
development of many cases of invasive aspergillosis and is now considered a risk factor
for it. A dysfunction of the immune system, namely systemic inflammation and immunodeficiency,
is described in cirrhosis with, in particular, an alteration of the phagocytic and
oxidative capacities of neutrophils. The latter is known to be the main immune effector
against Aspergillus. Therefore, we aim to study neutrophil’s responses from patients
with alcoholic cirrhosis at different stages of their disease against Aspergillus
fumigatus.
Methods: To achieve this objective, we conducted a prospective monocentric study involving
23 patients and 12 healthy controls. Patients were divided into 4 groups: stable cirrhosis
(n = 5), decompensated cirrhosis without severe alcoholic hepatitis (n = 3), decompensated
cirrhosis with severe alcoholic hepatitis (n = 11) and decompensated cirrhosis with
severe alcoholic hepatitis after 7 days of steroid treatment (n = 4). Interactions
between neutrophils and Aspergillus hyphae were analyzed by video microscopy, while
the oxidative burst of neutrophils before and after fungal stimulation and cytokine
production was determined by flow cytometry and enzyme immunoassay (EIA).
Results: Compared to healthy controls, patients with severe alcoholic hepatitis had
an increase in the production of reactive oxygen species by their neutrophils (1727
versus 2545, expressed as mean fluorescence intensity, p = 0.041). An increase in
plasma IL-8 levels (95 versus 378 ng/mL respectively, p = 0.006) was also observed
between these groups. Also among patients with severe alcoholic hepatitis, the production
of reactive oxygen species decreased after 7 days of corticosteroids compared to other
patients, both under basal conditions (937 versus 2545; expressed as mean fluorescence
intensity, p = 0.011) or after stimulation with germinating conidia (2031 versus 9272;
expressed as mean fluorescence intensity, p = 0.014). In addition, video microscopy
experiments (Figure 1) showed that an alteration in the ability of neutrophils to
kill A. fumigatus hyphae appears in the severe alcoholic hepatitis group after 7 days
of corticosteroid therapy compared to patients sampled before corticosteroid therapy
began (12% of mortality versus 46% respectively, p = 0.03) (Figure 2).
Conclusion: In conclusion, a pro-inflammatory state is present in patients with severe
alcoholic cirrhosis as well as in patients with stable cirrhosis. The introduction
of corticosteroids as a treatment for severe alcoholic hepatitis quickly leads to
a dramatic decrease in the inflammatory environment and the neutrophil’s ability to
kill Aspergillus fumigatus.
Frealle
E.
1
2
Pichavant
M.
1
Rouzic
O. Le
3
Reboux
G.
4
Bautin
N.
3
Willemin
M.-C.
3
Delourme
J.
3
Sendid
B.
2
Nseir
S.
5
Fry
S.
3
Gosset
P.
1
1Univ. Lille, CNRS, Inserm, CHU Lille, Institut Pasteur de Lille, U1019 – UMR8204
- CIIL - Center for Infection and Immunity of Lille, Lille, France
2Parasitology-mycology, CHU Lille, Lille, France
3Clinique Des Maladies Respiratoires, CHU Lille, Lille, France
4Mycology - Umr6249 Cnrs Chrono-environnement, University Hospital Besançon, Besançon,
France
5Pôle De Réanimation, CHU Lille, Lille, France
P364. Impact of mould exposure on the immune response in the course of chronic obstructive
pulmonary disease (COPD)
Objectives: Chronic obstructive pulmonary disease (COPD) is a complex chronic respiratory
disease which affects 8% of subjects over 45 years-old in France. It is mainly caused
by cigarette smoke, which induces chronic lung inflammation and is considered a key
etiological factor in the development and pathogenesis of COPD. Other environmental
factors, such atmospheric pollution, can also be involved in the evolution of the
disease, modulating inflammation and promoting exacerbations. Here, we assessed the
contribution of domestic mould exposure to lung and systemic inflammation in COPD
patients, during exacerbation or at stable state.
Methods: Sixty-two COPD patients were recruited during severe acute exacerbation requiring
hospitalization between August 2011 and November 2015 in Lille University Hospital.
Mycological analysis of sputa and anti-Aspergillus antibodies detection was performed
in order to assess Aspergillus colonization and sensitization status. CXCL8, TNFα
(recruiting/activation of monocytes and neutrophils), IL-1β, IL-6, IL-17, IL-22 (Th17
response) and CXCL4, CD62P, CD40L (platelet activation) levels were measured in the
sputum and in the serum. The inflammatory response was compared at inclusion (exacerbation)
and after an 18-months follow-up (at stable state) between (i) Aspergillus fumigatus
colonized or non-colonized patients, (ii) Aspergillus sensitized or non-sensitized
patients, and (iii) patient with or without mould exposure, which was assessed using
electrostatic dust collectors (EDCs) that were exposed during 10 weeks in the patient’s
home.
Results:
A. fumigatus colonization was detected in 16.9% of patients at inclusion (during exacerbation)
and 4% of patients at stable state. Anti-Aspergillus antibodies detection was positive
in 32.2% of patients at inclusion and 36.4% after the 18-months follow-up. No significant
differences were found between the cytokine levels during exacerbation or at stable
state when A. fumigatus colonized or non-colonized patients, and Aspergillus sensitized
or non-sensitized patients were compared. However, during exacerbation, patients with
mould exposure showed significantly higher levels of serum CXCL8 (19.6±41.2 pg/mL
vs <7 pg/mL for non-exposed patients, P = 0.001). Furthermore, at stable state, mould
exposure was associated with significantly higher levels of serum IL-6 (28.9±87.9
pg/mL vs <2 pg/mL, P = 0.016), and significantly lower levels of serum IL-22 (86.8±31.6
pg/mL vs 285.8±363.3 pg/mL, P = 0.0008).
Conclusion: These data suggest that domestic mould exposure could contribute to the
alteration of the inflammatory response in the course of COPD. Funding: Conseil Régional
Hauts-de-France, Gilead, MSD, Pfizer
Blaize
M.
1
Blez
D.
1
Soussain
C.
2
Boissonnas
A.
1
Meghraoui-Keddar
A.
1
Menezes
N.
3
Portalier
A.
4
Combadière
C.
1
Leblond
V.
4
Ghez
D.
5
Fekkar
A.
1
1Sorbonne Université, Inserm, CNRS, Centre d’Immunologie et des Maladies Infectieuses,
Cimi-Paris, F-75013, PARIS, France
2Hématologie, Institut Curie - Site de Saint-Cloud, 35 rue Dailly, F-92210, Saint-Cloud,
France
3AP-HP, Groupe Hospitalier Pitié-Salpêtrière, Service de Parasitologie-Mycologie,
F-75013, Paris, France
4AP-HP, Groupe Hospitalier Pitié-Salpêtrière, Service d’Hématologie, F-75013, Paris,
France
5Département d’Hématologie, Gustave Roussy, 114 rue Edouard Vaillant, F-94805, Villejuif,
France
P365. Effects of ibrutinib treatment on neutrophil response against A. fumigatus
Objectives: Ibrutinib, a small irreversible Bruton’s tyrosine kinase (Btk) inhibitor
has become a leading therapy against chronic lymphoid leukemia and has significantly
improved patient care. Recently, ibrutinib has been associated with the occurrence
of invasive fungal infections, in particular invasive aspergillosis. This observation
was surprising because patients with lymphoid malignancies are at low-risk of invasive
aspergillosis. The mechanisms underlying the increased susceptibility to fungal infections
associated with ibrutinib exposure are currently unknown. Polymorphonuclear neutrophil
is the cornerstone of anti-Aspergillus immunity. However, since the potential effect
of ibrutinib on the antifungal capacities of neutrophils has been studied to a very
limited extent, we decided to do so in more detail.
Methods: To answer this question, we studied the consequences of in vivo and in vitro
ibrutinib exposure on the neutrophil’s anti-Aspergillus responses. Thirty-three patients
receiving ibrutinib for lymphoid malignancies were included. A total of 63 blood samples
were collected from patients at different time points: just before ibrutinib treatment
initiation (n = 23) then approximately 1 month (n = 22) and 3 months later (n = 18).
Both flow cytometry and video-microscopy approaches were used to analyze neutrophils’
cell surface molecule expression, cytokine production, oxidative burst and killing
activity against Aspergillus.
Results: We observed different alterations between the patient group at 1 month of
treatment and the untreated group, as a decrease in the CD11b (major receptor for
beta-glucan) expression or IL8 production. Above all, we observed a decrease in the
production of reactive oxygen species (ROS) by neutrophils, both in the basal state
(2333 versus 1131, expressed as mean fluorescence intensity, p<0,05) or after stimulation
by Aspergillus germinating conidia (9330 versus 4569; p<0,05). Among the interactions
observed between germinating conidia and neutrophils in 16-hours video microscopy
co-cultures experiments, we focus on the engulfment and destruction of fungus by cells.
A decrease in the ability of neutrophils to spread over the fungus was observed between
treated and untreated patient groups (Figure 1). Regarding the destruction of Aspergillus
by neutrophils, we observed a dramatic decrease with 50% of germinating conidia killed
by neutrophils in the untreated group compared to only 1% in the group after one month
of treatment and 0.8% in the group after 3 months of treatment (p<0.01) (Figure 2).
The results were confirmed by the analysis of healthy neutrophils (from blood donors)
exposed (or not) in vitro to ibrutinib.
Conclusion: Ibrutinib is associated, both in vitro and in patients receiving this
treatment, with several functional defects in neutrophils, including a decrease in
the production of reactive oxygen species, a decrease in their ability to engulf the
fungus and an inability to effectively kill Aspergillus hyphae. Our results provide
a plausible explanation for the emergence of invasive aspergillosis in patients receiving
ibrutinib. Further work is needed to understand the exact mechanisms underlying this
effect, particularly if it is caused by inhibition of the Btk itself in neutrophils
or by an off-target effect. On May 2019, part of the data has been provisionally accepted
for publication in Haematologica journal.
Basmaciyan
L.
1
Garnaud
C.
2
Bon
F.
1
Maubon
D.
2
Aldebert
D.
2
Pape
P. Le
3
Cornet
M.
2
Dalle
F.
1
1Parasitology-mycology, University Hospital Dijon, Dijon, France
2Univ. Grenoble Alpes, CNRS, CHU Grenoble Alpes, Grenoble INP*, TIMC-IMAG, 38000 Grenoble,
France * Institute of Engineering Univ. Grenoble Alpes, GRENOBLE Cedex, France
3Ea1155 Iicimed, Nantes University Hospital, NANTES, France
P366. Host innate immune response to echinocandins-resistant Candida glabrata clinical
isolates: impact on the management of candidemia
Objectives:
Candida glabrata (C. glabrata) is a commensal yeast of the mucosal surfaces in humans.
Incidence of invasive candidiasis due to this species has recently increased. Thus
the high level of resistance that this species can present to different classes of
antifungals, including echinocandins is of major concern. Nowadays, the impact of
resistance acquisition in this opportunistic pathogen to the immune response of the
host remains poorly understood. Knowing the mechanisms by which Candida’s intrinsic
and adaptive resistance to antifungals can modulate (i.e. ’escape to’ or ’recognition
by’) the immune system of the host is crucial. Indeed, most of the antifungal resistance
mechanisms causes a direct or indirect modification of the fungal cell-wall. In addition,
by establishing the first contact with the host tissues, the fungal cell-wall is involved
in the recognition of C. glabrata by the host cells through interactions with Pattern
Recognition Receptors (PRRs). We hypothesize that changes in the fungal cell-wall,
as a result of resistance, could alter the recognition of C. glabrata by PRRs and
thus trigger a different immune response, from a qualitative (i.e. cytokines production
and immune cell recruitment) or quantitative (i.e. amplitude and duration of the immune
response) point of view. The purpose of this study was (i) to characterize the cell
wall modifications associated with resistance to echinocandins in C. glabrata and
then (ii) to investigate the impact of such modifications on the virulence of the
fungus and the host immune response.
Methods: Clinical isolates of C. glabrata included in the study displayed echinocandins’
resistance for which the mechanisms of resistance were molecularly characterized.
The virulence of these clinical isolates was performed using two in vitro models of
interaction of the fungus with the host compared to the ATCC reference strain: (i)
the Yeast-to-Enterocyte interaction model and (ii) the Yeast-to-Macrophages interaction
model. Cell wall characterization of the clinical isolates of C. glabrata was performed
using microscopy and flow cytometry.
Results: Data support a change in the virulence and recognition by the host of echinocandins-resistant
C. glabrata clinical isolates compared to the ATCC reference strain.
Conclusion: This study will allow (i) to better understand the prevalence of echinocandins-resistant
C. glabrata isolates in high-risk patients, (ii) to specify whether high levels of
in vitro resistance correlate or not to in vivo therapeutic failure and (iii) to establish
effective immunotherapeutic strategies to combat Candida resistance to antifungals.
Taraskina
A.
1
Shadrivova
O.
1
2
Boyko
I.
1
Frolova
E.
1
Uchevatkina
A.
1
Filippova
L.
1
Vasilyeva
N.
1
1Kashkin Research Institute of Medical Mycology, North-Western State Medical University
named after I.I. Mechnikov, Saint-Petersburg, Russian Federation
2Department of Clinical Mycology, Allergy and Immunology, North-Western State Medical
University named after I.I.Mechnikov, St. Petersburg, Russian Federation
P367. Gene variants of key TH-1 adaptive immune response proteins as predictors of
invasive aspergillosis in oncohematological patients
Objectives: Fungi of the genus Aspergillus are widely distributed in nature and are
relevant opportunistic pathogens for immunocompromised patients, causing a life-threatening
infectious fungal complication — invasive pulmonary aspergillosis (IPA). Disruption
of immune barriers associated with suppressive therapy, such as cytostatic polychemotherapy,
can affect both the mechanisms of the innate immune response and acquired, in particular,
the differentiation of T-helper cells (Th). The regulation of T-cell effector functions
occurs due to cytokine-induced intracellular signaling and transactivation of transcription
factors. Single nucleotide polymorphisms (SNPs) of cytokine genes that direct T cell
differentiation can lead to their different expression and / or affinity for complementary
receptors on immune cells, increasing the risk of infectious processes
The aim of this study
was to investigate gene variants of key protein-inductor of Th1 adaptive immune response
as biomarkers of IPA and effect on expression of IP-10 in serum in oncohematological
patients.
Methods: 178 oncohematological patients on the background of cytostatic polychemotherapy
with symptoms of lung injury were recruited. 86 oncohematological patients (44,5%)
either developed proven or probable IPA as defined by criteria of EORTC/MSG 2008 (median
age 43y ±14, range [22-77y], 57% males) whereas controls (92 oncohematological patients
(55,5%) without IPA comparable in age and sex) did not fulfill these criteria. Polymerase
chain reaction-restriction fragment length polymorphism (PCR-RFLP) was used to determine
the SNPs of rs458917, rs56061981 CXCL10 gene, rs1800629 TNFα gene and rs2430561 INFγ
gene. Chemokine IP-10 amount was determined by the enzyme-linked immunosorbent assay
with the use of commercial ELISA kit sets (Cloud-Clone Corp, USA) and is presented
in ng/ml serum. Statistical analysis was performed using SPSS21 (IBM, USA).
Results: The genotype and allele distribution of the study SNPs were no significant
differences between oncohematological patients with probable IPA and without IPA.
In the study we found that allele variants of G-135A (rs56061981) do not appear to
affect serum IP-10 levels although the presence of allele A is associated with a slight
increase in serum chemokine, while the presence of minor allele-1447G (rs4508917)
is associated with significantly higher concentrations of IP-10 in serum.
Conclusion: The presence of the CXCL10 -1447G allele in serum of oncohematological
patients was associated with increased IP-10 protein expression. The expansion of
the studied groups of patients will allow making conclusions about the prospect of
typing SNPs as a predictive marker of the risk of pulmonary mycosis development.
Bigeard
P.
Filleul
C.
Vahsen
T.
Zapata
L.
Botterel
F.
Guillot
J.
Arné
P.
Risco
V.
Ea Dynamyc Upec, Université de Créteil, Créteil, France
P368. In vitro phagocytosis of Aspergillus fumigatus conidia by chicken thrombocytes
and heterophils
Objectives: Avian respiratory diseases can be responsible for substantial economic
losses to the poultry industry. This is particularly true for aspergillosis, which
is pervasive in both domestic and wild avian communities, and is a common cause of
respiratory distress and morbidity. Mammal immune response against pulmonary aspergillosis
is characterized by an early interaction of Aspergillus conidia with phagocytic cells
such as macrophages and neutrophils, but knowledge remains limited regarding the avian
immune response after inhalation of Aspergillus conidia. Avian thrombocytes and heterophils
(homologues to mammal neutrophils) are known for their phagocytic activity, but nothing
has been described so far regarding their potential role during Aspergillus infection
in birds. This study aimed to investigate the phagocytic activity of chicken thrombocytes
and heterophils against Aspergillus conidia.
Methods: We used filtered GFP- or DsRed-expressing A. fumigatus conidia to inoculate
primary cultures of heterophils or thrombocytes previously purified by Percoll’s discontinuous
gradient density from whole blood of chicken. Cultures and conidia inoculation were
performed on poly-L lysine treated Labtek® slides or in suspension. When slides were
used, cultures were fixed at different time points to measure internalization rates
by immunolabeling with goat anti-chicken CD45 (leucocytes) and anti-chicken CD41 (thrombocytes)
antibodies. When cells were in suspension, they were inoculated with Aspergillus conidia
for 30 min, fixed and analyzed by flow cytometry (BD Accuri® C6) using the antibodies
above described. Finally, activation of phagolysosomal acidification was evaluated
by lysosome staining using the Lysotracker® method.
Results: We obtained cultures with up to 95.3% pure thrombocytes while heterophils
purification was less efficient (<50%) because of heterophil layer contamination with
erythrocytes and thrombocytes during the purification and heterophil aggregation during
culture. Nevertheless, identification of avian heterophils by morphology and CD45+
labeling demonstrated conidial internalization as soon as 15 min after culture inoculation.
Conidial internalization rate reached its peak 30 min after inoculation, with 22-28%
of thrombocytes containing at least one conidia, while heterophils showed up to 93%
of internalization. Eight hours after inoculation, conidial swelling and germination
was observed in almost all thrombocytes while internalized conidia in heterophils
remained quiescent. Lysotracker® staining confirmed phagolysosome fusion in heterophils
but not in thrombocytes containing conidia.
Conclusion: The results of the present study confirm for the first time the phagocytic
capacity of both chicken heterophils and thrombocytes to A. fumigatus conidia, and
suggest differences in internalization efficacy between these phagocytic cells. In
thrombocytes, phagocytic activity was lower than that detected in heterophils, which
allowed conidia swelling and germination inside the cells. Further studies shall determine
whether survival inside thrombocytes might be used by Aspergillus as a strategy to
ensure dissemination in birds.
Pacheco
I.
1
2
Costa-Barbosa
A.
1
2
Barbosa
A.M.
3
4
Dias
M.
1
2
Carvalho-Pereira
J.I.
1
2
Oliveira
M.E.C.D. Real
5
Pais
C.
1
2
Correia
A.
6
7
8
Torrado
E.
3
9
Sampaio
P.
1
2
1Institute of Science and Innovation For Bio-sustainability (ib-s), University of
Minho, Braga, Portugal
2Centre of Molecular and Environmental Biology (cbma), Department of Biology, University
of Minho, Braga, Portugal
3Life and Health Sciences Research Institute (icvs), School of Health Sciences, University
of Minho, Braga, Portugal
4I3s, Instituto De Investigação E Inovação Em Saúde, University of Porto, Porto, Portugal
5Centre of Physics (cfum), University of Minho, Braga, Portugal
6Ibmc, Instituto De Biologia Molecular E Celular, University of Porto, Porto, Portugal
7I3s, Instituto De Investigação E Inovação Em Saúde, University of Porto, Braga, Portugal
8G Dgaot, Faculdade De Ciências, University of Porto, Porto, Portugal
9Icvs/3b’s Pt Associate Laboratory, University of Minho, Guimarães, Portugal
P369. Evaluation of the liposome properties in the development of immune response
during an immunization protocol with C. albicans proteins loaded on DODAB:MO liposomes
Objectives:
Candida albicans is a normal colonizer of the human body, persisting in a harmless
state most of the time. However, given the opportunity, this species becomes one of
the most important opportunistic pathogens, which increases the risk of developing
systemic candidiasis. A promising antifungal strategy is the use of liposomal vaccine
delivery systems. Previous studies reported that cationic liposomes prepared with
DODAB:MO (1:2) and loaded with C. albicans cell wall surface proteins (CWSPs) were
stable, non-cytotoxic and avidly phagocytized by macrophages. However, standardizing
these liposomal formulations is challenging and complicated, since their wide range
of characteristics must be taken into consideration according to the application we
desire and the performance we expect. The liposome preparation method greatly influences
these characteristics. Our main objective was to analyze the implications of the liposome
preparation method on the biophysical properties of the DODAB:MO liposomes loaded
with C. albicans proteins and evaluate the consequences at the immune response level,
in order to develop a formulation that could be used in vaccination strategies against
systemic C. albicans infections.
Methods: CWSPs from Candida albicans SC5314 were encapsulated in DODAB:MO (1:2) liposomes.
The DODAB:MO liposomes were prepared using the thin lipid-film hydration method. The
lipid film was hydrated with ultrapure water (1º experiment) or with HEPES-buffer
and sonicated to reduce the vesicles’ size (2º experiment). For both, the final concentration
of CWSPs was 50 µg/mL in 25mM of HEPES-buffer and the final total lipid concentration
was 888 µg/mL. The mean size (nm), polydispersity (PDI) and surface charge (ζ-potential)
were evaluated with the Malvern ZetaSizer Nano ZS (Malvern, UK) particle analyzer
and the immunization procedure was carried as previously reported (Carneiro et al.,
2015). The production of IFN-γ, IL-17, IL-4 and IL-10 in splenic CD4+ T cells was
evaluated through flow cytometry. “ex vivo” cytokine production was evaluated through
re-stimulation of the splenocytes with CWSPs and CWSP-specific antibodies were quantified
by ELISA.
Results: Liposomes from the first experiment had a size around 666.23 ± 91,31 nm,
with a PDI of 0.36 ± 0.08, and a ζ-potential of -2.30 ± 0.31 mV. Changing the liposome
preparation method, we obtained smaller liposomes (249.69 ± 29.34 nm), with a PDI
of 0.20 ± 0.06 and a ζ-potential of -8.53 ± 0.66. While IL-4 was the prevailing cytokine
when taking into consideration the liposomes from the first experiment, the liposomes
from the second experiment led to much higher production of IFN-γ and IL-17, which
clearly shifted the immune response from a Th2 to a Th1/Th17 response. The liposomes
from the second experiment also demonstrated a significant increase (10x) in CWSP-specific
antibodies.
Conclusion: Our results indicate that the biophysical properties of DODAB:MO liposomes
are important in the type of adaptative cellular response, and that in reducing significantly
these liposomes sizes a shift from a Th2 to a Th1/Th17 response was observed, which
is known to be more protective against systemic candidiasis. Therefore, with this
liposome system, the liposome size has a huge impact in the immune response.
Teschner
D.
1
Plein
K.
1
Michel
C.
1
Pruefer
S.
2
Bros
M.
3
Beckert
H.
4
Wagner-Drouet
E.M.
1
Theobald
M.
1
Schild
H.
2
Radsak
M.P.
1
1Department of Hematology, Medical Oncology, & Pneumology, University Medical Center
of the Johannes Gutenberg-University, Mainz, Germany
2Institute of Immunology, University Medical Center of the Johannes Gutenberg-University,
Mainz, Germany
3Department of Dermatology, University Medical Center of the Johannes Gutenberg-University,
Mainz, Germany
4Clinic For Pneumology, Ruhrland Clinic, Faculty of Medicine of the University of
Duisburg-Essen, Essen, Germany
P370. rThe Impact of NFAT Inhibition on Neutrophil Effector Functions, Antifungal
Defense, and Myelopoiesis in Cyclosporine A-Treated and NFATc1LysM Mice and in Patients
after Allogeneic Hematopoietic Stem Cell Transplantation
Objectives: Calcineurin inhibitors substantially contribute to the risk for opportunistic
fungal infections in patients after allogeneic transplantation (HSCT). It is well
known that the nuclear factor of activated T cells (NFAT) is an important transcription
factor downstream of calcineurin especially in T cells. However, NFAT also seems to
play a role in innate antifungal immune responses by polymorphonuclear neutrophils
(PMN), as well as in regulation of myelopoiesis and myeloid differentiation.
Methods: Firstly, isolated PMN from CsA-treated patients after allogeneic HSCT (n
= 17) and healthy donors (n = 8) were analyzed were analyzed in vitro and ex vivo
regarding their different effector functions. Secondly, we used a murine IPA model
(C57BL/6) and treated CsA-treated or LysM-specific NFATc1 knockout (NFATc1LysM) mice
with Aspergillus fumigatus intratracheally. PMN recruitment to the lungs and pulmonary
fungal clearance were examined by analyzing bronchoalveolar lavages (BAL) and peripheral
blood (PB) using flow cytometry and cytometric bead array and murine lungs by fungal
culture assays and histopathologic examination. Furthermore, survival was studied
with neutropenic animals serving as positive controls. In addition, we investigated
myelopoiesis and myeloid differentiation by quantifying bone marrow derived myeloid
progenitor cells from CsA treated or NFATc1LysM mice using flow cytometry and simultaneously
counting PMN in PB under steady state conditions.
Results: CsA significantly enhanced phagocytosis of PMN in vitro and ex vivo in patients’
blood samples. Moreover, PMNs migratory capabilities were reduced in vitro, whereas
other effector functions or release of IL-8 were rather unaffected. PMNs of CsA-treated
patients showed increased activation, degranulation and production of inflammatory
mediators, but production of ROS was slightly decreased. In our in vivo model, IPA
was lethal in neutropenic mice whereas solely CsA or vehicle treated mice survived
the infection. CsA treatment resulted in enhanced PMN recruitment in BAL by trend,
while pulmonary inflammation and PMN counts in PB remained stable. Indeed, fungal
clearance was clearly constrained in CsA treated animals. In our murine knockout model,
NFATc1LysM mice infected with Aspergillus fumigatus showed unimpaired survival without
displaying detectable differences in PMN recruitment or fungal clearance. However,
pulmonary inflammation and PMN counts in PB seemed to be more pronounced in knockout
mice. Interestingly, BALs of CsA treated mice showed increased levels of IL-6 by trend
but decreased levels of MCP-1 and TNF-α. In contrast, MCP-1, RANTES and TNF-α were
enhanced by trend in BALs of NFATc1LysM mice, while IL-6 was reduced compared to wild
type controls. PMN counts in PB were unaffected in NFATc1LysM mice but distribution
of bone marrow derived murine myeloid progenitor cells was significantly impaired
especially in megakaryocyte-erythroid progenitor cells, whereas solely CsA treatment
had no influence.
Conclusion: Results from our in vitro and ex vivo studies on patients’ blood samples
as well as from our murine in vivo IPA model indicate that NFAT regulates not only
myelopoiesis, but also PMN functionalities in mice and humans. Nevertheless, these
interactions are obviously multidimensional and potentially derive from involvement
of different pathways. The underlying molecular mechanisms and clinical relevance
of our findings in HSCT remain to be determined.
Naruse
H.
Ito
T.
Noshi
E.
Tanaka
Y.
Maki
H.
SHIONOGI & CO., LTD., Osaka, Japan
P375. Novel assay for sterol 24-C-methyltransferase enzymatic activity, a primary
antifungal target of Abafungin
Objectives: Abafungin is a broad-spectrum antifungal agent. It has been presumed that
abafungin inhibits sterol 24-C-methyltransferase (SMT) from the report that abafungin
decreased L-[Methyl-14C]methionine incorporated intracellular sterol. SMT is one of
the enzymes involved in ergosterol biosynthesis pathway which is well-known antifungal
target (e.g. azoles). As erg6 gene encoding SMT is conserved in many fungal species,
SMT could be a promising drug target. However, it is not known whether abafungin actually
inhibits SMT enzymatic activity, and there is no high throughput SMT enzyme assay
for drug discovery. Therefore, we developed a novel SMT enzymatic assay by homogeneous
time-resolved FRET (HTRF). We also evaluated the effects of SMT inhibition on antifungal
activity.
Methods: HTRF is based on a competitive immunoassay between SAH, byproduct of SMT
reaction, and d2 labelled SAH (SAH-d2) towards Lumi4-Tb cryptate labelled anti-SAH
antibody. A. fumigatus SMT and its substrates, lanosterol and SAM, were mixed and
incubated at room temperature, then SAH-d2 and anti-SAH antibody were added. After
incubation at room temperature, HTRF signal was measured. The HTRF ratio was calculated
as a two-wavelength signal ratio (665 nm/620 nm). SMT inhibitory activity of abafungin
and its derivatives were calculated as IC50 values. MICs of these compounds against
A. fumigatus and C. albicans were measured according to the method of the CLSI.
Results: SAH was detected by 0.001 μM to 0.1 μM in the optimized condition and SMT
produced SAH in a dose dependent-manner. SMT of microsomal fraction indicated 3-fold
higher enzymatic activity than that of cytoplasmic fraction. In this HTRF assay abafungin
inhibited SAH production and exhibited IC50 of 0.21 μM. The MICs of abafungin against
A. fumigatus and C. albicans were 2 μg/ml and 0.0625 μg/ml, respectively. The derivatives
without SMT inhibitory activity didn’t exhibit antifungal activity against A. fumigatus
and C. albicans.
Conclusion: We established a novel assay to evaluate A. fumigatus SMT activity. Abafungin
was revealed to inhibit SMT enzymatic activity, which led to antifungal activity.
This result would help us develop new antifungals targeting SMT.
Kress
M.R. Von Zeska
1
Tonani
L.
1
Fonseca
L.M.
1
Almeida
O.G.G.
1
Capizzani
C.P.D.C.
1
Martinis
E.C.P. De
1
Torres
L.A.G.M.M.
2
1Faculdade De Ciencias Farmacéuticas De Ribeirao Preto, Universidade de Sao Paulo,
Ribeirao Preto-SP, Brazil
2Faculdade De Medicina De Ribeirão Preto, Universidade de São Paulo, Ribeirao Preto-SP,
Brazil
P376. Metagenomic analysis and culture-dependent diagnosis of cystic fibrosis patient
airways
Case Report: Cystic fibrosis (CF) is a debilitating autosomal recessive disease in
which the patient carries a mutation in the Cystic Fibrosis Transmembrane Conductance
Regulator (CFTR) gene, which encodes a chloride channel involved in electronic exchanges
through the plasma membrane of epithelial cell types. Consequently, a defective lung
mucociliary clearance occurs, producing thick and sticky bronchial mucus, suitable
for entrap airborne bacteria and fungi. The culture-dependent diagnostics of CF patients
airways show the frequent colonization by Haemophilus influenzae, Staphylococcus aureus,
Pseudomonas aeruginosa, Burkholderia cepacia complex and atypical mycobacteria. Additionally,
prolonged antibiotic treatments contribute to the colonization by multidrug-resistant
pathogens such as Achromobacter xylosoxidans and Stenotrophomonas maltophilia. On
the other hand, culture-independent technologies, as the metagenomic approach, revealed
that the CF patients airways are not inhabited by these few pathogens, but by complex
polymicrobial communities. Fungal species are usually found colonizing the CF patients
airways (e.g.Aspergillus fumigatus and Candida albicans), however, few metagenomics
studies are reported in the literature. Here, we show the culture-dependent identification
and metagenomic approach (16S ribosomal DNA and the internal transcribed spacers (ITS)
of the nuclear ribosomal DNA) of CF patient sputum. The detailed description of the
CF lung microbiome is presented and compared with culture-dependent identification.
The isolation of microorganisms on media culture from the sputum of the CF patient
revealed the bacteria Stenotrophomonas maltophilia, Pseudomonas aeruginosa, Achromobacter
xylosoxidans and Staphylococcus aureus, and the fungi Aspergillus fumigatus and Candida
albicans. The identification was performed by classical and molecular methods, respectively.
As the culture-based identification, the 16S and ITS metagenome revealed the bacteria
Stenotrophomonas sp., Pseudomonas putida, Achromobacter sp. and Staphylococcus aureus,
and the fungi Aspergillus fumigatus and Candida albicans. But metagenome additionally
identified Streptococcus sp., Neisseria sp. and Saccharomyces cerevisiae in high relative
abundance, and Tectona grandis, Actinobacillus sp., Schizophyllum commune, Agaricales
order and Halosphaeriaceaefamily in intermediate and low relative abundance. Thus,
the culture-based analyses correlated qualitatively with the relative abundance of
data on obtained bacterial and fungal taxa. However, additional identifications were
obtained by metagenome data. Here we observed that the taxonomic identification by
Regarding 16S and ITS metagenome to the species level were not achieved for the fungal
kingdom and the estimation of the relative abundance of species in the community is
imprecise. This is justified by the primer-based amplicons sequencing method which
has a limited ability to resolve this taxonomic identification. Thus, although we
have identified several species colonizing the CF patients airways, the metagenome
shotgun approach is indicated to provide information about the composition of the
community up to the level of clonal complexes.
Meir
Z.
1
Sandovsky
H.
1
Shadkchan
Y.
1
Masrati
G.
2
Wiemann
P.
3
Ben-Tal
N.
2
Keller
N. P.
3
Osherov
N.
1
1Clinical Microbiology and Immunology, Tel Aviv University, Tel Aviv, Israel
2Biochemistry & Molecular Biology, Tel Aviv University, Tel Aviv, Israel
3Medical Microbiology and Immunology, University of Wisconsin, Madison, United States
of America
P377. Mechanisms of resistance to elevated copper in the pathogenic mold Aspergillus
fumigatus
Objectives:
Aspergillus fumigatus is the most common opportunistic mold pathogen in the growing
immunocompromised population. Patients with weak immune systems are at risk to develop
Invasive Aspergillosis (IA), a deadly type of infection with high mortality rates
caused by A. fumigatus. Fungi use unique pathways to control the levels of essential
metals inside the cell. Copper is a transition metal essential for all living systems
and its homeostasis in A. fumigatus under copper excess is dependent on the copper
sensing transcription factor AceA and its downstream target, the copper transporter
CrpA. Studies suggest that genes participating in copper homeostasis can be promising
drug targets. Our goals were to reveal the detailed function of the copper transporter
CrpA and to identify and characterize additional participants acting in the Cu-resistance
pathway of A. fumigatus and assess their relevance to virulence.
Methods: To achieve these goals we constructed a model structure of A. fumigatus CrpA,
in collaboration with Prof. Nir Ben-Tal, and identified evolutionarily conserved fungal-specific
domains in the protein. We generated GFP-tagged CrpA and studied its subcellular localization.
We performed RNAseq analysis of A. fumigatus exposed to high copper levels and deleted
selected upregulated genes. Finally, we performed a screen to identify genes that
provide Cu-resistance when overexpressed.
Results: Significant progress was made in the localization of CrpA protein. We located
the protein mostly in the ER but also in the membrane. We also created an inducible
overexpression strain of CrpA using the xylose-inducible promoter xylP and showed
that CrpA overexpression confers increased copper resistance when exposed to copper
in vitro but not in vivo. We generated deletion strains for six genes that were up-regulated
in WT vs AceA-null strains following exposure to copper as identified by RNA-seq.
These genes are chaperones and metal-binding proteins that may participate in A. fumigatus
copper resistance. The phenotypes of these deletion strains will be described. We
performed a genetic screen using an overexpression library in which A. nidulans was
transformed with the AMA-1-based overexpression genomic library and plated on excess
Cu. We found four new genes that confer resistance to A. nidulans
Conclusion: Our results provide a more detailed picture of copper resistance in A.
fumigatus, indicating that is dependent on the combined activities of the CrpA copper
exporter, Cu-chaperones and accessory stabilizing proteins.
Nascimento
E.
1
Barião
P.H.G.
2
Kress
M. Von Zeska
2
Martinez
R.
1
1Department of Internal Medicine, Medical School /University of Sao Paulo/Ribeirao
Preto, Ribeirão Preto, Brazil
2school of Pharmaceutical Sciences of Ribeirao Preto, University of Sao Paulo, Ribeirão
Preto, Brazil
P378. Genotyping of Cryptococcus spp. isolated from HIV-negative patients with Neurocryptococcosis
Objectives: The aim of this study was to determine the species and molecular types
of Cryptococcus spp. isolates from patients not co-infected with HIV from the Medical
Hospital of Ribeirao Preto - Medical School of Ribeirão Preto - University of Sao
Paulo – Brazil, from 2000 to 2017, and correlate with the comorbidity of the patients.
Methods: A total of 43 strains of Cryptococcus spp. isolated from 43 patients not
co-infected with HIV were genotyped. The species were determined by PCR with the primers
CN70 and CN49. The molecular types C. neoformans VNI, VNII, VNIII and VNIV, and C.
gattii VGI, VGII, VGIII and VGIV were determined by PCR-RFLP with the amplification
of the URA5 gene and subsequent enzymatic restriction with HhaI and Cfr13I. The identification
of C. gattii and C. laurentii was confirmed by the sequencing of the rDNA ITS regions.
Results: From a total of 43 clinical isolates, 28 were identified as C. neoformans,
13 as C. gattii and 2 as C. laurentii. Among C. neoformans clinical isolates, 27 were
identified with the molecular type VNI and 1 VNII. Additionally, 2 C. gattii VGI and
11 C. gattii VGII were identified. The correlation between species and patient comorbidity
revealed 28 clinical cases (Group 1) of patients not coinfected with HIV but with
other underlying diseases (e. g. diabetes, adenocarcinoma, chronic lung disease, transplants,
systemic lupus erythematosus and others) and 15 clinical cases (Group 2) of immunocompetent
patients. Most of the isolates identified in Groups 1 were C. neoformans (75%) and
in Group 2 were C. gattii (47%). C. laurentii were isolated from the Group 2.
Conclusion: Most of the clinical isolates were identified as C. neoformans and were
more present in individuals not co-infected with HIV but who had another underlying
disease. On the other hand, C. gattii is more frequent in the group of immunocompetent
patients. The molecular types VNI and VGII were the most identified in our study,
which corroborate with other studies carried out in Southeast Brazil.
Sacheli
R.
1
Tomé
C.
1
Adjetey
C.
1
Darfouf
R.
1
Palmeira
L.
2
Hayette
M.-P.
1
1Clinical Microbiology- Nrc For Mycoses, University Hospital of Liège, Liège, Belgium
2Human Genetics, University Hospital of Liège, Liège, Belgium
P379. Classification of dermatophytes by a multilocus phylogenetic approach based
on Tef-1α, beta tubulin and ITS genes.
Objectives: Identification of dermatophytes to the species level is epidemiologically,
ecologically and therapeutically significant. The use of phylogenetic species concepts
based on rDNA internal transcribed spacer (ITS) regions have improved the taxonomy
of dermatophytes. However, it has been shown that for some species, confirmation and
refinement using other genes are needed. Indeed, there are problems with accurate
definition and characterization of dermatophytes especially among the Trichophyton
mentagrophytes series. Intra- and interspecies variations of the translation elongation
factor 1-α (Tef-1α) gene were evaluated as a new identification marker in a wide range
of dermatophytes. The aim of this study was to evaluate the discriminatory power of
a phylogenetic tool based on concatenated sequences of three genomic regions including
Tef-1α, ITS (ITS1 to ITS2) and beta-tubulin to differentiate: 1/ the anthropophilic
Trichophyton interdigitale species from the zoophilic Trichophyton mentagrophytes
2/ the two closely related African anthropophilic species Trichophyton violaceum from
Trichophyton soudanense.
Methods: 26 well characterized strains of T. interdigitale/mentagrophytes have been
selected including 3 IHEM reference strains; 30 well characterized strains of T. soudanense/T.violaceum
including 2 IHEM reference strains were also included. All strains were submitted
to ITS, beta-tubulin and Tef-1α PCR/sequencing. Moreover, identification was completed
by real time PCR DermaGenius® (PathoNostics). Alignments were performed using MUSCLE
in Seaview. The alignments for each of the three genes were then concatenated together
based on the name of the sample and gaps were added between genes. Phylogenies were
then inferred on the concatenated alignment using PhyML.
Results: After generation of the phylogenetic tree by concatenation of the three sequence
genes, the differentiation between T. interdigitale and T. mentagrophytes was clear.
The 16 strains of T. interdigitale were well classified into one distinct group on
the dendrogram. The 10 strains of zoophilic T. mentagrophytes were well distinct of
the anthropophilic group and defined by another clade on the dendrogram. Reference
strains were correctly classified into the two previously defined groups. Regarding
the differentiation between T. soudanense and T. violaceum, the concatenation permitted
to define two well distinct clades on the dendrogram. One clade containing the 10
T. violaceum tested and the other containing the 20 T. soudanense included in the
study. Reference strains were correctly classified into each corresponding clade.
Conclusion: The concatenation of ITS, beta-tubulin and Tef-1α genes sequences allows
the generation of a discriminating dendrogram between the zoophilic T. mentagrophytes
and the anthropophilic T. interdigitale and between the two closely related African
anthropophilic species T. violaceum and T. soudanense. These species are not clearly
well defined by ITS analysis only. This multilocus phylogenetic approach allows to
better define the species boundaries between these dermatophytes species and facilitates
the molecular characterization of these species in routine diagnostic.
Oliveira
M.
1
2
Pinto
M.
3
Verissimo
C.
4
Sabino
R.
2
1Animal Biology Department, Faculty of Sciences of the University of Lisbon, Lisbon,
Portugal
2Reference Unit For Parasitic and Fungal Infections, Infectious Diseases, National
Health Institute Dr. Ricardo Jorge, Lisbon, Portugal
3Bioinformatics Unit, Infectious Diseases, National Health Institute Dr. Ricardo Jorge,
Lisbon, Portugal
4Infectious Diseases, Reference Unit for Parasitic and Fungal Infections, National
Health Institute Dr. Ricardo Jorge, Lisbon, Portugal
P380. rPulmonary mycobiome of patients with suspicion of respiratory fungal infection
– an exploratory study
Objectives: This pilot study aimed to characterize the pulmonary mycobiome of patients
with suspicion of fungal infection of the respiratory tract as well as to identify
potentially pathogenic fungi colonizing/infecting their lungs.
Methods: A cohort of 10 patients was analyzed, including HIV+ patients and patients
with active infection caused by Mycobacterium species. Their respiratory samples (bronchoalveolar
lavage fluid/ bronchial secretions) were pre-treated with lyticase and proteinase
K; DNA was extracted using the High Pure PCR Template Preparation kit following the
manufacturer’s instructions. The internal transcribed spacer region 1 (ITS1) and calmodulin
gene were amplified by PCR and the resulting amplicons were sequenced using the Illumina
MiSeq platform with pair-end reads of 150 bp. The obtained results were analyzed using
the PIPITS pipeline as described by Gweon et al. [1]. Operational taxonomic units
(OTU) to which less than 0.1% of the total reads attributed were disregarded.
Results: Thirty-seven different OTU were identified from which two belonged to the
Plantae kingdom, 11 had less than the 0.1% threshold of the total reads and were therefore
disregarded. The remaining 24 different OTU (grouped in 17 phylotypes), were considered
as part of the pulmonary mycobiome of patients. Two phyla were identified: Basidiomycota
(33.3%) and Ascomycota (54.2%). Regarding the Basidiomycota phylum, reads were classified
in three classes (Agaricomycetes, Tremellomycetes and Walleomycetes), while for the
Ascomycota phylum four different taxonomical classes were identified: Pneumocystidomycetes,
Dothideomycetes, Eurotiomycetes and Saccharomycetes, with the latter being the most
frequent class. Twelve fungal genera were identified, being Candida the most frequently
detected. The median number of fungal genera detected in patients’ pulmonary mycobiome
was six (ranging from two up to nine). The genus Papilotrema and the potentially pathogenic
genera Cryptococcus and Pneumocystis were exclusively found in the pulmonary mycobiome
of HIV+ patients. Other potentially pathogenic fungi such as Aspergillus spp., Trichosporon
spp., Saccharomyces spp. and Schizophyllum spp. were also detected.
Conclusion: This pilot study illustrates how the pulmonary mycobiome is rich and highly
variable in patients with fungal infections. The obtained results suggest that the
described metagenomic analysis may possess a great ability to quickly and effectively
detect potentially pathogenic fungi in the mycobiome of patients, making it a promising
future diagnostic tool. Thus, further optimization, standardization and clinical validation
of these NGS methodologies should be warranted in the future. Reference: Gweon HS,
Oliver A, Taylor J, Booth T, Gibbs M, Read DS, Griffiths RI, Schonrogge K. PIPITS:
an automated pipeline for analyses of fungal internal transcribed spacer sequences
from the Illumina sequencing platform. Methods Ecol Evol. 2015 Aug;6(8):973-980.
Duvaux
L.
1
2
Shiller
J.
3
Vandeputte
P.
2
Bahut
M.
4
Lemaire
C.
1
Meyer
W.
5
Oyant
L. Hibrand Saint
4
Renou
J.P.
3
Cam
B. Le
1
Bouchara
J.-P.
2
Gastebois
A.
2
1Irhs-ecofun, INRA, Beaucouzé, France
2Geihp, Université d’Angers, Angers cedex, France
3Irhs-bio-info, INRA, Beaucouzé, France
4Sfr-quasav-anan, Université Angers, Beaucouzé, France
5Molecular Mycology Research Laboratory, University of sydney, Sydney, Australia
P382. Comparative genomic analysis of Scedosporium species and identification of pathogenicity
determinants.
Objectives:
Scedosporium species are soil saprophytes that may cause a large variety of infections
in human. Especially, some of them are capable to chronically colonize the lungs of
patients suffering from cystic fibrosis (CF) which constitutes a major risk of disseminated
infection in case of lung transplantation. Molecular and biochemical analysis revealed
that the genus Scedosporium comprises at least ten distinct species with differences
in their susceptibility to antifungals and in their pathogenicity. Among them, three
species, S. boydii, S. apiospermum and S. aurantiacum are predominant in CF patients,
while other species like S. minutisporum are occasionally encountered in this clinical
context. Conversely, S. dehoogii has never been reported from respiratory secretions
of CF patients whereas some environmental studies revealed its common occurrence in
soils where it accounts for up to 40% of all Scedosporium isolates. The aim of our
project was to identify by a comparative genomic approach, regions of the genome or
genes that might be responsible for the ability of Scedosporium species to establish
within the respiratory tract of CF patients and to cause chronic colonization of the
airways.
Methods: Five clinical or environmental isolates from each of the five species S.
boydii, S. apiospermum, S. aurantiacum, S. minutisporum, and S. dehoogii were sequenced.
To identify candidate proteins that may enable Scedosporium species to establish within
the respiratory tract of CF patients, we first searched clusters of orthologous proteins
conserved in all isolates of the pathogenic species, but absent in S. dehoogi isolates.
Genomic data were also analyzed searching for genes present in all pathogenic species,
as well as in S. dehoogii, but exhibiting in this last species point mutations potentially
leading to a loss of function.
Results: At the beginning of the project, the whole genome of a first strain of S.
apiospermum sensu stricto (Vandeputte et al., 2014) and of S. aurantiacum (Pérez-Bercoff
et al., 2015) were already available. The whole-genome sequencing project allowed
us to obtain a total of 2.4-8.9 mega-reads per isolate which permitted good quality
draft genome assemblies. For 3 species, we did de novo sequencing, genome assembly
and performed comparative genomic analysis with the 25 strains. Finally, comparative
genomics allowed us to identify a series of 134 genes lost or with a potential loss
of function in S. dehoogii but still functional in all other species.
Conclusion: This comparative genomic analysis of the main Scedosporium species allowed
us to identify a set of candidate genes potentially involved in the adaptative mechanisms
developed by pathogenic Scedosporium species to establish within the respiratory tract
of patients suffering from CF. Functional genomic approaches focused on the genes
here identified will now be developed to elucidate their role in pathogenesis. Acknowledgment
This project was financed by Angers University with the AAP-CS program.
Alimu
Y.
Tanaka
R.
Ban
S.
Yaguchi
T.
Division of Bio-resource, Medical Mycology Research Center, Chiba University, Chiba,
Japan
P383. Molecular phylogenetic study of strains morphologically identified as Exophiala
dermatidis from clinical and environmental specimens in Japan
Objectives: With support from the National Bio-Resource Project, at the Medical Mycology
Research Center (MMRC), Chiba University, the collection, storage and distribution
of pathogenic fungi are carried out mainly on clinical isolates. The molecular phylogenetic
position of the isolates, the mycological characterization, the genomic and the clinical
information are managed and added to the database to increase the value of the stock
and lead to an increase in the number of aliquots. Since old isolates are morphologically
identified and conserved, their identifications are reviewed as needed based on the
base sequence of the ITS region of rDNA. We report here on Exophiala dermatitidis,
which is an important pathogen of deep-skin dermatomycosis, it has been reconfirmed
with identification and fitted with the latest classification. E. dermatitidis has
been reported to have a molecular phylogenetically polymorphism in strains from South
America. However, the investigation of the distribution in Japan has not been reported.
The aims of this study, is investigate the phylogenetic distribution of E. dermatitidis
in Japan.
Methods: In this investigation, we examined 66 clinical and environmental isolates
morphologically identified and conserved as E. dermatidis previously, which were stocked
in Bioresource Management Office in MMRC. The identification based on the ITS region.
The sequences were aligned and compared through neighbor-joining phylogenetic tree
analyses on the ITS1 region, using the Molecular Evolutionary Genetics Analysis (MEGA)
version 7.0.26 software package. Furthermore, the strains of different gene types
will test for mycological characterization such as the growth temperature, the growth
rate, and the drug sensitivity.
Results: The examined 66 clinical and environmental isolates were composed of 47 ones
from Japan, 4 ones from USA, 5 ones from Brazil, 6 ones from Venezuela and 4 ones
from China. According to establish a phylogenetic tree for the ITS1 region, we knew
that 45 of 47 isolates from Japan, 2 of 4 ones from China, 2 of 6 ones from Venezuela
and all of 4 ones from the USA were classified to the gene type A1. Only 2 of Japan
and China‘s isolates belonged to the gene type A2, respectively. Interestingly, all
of the isolates from Brazil were part of the gene type B and most of the Venezuela
samples were close to the gene type C. The relationship between the types and their
mycological characterizations was examined.
Conclusion: In our study, it is confirmed that, in South American strains, polymorphisms
were found molecularly within species, and molecular phylogenetic diversity of the
isolates in Japan was relatively uniform.
Lackner
M.
1
Keniya
M.V.
2
Monk
B.
2
1Division of Hygiene and Medical Microbiology, Medical University of Innsbruck, Innsbruck,
Austria
2Sir John Walsh Research Institute, University of Otago, Dunedin, New Zealand
P384. Investigation of the impact of amino acid changes in the substrate binding site
of fungal sterol 14α-demethylase on protein structure and function using Saccharomyces
cerevisiae as an expression model.
Objectives: Mucormycetes are intrinsically short-tailed azoles resistant. We used
the high resolution X-ray crystal structure of S. cerevisiae lanosterol 14α-demethylase
(LDM) in complex with voriconazole (VCZ) to postulate that the amino acid substitution
Y129F in Rhizopus arrhizus LDM (RaLDM) F5 is associated with their intrinsic resistance.
Our aim was to experimentally test this hypothesis.
Methods: Full-length recombinant C-terminal hexahistidine-tagged LDM (F1 and F5) of
R. arrhizus were overexpressed from the PDR5 locus in a S. cerevisiae host lacking
7 drug efflux pumps. Their cognate NADPH-cytochromeP450 reductase (RaNCP) was overexpressed
from the PDR15. Phenotypic and biochemical analysis were used to determine structural
and functional features of RaLDMF1, RaLDMF5, without and with co-expression of RaNCP.
Results: RaLDMF1 and F5 were functionally overexpressed in S. cerevisiae. RaLDMF5
appeared to be the major contributor to azole resistance, with its expression resulting
in a 32x higher VCZ minimal inhibitory concentration (MIC) than the recipient strain.
Co-expression of RaLDMF5 with NCP resulted in additional 4-fold increase of VCZ MIC
when compared with RaLDMF5 single expression. MICs for long-tailed azoles were unaffected
by the LDM supplementation. S cerevisiae co-expressing RaLDMF1 with NCP had similar
MIC values as the recipient strain. Azole-susceptibility patterns of S. cerevisiae
co-expressing RaLDMF5 and RaNCP closely matched those of R. arrhizus. Phenotypic analysis
of recombinant LDMs expressed in S. cerevisiae provides insights on the relevance
of mutations located in the substrate binding pocket. This system allows assessment
of effects on the drug target without interference from drug efflux pathways.
Conclusion: LDMF5 was found to be the dominant isoform in R. arrhizus that confers
short-tailed azole resistance. Biochemical and structural analysis of the purified
protein are expected to provide deeper understanding of the observed phenotypes.
Gangneux
J.P.
1
Vandenborght
L.-E.
2
Bidon
B.
3
Belleguic
C.
4
Neuville
E. De
4
Guegan
H.
1
Leroy
S.
5
Laviolle
B.
4
Horsley
A.
6
Denning
D.
7
Cuomo
C.
8
Bouchara
J.-P.
3
Delhaes
L.
9
Papon
N.
10
1Laboratoire De Parasitologie-mycologie, CHU de Rennes, Rennes, France
2Genoscreen, Lille, France
3CHU d’Angers, Angers, France
4CHU de Rennes, Rennes, France
5CHU de Nice, Nice, France
6University Hospital of South Manchester, Manchester, United Kingdom
7University of Manchester, Manchester, United Kingdom
8Broad Institute of MIT and Harvard, Cambridge, United States of America
9Parasitology Mycology, Univeristy of Bordeaux, Centre de Recherche Cardio-Thoracique
de Bordeaux, U1045, F-33000, Bordeaux, France, Bordeaux, France
10Ea Geihp, UFR Pharmacie d’Angers, Angers, France
P385. A metagenomic and whole genome sequencing comparative approach to study the
impact of azole therapy on chronic colonisation with Aspergillus fumigatus during
cystic fibrosis.
Objectives: The impact of early azole therapy during cystic fibrosis (CF) remains
debated. The ATCF (Antifungal Therapy during Cystic Fibrosis) is a longitudinal phase
III study with the following objectives: - to assess the microbiological and clinical
effects of azole therapy: negativation of sputum cultures for Aspergillus, course
of FEV1, clinical signs and quality of life, and the impact on co-prescription of
antibiotic courses and/or steroid therapies. - to study the genomic impacts of azole
treatment on the evolution of micro- and mycobiota and on genomic evolution of Aspergillus
isolates.
Methods: The ATCF study followed CF patients with a chronic colonisation with A. fumigatus
and randomized to receive an early oral azole therapy with itraconazole (ITRA) or
voriconazole (VORI) for 4 to 6 months in a phase III study. A long-term follow-up
was conducted until 2 months after end of treatment (EOT). All sputa were submitted
to DNA extraction for further metagenomic approach to determine the fungal (ITS2)
and bacterial (16S) microbiota. Besides, twenty isolates of Aspergillus fumigatus
recovered before and after treatment were sequenced with a shotgun strategy.
Results: Eleven patients were included and followed until 2 months after EOT. All
assessable patients had a successful outcome, with a negativation of sputa, an increase
in FEV1 and a decrease in the number of antibiotic/steroids co-prescriptions. However,
2 months after EOT, most of clinical assessment criteria were similar to the baseline
values. Fungal diversity increased under treatment with a large decrease of the more
abundant fungal taxa, particularly Aspergillus. However, 2 months after EOT the fungal
diversity returned to baseline values and Aspergillus reappeared, mostly in a larger
proportion. Besides, Streptococcus, Pseudomonas and Staphylococcus were the main genera
detected and their proportion increased under azole therapy. The bacterial communities
were similar after EOT compared to before. The analysis of micro – and mycobiota must
be further documented. All SNPs and InDels variants were determined for 20 isolates.
Isolates from a single sputum and more generally from a single patient during treatment
were closely related. By contrast, isolates obtained 2 months after EOT were far different
suggesting reinfections with new genotypes. In particular, one azole resistant A.
fumigatus was recovered with a TR34/L98H mutation of the CYP51A gene, suggesting an
environmental origin rather than an acquired resistance under azole drug pressure.
Conclusion: When a chronic colonisation with A. fumigatus is associated with clinical
deterioration, EOT can temporarily reduce airway infection and inflammation, and extend
periods of stable disease status. While only a limited number of patients were included,
the longitudinal phase III design of ATCF study combined to a genomic approach underlines
the need for azole therapy rationalization during CF. Indeed, the reversible effects
2 months after the EOT and the limited tolerance of both ITRA and VORI encourages
short courses of therapy driven by the clinical improvement. The longitudinal changes
of the mycobiota were restored 2 months after EOT and genetic characterization of
isolates showed that reinfection with new genotypes is more frequent than Aspergillus
in host evolutions under azole treatment.
Garcia-Rubio
R.
1
Clear
A.
1
Healey
K.R.
2
Shor
E.
1
Perlin
D.S.
1
1Center For Discovery and Innovation, Hackensack Meridian Health, Nutley, United States
of America
2Department of Biology, William Paterson University, Wayne, United States of America
P386. Role of Candida glabrata candidate genes in echinocandin tolerance and resistance
development
Objectives:
Candida glabrata is the second most prevalent bloodstream fungal pathogen in North
America and Europe, and its prevalence is rising. Echinocandin class compounds are
first-line agents for the treatment of these infections, but therapeutic failures
are increasingly encountered. However, the reasons why C. glabrata shows elevated
potential to develop echinocandin resistance, relative to other Candida sp., are currently
not well understood. Although echinocandins are fungicidal drugs, multiple cellular
mechanisms can stabilize C. glabrata during drug exposure and promote drug tolerance,
allowing cells to survive long enough to acquire mutations leading to stable clinical
drug resistance. Interestingly, genomic analyses of different C. glabrata clinical
isolates have revealed a high level of genetic diversity both within the same MLST
sequence type (ST) clade and between them. Depending on the population subset, genomic
plasticity may be responsible for differences in tolerance phenotype. The objective
of our research is to elucidate the factors involved in promoting echinocandin drug
tolerance in C. glabrata.
Methods: Because echinocandins target the fungal cell wall, we have focused on interrogating
the role of cell wall integrity pathway components in promoting echinocandin tolerance.
We have used an in vitro killing assay developed in our group to measure tolerance
over a range of micafungin concentrations (0.016-32 µg/ml). Multiple knock-out mutants
(∆slt2, ∆snf1, ∆rlm1, ∆sbe2, ∆mid2, ∆cka1, ∆cbk1, ∆ypk2, ∆slg1, ∆yps1, ∆ada2, ∆mot3,
∆gas1, ∆rta1, ∆chs5, ∆crz1, ∆pdr1, ∆ssd1, ∆sbe22, ∆chs3) were tested in this assay
looking for significant changes in tolerance phenotypes. Additionally, to identify
genetic polymorphisms that may be influencing echinocandin tolerance, we have sequenced
some of the most relevant cell wall integrity pathway genes, particularly focusing
on those whose deletion was found to affect drug tolerance in the killing assay.
Results: Most of the knock-out mutants showed no or minor changes in echinocandin
tolerance. However, three mutants – ∆yps1, ∆ypk2 and ∆slg1 – resulted in significantly
reduced tolerance to micafungin. These genes were sequenced in a number of C. glabrata
echinocandin sensitive clinical isolates. Different polymorphism combinations were
found in these strains, with specific polymorphisms being characteristic of particular
STs. Several of these genetic polymorphisms resulted in amino acid changes.
Conclusion: Changes in micafungin tolerance phenotype were found in mutants lacking
certain cell wall integrity pathway genes, implicating them in promoting drug tolerance
in C. glabrata and representing potential targets to limit development of antifungal
drug resistance. Further studies will evaluate whether polymorphisms in these genes
are responsible for variation in drug tolerance among clinical strains.
Nagy
G.
1
2
Szebenyi
C.
1
2
Vaz
A.
1
2
Jáger
O.
1
2
Ibragimova
S.
1
Gu
Y.
3
Ibrahim
A.S.
3
Vágvölgyi
C.
1
Papp
T.
1
2
1Deapartment of Microbiology, University of Szeged, Szeged, Hungary
2MTA-SZTE “Lendület” Fungal Pathogenicity Mechanisms Research Group, Szeged, Hungary
3Los Angeles Biomedical Research Institute at Harbor-UCLA Med Center, Torrance, United
States of America
P387. Genetic modification of opportunistic pathogenic Mucoromycotina species using
a plasmid free CRISPR/Cas9 system
Objectives: Most fungal species, which can cause human infections belong to the genera
Candida and Aspergillus, but in the recent years, the number of infections caused
by Mucoromycotina species (e.g., Rhizopus oryzae, Lichtheimia corymbifera and Mucor
circinelloides) has significantly increased. The properties and potential virulence
factors that make the Mucoromycotina species enable to cause disease (mucormycosis)
in patients, are not known. Potential virulence factor genes may play role in fungal
dimorphism, proteolytic enzyme production, iron uptake, and production of cell surface
proteins.
Methods: Genetic manipulation of Mucoromycotina species, based on homologous recombination
still can be a great challenge. In this study, a plasmid free CRISPR/Cas9 system was
used for the genetic manipulation of Mucor, Lichtheimia and Rhizopus species. PEG-mediated
protoplast transformation was used to introduce the Cas9 enzyme and the synthesized
gene-specific gRNA with or without a deletion cassette into the fungus.
Results: In case of Lichtheimia and Rhizopus, the plasmid free CRISPR/Cas9 system
was successfully used to disrupt the pyrG gene by NHEJ. Our results suggested that
the disruption of the pyrG gene has no effect on the growth of fungal strains. These
strains and the developed methods will be suitable for further transformation procedure
in the future.
Conclusion: Based on the potential virulence factor genes (cotH, svf, cyp51, hsbA),
a mutant library has been constructed from M. circinelloides. We have started to analyse
the function of these genes in the pathogenesis and azole resistance of Mucor. This
study was supported by the “Lendület” Grant of the Hungarian Academy of Sciences (LP2016-8/2016)
and the Hungary grant 20391-3/2018/FEKUSTRAT of the Ministry of Human Capacities.
Dellière
S.
1
Angebault
C.
1
2
Fieux
M.
3
Bonfils
P.
3
Podglagen
I.
4
Woerther
P.-L.
2
5
Dannaoui
E.
4
Botterel
F.
1
2
1Mycology and Parasitology, Henri-Mondor, Créteil, France
2Ea Dynamyc Upec, Université de Créteil, Créteil, France
3Ent, HEGP, Paris, France
4Microbiology, HEGP, Paris, France
5Microbiology, Hôpital Henri-Mondor, Créteil, France
P388. Mycobiome and microbiome in chronic fungal rhinosinusitis
Objectives: Fungal ball rhinosinusitis is the main type of non-invasive fungal rhinosinusitis,
presenting as a dense mass of mycelium obstructing the sinus. Despite fungal hyphae
being observed on direct examination of biopsy samples, culture remains negative in
more than 60% of cases.1 The aim of this study was to evaluate the performances/efficacy
of targeted metagenomics to describe and analyze bacterial and fungal microbiota in
fungal balls compared to other types of sinusitis and find possible microbial co-occurrence/associations
intra or extra kingdom.
Methods: A total of 46 sinus biopsies were studied from 46 patients who underwent
surgery for chronic rhinosinusitis from 2015 to 2017 in Paris, France. Direct examination
of biopsies was performed by calcofluor and culture was performed on Sabouraud media.
DNA was extracted and fungal ITS1 and ITS2 loci and bacterial V3-V4 16S ribosomal
DNA locus were amplified and sequenced (MiSeqTM Illumina, V2 kit, 500 cycles). To
analyze the microbial diversity, operational taxonomic units were defined via QIIME
and assigned to SILVA and UNITE databases for 16S and ITS, respectively. Statistical
analyses were performed using SHAMAN. These data were compared to microbiological
and clinical data.
Results: Among these 46 patients, 38 had fungal balls and 8 had non-fungal chronic
rhinosinusitis and were considered as controls. Among the 38 fungal balls, direct
examination and culture were positive for fungi in 95% and 30% of biopsies, respectively.
By contrast, targeted metagenomic analysis of the 38 fungal balls yielded one or more
fungal genera in 100% of cases, with Aspergillus spp. alone or in combination with
other fungi in 92.1% (35/38) of patients. Control samples (n = 8) generated only a
limited number of fungal reads, mainly from the genus Malassezia (4/8). Overall, the
main bacterial genera found by targeted metagenomics were Haemophilus (20.1%), Staphylococcus
(14.6%), Pseudomonas (7.66%) and Enterobacter (5.84%). Using principal coordinate
analysis (Bray-Curtis distance; permanova test), we found significantly different
bacterial and fungal metagenomic profiles in patients with fungal balls compare to
controls (p = 0.047 and p<0.001, respectively) (Figure 1). One bacterial taxon, Haemophilus,
was significantly over-represented in patients with fungal balls compared to controls
(p<0.001).
Conclusion: Targeted metagenomics allowed identification of fungal genera responsible
for chronic rhinosinusitis when the culture remains negative. Aspergillus was the
most commonly fungus identified. The genus Haemophilus appeared statistically associated
with fungal balls. Fungal balls pathogenesis could result from microbial interactions
between fungi and bacteria in mixed biofilm-like structure. 1. Jiang, R.-S., Huang,
W.-C. & Liang, K.-L. Characteristics of Sinus Fungus Ball: A Unique Form of Rhinosinusitis.
Clin Med Insights Ear Nose Throat (2018).
Perez-Cuesta
U.
Guruceaga
X.
Ramirez-Garcia
A.
Abad-Diaz-De-Cerio
A.
Hernando
F.L.
Rementeria
A.
Immunology, Microbiology and Parasitology, University of the Basque Country (UPV/EHU),
Leioa, Spain
P389. From swollen to germination; a transcriptomic approach in Aspergillus fumigatus
Objectives: The germination is the first adaptive process that Aspergillus fumigatus
must overcome to colonize the environment or infect animals. The A. fumigatus germination
process involves a first step known as swollen and the subsequent hyphae elongation.
Morphologically, both are well-characterized processes but molecularly there are only
a few studies describing the transition from swollen to germination in the literature.
This study, therefore, focuses on the transcriptomic analysis of the genes differently
expressed between swelling conidia and early hyphae output to understand the mechanisms
involved in these steps.
Methods: The A. fumigatus reference strain Af293 was incubated at 37°C without agitation
in RPMI supplemented with 5% fetal bovine serum for 4.5 and 6 hours to obtain swelling
conidia or early hyphae respectively. Total RNA was extracted and maintained in RNA
later until use. Finally, RNA samples were studied using the AWAFUGE microarray (Agilent
Whole A. fumigatus Genome Expression 44K v.1) designed by our research group.
Results: The transcriptomic analysis detected 158 and 256 genes overexpressed in the
swelling and early hyphal stages, respectively. GO enrichment analysis showed that
transport (13.2%), organelle organization (11.9%) and regulation (10.7%) were the
most common categories in the swelling stage, and transport (10.2%), regulation (10.2%)
and filamentous growth (7.4%) in early hyphal stage. Unfortunately, only 36% and 53.5%
of the overexpressed genes had known molecular function in swelling or hyphal stages,
respectively. Among them, the oxidoreductase (17.6%), hydrolase (8.8%) and transferase
(8.8%) were the most usual activities detected in swelling stage, while hydrolase
(14.1%), oxidoreductase (10.2%) and transferase (6.3%) activities were the most abundant
in early hyphal stage. According to component ontologies, the most overexpressed genes
in the swelling conidia codified proteins located in mitochondrion (24.5%), membrane
(23.3%) and nucleus (13.8%), while the ones in the early hyphae stage codified proteins
of the membrane (13.7%), extracellular region (5.9%) and plasma membrane (4.7%). On
the one hand, in the swelling step highlights the primary metabolism being the production
of energy the principal priority. In addition, the regulation of glutamate homeostasis
as well as the iron metabolism may also play a crucial role. On the other hand, in
hyphal output, an overexpression of genes implicated in vitamins and cofactors production,
calcium regulation and cell wall were detected.
Conclusion: This work might enable a better understanding of the germination process
of Aspergillus fumigatus from a transcriptomic point of view. This project could involve
important advances in the knowledge of the ecology and the mechanisms of adaptation
of the fungus that could be exploited by both the biotechnological and the biosanitary
sector.
Li
J.
1
Coste
A.T.
1
Bachmann
D.
1
Sanglard
D.
1
Lamoth
F.
2
1Microbiology Institute, University Hospital Lausanne, University of Lausanne, Lausanne,
Switzerland
2Infectious Diseases Service, CHUV, Lausanne, Switzerland
P390. rGenotyping of azole resistance in Candida auris
Objectives: Since 2009, Candida auris has emerged as a multidrug-resistant yeast pathogen
in all continents. Its ability to cause nosocomial outbreaks with high mortality rate
has attracted worldwide attention. Four distinct geographic and genetic clades have
been described: I: South Asia (India, Pakistan), II: East Asia (Japan, South Korea);
III: South Africa; IV: South America (Venezuela, Colombia). Remarkably, rapid development
of fluconazole resistance is a hallmark of most C. auris isolates. However, mechanisms
of azole resistance and type of genetic mutations still remain unclear. Hotspot mutations
in the target gene (ERG11) are classical cause of azole resistance in Candida spp,
but other mechanisms exist, such as overexpression of drug transporters induced by
gain-of-function mutations in transcription factors like TAC1 and MRR1, both of which
are important regulators of efflux systems in Candida albicans. Nevertheless, TAC1
and MRR1 have not been studied in C. auris yet. The objective of the study was to
analyze the genotype of these genes in C. auris isolates from different clades and
with different levels of azole resistance.
Methods: Twenty-two C. auris strains from India, Japan, Israel, Colombia and Switzerland
were collected. Susceptibility to fluconazole (FLC) and voriconazole (VOR) was tested
according to CLSI protocol. Minimal inhibitory concentration (MIC) thresholds for
resistance were defined according to arbitrary cut-offs previously reported by Lockhart
et al.: MIC ≥32 µg/mL for FLC and ≥2 µg/mL for VOR. Entire sequencing of ERG11, TAC1
and MRR1 was performed for all 22 isolates.
Results: By antifungal susceptibility testing, 2 isolates were classified as azole
susceptible (MIC FLC 4 µg/mL and VOR ≤1 µg/mL), 13 as resistant to FLC only and 7
as resistant to both FLC and VOR. ERG11 sequencing revealed three hotspot mutations
that have already been described in C. auris (F126T, Y132F and K143R). These isolates
(n = 8) exhibited high level resistance to FLC and variable VOR susceptibility. In
addition, we identified two new hotspot mutations: V125A from Israeli and Swiss strains
(n = 3) with high resistance to both FLC and VOR, and F444L from Colombian strains
(n = 4) with high resistance to FLC and variable VOR susceptibility. Putative TAC1(QG37_07465)
sequencing uncovered twenty mutations possibly associated with azole resistance (i.e.
present in azole-resistant isolates, but not in susceptible ones): S89Y, E200K, F214L/S,
R215K, K225N, K247E, I268V, Q298K, T346I, Q503R, F580L, P595H, P607S, S611P, A640V,
Y647C, I772-, N773- and L774M. Compared to ERG11 mutants exhibiting high FLC MIC (≥64
µg/mL) and relatively high VOR MIC (≥1 µg/mL), ERG11 wild-type isolates harboring
TAC1 mutations exhibited intermediate level of FLC resistance (MIC: <64 µg/mL) and
low VOR MIC (<1 µg/mL). Putative MRR1(QG37_07783) sequencing unveiled three mutations:
N167K, G280R and N647T. MRR1 mutations were always associated with ERG11 mutations
and their role in azole resistance thus remains unclear.
Conclusion: Overall, we identified two new mutations in ERG11 (V125A and F444L) within
conserved hotspot regions. Moreover, we identified twenty mutations in TAC1-like that
were associated with intermediate level of fluconazole resistance in the absence of
ERG11 mutations. Their putative role in azole resistance deserves further investigations.
Romald
N.
1
Kindo
A.J.
2
Veeraragavan
M.
3
1Department of Microbiology, Sri Ramachandra Institute of Higher Education and Research,
Chennai, India
2Microbiology, Sri Ramachandra Medical College and Research Institute, SRIHER, Chennai,
India
3Dermatology, Sri Ramachandra Medical College and Research Institute, Chennai, India
P391. Comparison of phenotypic and genotypic methods of speciation and gene sequencing
of Malassezia from the skin samples in a tertiary care hospital
Objectives: Speciate Malassezia phenotypically. Confirm the phenotypic identification
through molecular method of speciating Malassezia by PCR and Gene Sequencing. Comparison
between phenotypic and genotypic result.
Methods:
Malassezia species was isolated from the skin scrappings of patients with hypo/hyper
pigmented lesions. Microscopic Examination was done with 10%KOH. Samples showing meat
ball and spaghetti appearance were cultured onto Sabourauds Dextrose Agar (SDA), SDA
with olive oil overlay (SDA-O) and Modified Dixon’s agar (MDA) containing chloramphenicol
and incubated at 32o C upto 3 weeks. Phenotypic Identification Was done based on the
colony growth characteristics, gram staining, urease test, catalase test, bile esculin
with tween 60 hydrolysis and tween assimilation. Molecular Identification The isolates
which were cultured and identified by phenotypic methods were confirmed by Molecular
methods DNA was extracted by Phenol: Chloroform method. Extracted DNA was amplified
by Polymerase chain reaction (PCR) using thermocycler Primers used Pan-fungal PCR
primers:- PCR amplification of ITS1 and ITS4 regions ITS1 5΄ TCC GTA GGT GAA CCT GCG
G 3΄ and ITS4 5΄ TCC TCC GCT TAT TGA TAT GC 3΄ Malassezia specific nested primers
Pan fungal PCR positive samples were subjected to species specific nested PCR using
Malassezia specific primer ITSIF-N 5΄GGATCATTAGTGATTGCCTTTATA 3΄ and ITS4-R 5΄ TCCTCCGCTTATTGATATG
3΄ Agarose Gel Electrophoresis was done and band obtained PCR products were sequenced
by Sanger’s sequencing and NCBI blast was done and species identified.
Results:
Phenotypic method of speciation of Malassezia Out of 31 samples 7 isolates were Malassezia
furfur 12 isolates were Malassezia sympodialis 9 samples grew Malassezia globosa 3
samples grew Malassezia obtusa MOLECULAR IDENTIFICATION Pan-fungal PCR PCR by Pan
fungal primers ITS 1 and ITS 4 shows band between 600-1000bp (fig). All samples showing
positive band was subjected to Malassezia specific nested PCR. Malassezia specific
nested PCR Malassezia specific nested PCR using Malassezia specific primers showed
band at 200-300bp.(fig) Sequence result(table)
Conclusion: Phenotypic speciation showed Malassezia sympodialis as the predominant
species Genotypic speciation showed Malassezia furfur as the predominant species and
the least was Malassezia sympodialis. Genotypic speciation is more confirmatory than
phenotypic method Speciation of Malassezia is important because of variation in resistant
pattern to anti-fungals among different species.
Cabeza
E.
Arteta
Z.
Betancor
L.
Montevideo, Hygiene institute, Faculty of medicine, University of the Republic, Montevideo,
Uruguay
P392. First molecular characterization of Uruguayan Cryptococcus spp clinical isolates.
Objectives: To characterize Uruguayan Cryptococcus spp isolates from meningoencephalitis
by PCR fingerprinting and MLST.
Methods:
Clinical isolates Our laboratory receives clinical samples from fungal infections.
From the analyzed period, between 1th of January of 2000 and 31th of december of 2015,
55 strains of Cryptococcus spp were selected to be studied. Phenotypic identification
was performed in all cases. 52 C. neoformans complex and 3 C.gattii complex, were
recovered from cerebrospinal fluid (CSF) samples (n = 51), bornquioloalveolar lavage
(n = 2), blood culture (n = 1) and pleural effusion (n = 1). Characterization by PCR
fingerprinting Molecular type was determined by PCR fingerprinting as previously described
(Meyer 1993). The specific sequence used was the one from wild-type M13 minisatelite
phage (5-GAGGGTGGCGGTTCT-3). PCR fingerprint patterns were assigned according to the
main bands of the band pattern of the reference strains. MLST (Mutilocus sequence
type) and phylogenetic analysis. MLST analysis was performed by the individual application
of the six housekeeping genes CAP59, GPD1, LAC1, PLB1, SOD1 and URA5 along with the
IGS1 region according as previously described (Meyer 2009). The allele types and the
sequence type (ST) were identified via sequence comparisons with the Cryptococcus
MLST database. The allelic profile of the strains and the global dataset were applied
to the goeBURST algorithm in PHILOVIZ software. Clona complex (CC) was defined when
the strain had no more than one difference in housekeeping gene allele. Statistical
analysis X2 and Fischer’s exact test were performed for categorical variables and
student’s T test for continuous variable.
Results:
Molecular types of a total of 55 clinical isolates, molecular types were identified
in 50, in the 5 remaining the pattern of bands is reproducible but not concordant
with the patterns of the references strains. Three of them corresponded to C.gattii
complex. 28 (56,0%) isolates were C.neoformans var grubii, VNI (now C. neoformans),
16 (32,0%) were C.neoformans var neoformans, VNIV (now C. deneoformans), 6 (12 %)
were VNIII AD hybrids. The strains that were not identified by PCR fingerprinting
were studied by MLST. MLST (Mutilocus sequence type) and phylogenetic analysis. 20
strains selected by their genetic variability and phenotipic characterization were
analyzed by MLST. 7 different allele were identified for CAP59, 4 for GPD1,11 for
LAC1, 7 for PLB1, 5 for SOD1, 7 for URA5 and 8 for IGS1 region. A total of 16 ST were
observed, 13 C. neoformans complex isolates and 3 STs for 3 C. gattii complex isolates
(Table 1). of the 13 STs corresponding to C. neoformans complex, 8 were not reported
until now and were included in the database (ST605, ST606, ST607, ST608, ST609, ST610,
ST611 and ST612). The more frequent ST were ST32 (n = 3), ST23 (n = 2), ST63 (n =
2).
Conclusion: This is the first molecular characterization of Uruguayan isolates of
Cryptococcus spp from cases of meningoencephalitis. We demonstrated the great genetic
variability of circulating Cryptococcus spp strains in our environment that cause
meningoencephalitis. The new STs reported are closely related with each other and
with the rest of the STs, although they did not belong to the same CC.
Delarze
E.
1
Trachsel
E.
1
Brandt
L.
1
DEnfert
C.
2
Sanglard
D.
1
1Institute of Microbiology, Lausanne University Hospital and University of Lausanne,
Lausanne, Switzerland
2Pasteur Institute, Paris, France
P393. Identification of genetic mediator of fluconazole tolerance in Candida albicans
Objectives: Fluconazole (FLC) resistance in Candida albicans has long been reported
and is involving several well described molecular resistance mechanisms. FLC resistance
is based on measurement of MIC values and threshold values have been provided to distinguish
between wild-type susceptible isolates and resistant isolates. Another phenomenon,
called FLC tolerance (known also as “trailing effect” is susceptibility assays) is
characterized by the ability of C. albicans to grow persistently at supra-MIC drug
concentrations without acquisition of drug resistance. Little is known about molecular
mechanisms behind FLC tolerance and we aimed here to undertake identification of genetic
mediators of FLC tolerance.
Methods: We used in this study a collection of 579 C. albicans strains, each of which
could overexpress a specific C. albicans gene in a tetracycline-dependent manner and
was tagged by a distinct sequence barcode. The whole collection was pooled in RPMI
and, after repeated subcultures under FLC pressure at 32 µg/ml for 5 consecutive days
in RPMI with 2% glucose, the relative population of each barcode was determined by
barcode sequencing. In addition, each barcoded strain was subjected to single tolerance
assays yielding tolerance indexes reflecting relative growth of strains at a single
drug concentration (8 µg/ml FLC) after 24 h growth in RPMI in 2% glucose (growth conditions
similar to EUCAST susceptibility assays).
Results: Combining this genetic selection with single isolates testing, two transcription
factors (CRZ1 and GZF3) and a protein kinase (YCK2) were confirmed to be positive
mediators of FLC tolerance when overexpressed. CRZ1 s part of the calcineurin pathway
that is known critical for C. albicans in response to antifungal stress, while GZF3
and YCK2 are less well characterized. Using overexpression of these genes in deletion
mutants, we showed that GZF3 acts probably upstream of CRZ1. CRZ1 and GZF3 were deleted
in azole-susceptible clinical strains but exhibiting different levels FLC tolerance.
Our results demonstrate that CRZ1 plays a critical role in FLC tolerance in these
strains while GZF3 deletion has minor effects. Consistent with these data, transcriptional
profiles of clinical strains in the presence of FLC at supra-MIC drug concentrations
was reminiscent of a calcineurin-like transcriptional response.
Conclusion: A genetic screen permitted the identification of mediators of FLC tolerance,
one of which (CRZ1) is known to be part of the calcineurin pathway. FLC tolerance
of clinical strains is dependent on the presence of at least CRZ1. The precise mechanisms
of FLC tolerance mediated by CRZ1 and the other identified genes still need further
investigations.
Premamalini
T.
1
Rajyoganandh
S.V.
2
Veena
H.
2
Vijayakumar
R.
2
Kindo
A.J.
2
Rungmei
S.K.M.
3
Sridharan
K.S.
2
Vijayalakshmi
B.
4
1Microbiology, Sri Ramachandra Medical College and Research Institute, SRIHER, chennai,
India
2Microbiology, Sri Ramachandra Medical College and Research Institute, SRIHER, Chennai,
India
3Microbiology, SG-PGI Lucknow, Lucknow, India
4Infecious Disease Consultant, SIMS, Chennai, India
P394. Genotypic characterization of Trichosporon sp. from a Tertiary care centre in
South India
Objectives: To determine the species distribution of the Trichosporon sp. based on
genotypic characterization. To assess the genetic relatedness of the most commonly
isolated Trichosporon asahii strains using RAPD primers GAC1 and M13.
Methods: Seventy two Trichosporon clinical isolates recovered from patients of SRMC
& RI, a few hospitals in Chennai, and a hospital in Lucknow, were considered for the
study. PCR was performed initially with Trichosporon genus specific primers to double
check for accurate identification of this genus. Genotypic characterization was done
by PCR amplification of rRNA gene sequences by T. asahii specific primers which identified
only T. asahii isolates. Species confirmation was done by IGS1 sequencing and phylogenetic
analysis. Maximum parsimony clustering was used for construction of phylogenetic tree.
Its stability was assessed by parsimony bootstrapping with 1,000 pseudo replications.
Phylogenetic analysis was done by comparing the obtained nucleotide sequences with
the nucleotide sequences of Trichosporon reference strains obtained from the GenBank
database. DNA fingerprinting of the T. asahii isolates was carried out by RAPD analysis,
using primers GAC-1 and M13.
Results: Distribution of sample source of the 72 isolates were as follows: 43 (59.7%)
urine, 12 (16.7%) blood, 7 (9.7%) Percutaneous nephrostomy (PCN) tube, 5 (6.9%) respiratory
isolates, 4 (5.6%) pus, and 1 (1.4%) peritoneal dialysate fluid. Trichosporon specific
PCR confirmed all the 72 isolates as belonging to this genus. Trichosporon asahii
specific PCR identified 65 isolates as T. asahii (Fig. 1). The remaining 7 isolates
were identified further by phylogenetic analysis of the IGS1 sequences. In phylogenetic
analysis, six representative T. asahii clinical isolates clustered together with T.
asahii KR265116.1 and T. asahii KR872659.1 (bootstrap ≥95), confirming the molecular
identification results. One isolate identified as Trichosporon species clustered in
the same clade (bootstrap ≥79) was found to be closely related to T. ovoides. All
these isolates belonged to the Ovoides clade. Two isolates clustered along with T.
loubieri AB066428 & T. loubieri KT936593.1 (bootstrap ≥99). They belonged to the Gracile
clade. The remaining 4 isolates clustered together in Cutaneum clade along with T.
cutaneum AB 066415.1 (bootstrap ≥47) (Fig. 2). Finally, the 72 Trichosporon clinical
isolates were identified as 65 Trichosporon asahii, 4 Trichosporon cutaneum, 2 Trichosporon
louberi and one Trichosporon sp. closely related to T. ovoides. DNA fingerprinting
of the mostly commonly isolated T. asahii (64/72) isolates carried out by RAPD analysis,
identified more heterogeneity among the T. asahii isolates by GAC1 than M13 primer.
Conclusion:
Trichosporon asahii is the most common species causing human trichosporonosis, though
other members of the genus may also be involved. The members of the genus Trichosporon
are most frequently under-reported due to inconsistency of the phenotypic methods
used routinely in most laboratories. Trichosporon infections when treated with fluconazole
or caspofungin, which is commonly used to treat yeast infections, results in poor
clinical outcome. Hence, correct identification of the Trichosporon species using
molecular methods, helps the clinician in providing appropriate treatment.
Rubio-Portillo
E.
1
Orts
D.
2
Llorca
E.
2
Fernández
C.
3
Ferrer
C.
4
Gálvez
B.
5
Esteban
V.
6
Revelles
E.
4
Pérez-Martín
C.
4
Gómez-Imbernón
E.
4
Adsuar
J.
4
Piqueras
P.
4
Amat
B.
5
Antón
J.
1
Colom
M.F.
4
1Physiology, Genetics and Microbiology, University of Alicante, San Vicente del Raspeig,
Spain
2Pneumology, Hospital General Universitario de Elda, Elda, Spain
3Pneumology, Hospital General Universitario de Alicante, Alicante, Spain
4Medical Mycology Lab, University Miguel Hernandez, Sant Joan d’Alacant, Spain
5Pneumology, Hospital Universitario del Vinalopó, Elche, Spain
6Pneumology, Hospital Clínico Universitario de Valencia, Valencia, Spain
P395. The lung mycobiome and the domestic environment
Objectives: The goal of the study was to assess the relationship between the fungal
mycobiome detected in samples from the Lower Respiratory Tract (LRT) of non -infectious
patients, and the mycobiota detected in the air and dust of their dwellings.
Methods: The mycobioma of the LRT was analysed by the study of Bronchoalveolar Lavage
(BAL) samples taken from patients with indication of bronchoscopy. After informing
and getting their consent for the study, they were asked about the possibility of
a domestic sampling that was scheduled. Negative controls were included for every
laboratory procedure and samples from the bronchoscopes were also taken as controls.
All samples were processed for DNA extraction and for cultures. The fungal Internal
Transcribed Spacer (ITS2) of the extracted DNA was amplified and sequenced by the
illumina system. Results of the massive sequencing were analyzed by QIIME 1.8.0 and
compared with the ones in the UNITE Database. NCBI nucleotide database was also used
for identification. Cultures of samples were incubated at room temperature and 37ºC
and the fungal isolates were studied for identification.
Results: Forty-six BAL, 4 samples from bronchoscope and 19 domestic environmental
(MA) samples, together with a negative control from the PCR were submitted to massive
sequencing analysis (Solexa/Illumina system). The first results yielded over 7000000
sequences (5060431 from BAL, 108178 from bronchoscopes and 10861275 from MA samples).
In order to filter contaminants, we used a negative control from PCR and three negative
controls from each sequencing run. Samples were clustered in different fungal Operational
Taxonomic Units (OTUs) at 99% identity and sequences clustered in OTUs present in
control samples were remove from the analysis. After contaminant filtering, we obtained
2840808 clean sequences belonging to 122 different fungal Operational Taxonomic Units
(OTUs) at 97% identity. The similarity among different microbial communities was assessed
using phylogenetic information. Among the fungal species detected by massive sequencing,
6 OTUs were detectable in more than 75% of the BAL and 75% of the MA samples. These
sequences corresponded to Cladosporium angustisporum; Candida orthopsilopsis; Rhodotorula
mucilaginosa; Cryptococcus neoformans sensu stricto; Naganishia liquefaciens and Malassezia
restricta. Most of these species could be detected also by culture in the domestic
environment, but almost none in BAL samples cultures. When comparing the mycobiome
of the LRT of patients with the one of their domestic environment, important coincidences
can be seen for specific species of Candida, Cryptococcus and Malassezia.
Conclusion: The results show an important similarity between the mycobiota of the
environment and the mycobiome of the LRT samples of the patients. Most of the fungal
species detected in both environments correspond to fungi which are important opportunistic
pathogens like Cryptococcus neoformans and Candida orthopsilosis.
Okeke
O.F.I.
1
Fapohunda
S.O.
2
Soares
C.
3
Lima
N.
4
Ayanbimpe
G.M.
1
1Medical Microbiology, University of Jos, Plateau State, Nigeria
2Bioscience and Biotechnology, Babcock University, Ogun State, Nigeria
3Centre of Biological Engineering, Micoteca da Universidade do Minho, University of
Minho, Campus of Gualtar, Braga, Portugal
4Centre of Biological Engineering, Micoteca da Universidade do Minho, University of
Minho, Campus of Gualtar, Braga, Portugal
P396. Identification of Mycotoxigenic Fungi from Grains in a Nigerian Region Using
the Modern Polyphasic Methodology.
Objectives: Mycotoxins are poisonous substances produced by mycotoxigenic fungi which
contaminate agricultural commodities. Many foods and feeds can become contaminated
with mycotoxins since they can form in commodities before harvest, during the time
between harvesting and drying, and in storage. Mycotoxins may also be carried over
to animal products due to consumption of contaminated feed. Maize (Zea mays) and guinea
corn (Sorghum bicolor) form a major staple of the study area and are high risk commodities
for contamination with mycotoxigenic fungi. This study was carried out to identify
mycotoxigenic fungi from maize and guinea corn in Plateau state, north-central of
Nigeria.
Methods: In a multistage sampling technique, markets for the study were selected.
Using simple random sampling method, 270 samples were collected from various sampling
points within selected markets where these grains are sold. Isolation and identification
of the mycotoxigenic fungi employed the modern polyphasic methodology for filamentous
fungi identification.
Results: The mycotoxigenic fungi Aspergillus, Fusarium, and Penicillium were isolated.
Further work on these mycotoxigenic fungal genera identified them as follows: A. niger,
A. aculeatus, A. tamari, A. flavus, F. verticillioides and F. equiseti. The Penicillium
isolate was identified to belong to the section Sclerotiora and closely related to
P. malochii. Aspergillus accounted for 75% of all mycotoxigenic fungi isolated while
Fusarium and Penicillium accounted for 23% and 2% respectively.
Conclusion: Further work is also ongoing to determine if our strain of Penicillium
is a P. malochii or an entirely novel species. Our findings indicate contamination
of maize and guinea corn in the study area with mycotoxigenic fungi which may pose
serious health risks for the human and animal population and also have implications
for food safety and public health in Nigeria. In view of this, further investigation
is necessary to assess the health risks linked with the consumption of these grains.
Key words: Mycotoxigenic fungi, grains, Nigeria
Batista
W.
1
Navarro
M.
2
Castilho
D.
2
Calado
J.
2
Conceição
P.
1
Castro
B.
1
Silva
R.
2
Chaves
A.
2
1Pharmaceutical Sciences, Universidade Federal de São Paulo, São Paulo, Brazil
2Microbiologia E Imunologia, UNIFESP, São Paulo, Brazil
P397. Regulation of DNA repair machinery and Ras pathway in Paracoccidioides brasiliensis
during oxidative stress
Objectives: Paracoccidioidomycosis is a granulomatous disease caused by fungi of the
genus Paracoccidioides and widely distributed in Latin America. The disease has high
disabling potential and yet is still absolutely neglected. These fungi undergo cell
differentiation induced by changes in temperature and this event is critical to the
ability to cause disease. The knowledge of which molecular patterns of the fungus
may be true virulence factors is still limited by the difficulties inherent to genetic
manipulation of these fungi. Studies that have tried to understand these molecular
aspects rely on the method of transformation mediated by Agrobacterium tumefaciens.
To overcome these barriers, we need to understand the mechanisms of DNA repair used
by the fungus. This is because the establishment of a knockout strain depends on the
occurrence of homologous recombination events. The aim of the present communication
was to evaluate the role of the DNA repair machinery of P. brasiliensis in response
to oxidative stress.
Methods:
P. brasiliensis yeast cells were treated with different concentrations of H2O2, in
the presence or absence of pharmacological inhibitors (Ras, Hog-1 or DNA ligase 4
pathways). We used the RBD-GST (Ras Binding Domain fused with GST) probe to detect
the Ras activation and western blot to evaluate the Hog-1 phosphorilation levels.
Gene expression was analyzed by qRT-PCR. Cell viability were assessed by MTT assay
or cell counting.
Results: The double-strand breaks repair pathways communicate with the Ras GTPase
and Hog1 Map kinase pathways. The genes PbRAD51 and PbRAD52 are active elements during
DNA repair and participate in the response to oxidative stress in P. brasiliensis.
In addition, inhibition of the non-homologous end joining (NHEJ) mediated repair pathway
has been observed to provide a protective response to P. brasiliensis yeasts during
a normally lethal oxidative stress condition for the fungus. Perhaps, this event is
related to the greater efficiency of the repair by homologous recombination induced
by the inhibition of NHEJ. Finally, it was observed that the combination of the damping
of the NHEJ pathway, through the inhibition of DNL4 or GSK3, together with the induced
oxidative stress do increase the efficiency of transformation processes in yeasts
of P. brasiliensis. Indeed, inhibitors of NHEJ pathway caused a protective effect
on yeast cells challenged with high concentration of H2O2. This effect correlates
with an increase in expression of scavenger genes like catalase and superoxide dismutase.
Conclusion: Taken together, our results indicate that there is a significant crosstalk
between two major cellular damage response pathways, ROS signaling and DNA repair,
for P. brasiliensis survival.
Salmanton-Garcia
J.
1
Seidel
D.
1
Köhler
P.
1
Mellinghoff
S.
1
Wisplinghoff
H.
2
Vehreschild
J.-J.
1
Cornely
O.A.
1
Vehreschild
M.J.G.T.
1
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany
2Antibiotic Resistance, Molecular Epidemiology, Labor Wisplinghoff, Cologne, Germany
P400. rMatched-paired analysis of patients treated for invasive mucormycosis - standard
treatment vs. posaconazole new formulations (MoveOn)
Objectives: Current first-line (1st) antifungal treatment for invasive mucormycosis
(IM) consists of liposomal amphotericin B (AMB). Salvage (SAL) treatment options are
limited and often based on posaconazole oral suspension (POSsusp). However, with the
approval of posaconazole new formulations (POSnew), patients could benefit from improved
pharmacokinetics, safety and tolerability. Therefore, our aim is to assess the effectiveness
of POSnew as first-line and SAL treatments for IM.
Methods: We performed a case-matched analysis with proven or probable IM patients
from the FungiScope® Registry. 1st-POSnew and 1st-AMB+POSnew cases were matched with
1st-AMB-based treatment controls, and SAL-POSnew cases were matched with SAL-POSsusp
controls. Each case was matched with up to three controls based on severity, haematological/oncological
malignancy, surgery and/or renal dysfunction.
Results: Five patients receiving first-line POSnew alone, 18 receiving first-line
POSnew combined with AMB, and 22 receiving salvage POSnew were identified. By day
42, favourable response was reported for 80.0% (n = 4/5) of patients receiving first-line
POSnew, for 27.8% (n = 5/18) receiving first-line POSnew plus AMB, and for 50.0% (n
= 11/22) receiving salvage POSnew. Day-42 all-cause mortality of patients receiving
POSnew was lower compared to mortality in their respective controls (20.0% (n = 1/5)
in 1st-POSnew vs. 53.3% (n = 8/15) in 1st-AMB; 33.3% (n = 6/18) in 1st-AMB+POSnew
vs. 52.0% (n = 26/50) in 1st-AMB; 0.0% (n = 0/22) in SAL-POSnew vs. 4.4% (n = 2/45)
in SAL-POSsusp).
Conclusion: In the observed patients, POSnew was effective in terms of treatment response
and associated mortality of IM. POSnew may be an alternative for treatment of IM.
Burger
E.
1
Santos
L.A.
1
Grisolia
J.C.
1
Dias
N.A.
1
Paula
F.B.D.A.
2
Oliveira
A.M. De
3
Rodrigues
A.M.
4
Camargo
Z.P.D.
4
1Department of Microbiology and Immunology, Federal University of Alfenas, Alfenas,
Brazil
2Department of Clinical and Toxicological Analysis - Pharmaceutical Sciences Faculty,
Federal University of Alfenas, Alfenas, Brazil
3Laboratory of Pathology - José Do Rosário Vellano University, UNIFENAS, Alfenas,
Brazil
4Department of Microbiology, Immunology and Parasitology, Federal University of Sao
Paulo, Sao Paulo, Brazil
P401. Efficacy of Celecoxib solely or in combination with Itraconazole in the treatment
of murine paracoccidioidomycosis
Objectives: Our objective is to evaluate the effectiveness of Celecoxib, of Itraconazole
and both, as a combined treatment for paracoccidioidomycosis, a highly prevalent granulomatous
mycosis in Latin America using a murine model.
Methods: Mice were intraperitoneally infected with a virulent P. brasiliensis isolate
and three days later, daily administration by gavage of either 3mg/mL Itraconazole
(I), or 6mg/mL Celecoxib (C) or both 3mg/mL Itraconazole and 6mg/mL Celecoxib (I+C)
was initiated and kept for 15 days. Controls included infected, non-treated mice (Pb)
and non-infected mice. Body weight and survival rate were monitored daily. At 7 and
15 days of infection, delayed type hypersensitivity (DTH) was evaluated and serum
was obtained for specific anti-P. brasiliensis IgG antibody determination. At the
last day of treatment, the mice were sacrificed and spleen, lungs, liver and epiploo/pancreas
were collected and their macroscopic characteristics were analyzed. Following, the
organs were either processed and stained with H/E for general histology, or macerated,
for quantification of viable fungi.
Results: There was no difference in the body weight of infected, treated or uninfected
controls and there were no deaths registered during this early phase of the infection
in mice from any group. In all experimental groups, DTH decreased from day 7 to day
15 and, in contrast, specific IgG titers increased from day 7 to day 15. But At 15
days, both, DTH reactivity and IgG titers were the highest in the Pb group, lower
in the I group and in the C group, and the lowest in the I+C group. This decrease
in both cellular and humoral specific immunity in the groups most treatment-covered
may be explained by the elimination of viable P. brasiliensis by the drugs, rendering
a full immune response less necessary. The histological aspects demonstrated that
treatment with I+C was the one that reduced the most the number of lesions and the
severity of the granulomatous response in all organs. When analyzing the control of
fungal growth in the tissues, both mice from the I and from the C groups showed less
fungi scattered in the tissue than mice from the Pb group; however the lowest numbers
of fungi were found in mice from the I+C group. In the epiploo, which is the organ
of shock for this model of P. brasiliensis infection, the animals of the I+C group
had the fewest fungi with preserved morphology. The same was found in terms of viable
fungi, as the number of CFUs was lower in all organs of treated mice, and more markedly
in those receiving the combined I+C therapy.
Conclusion: We showed the effect of the antifungal drug Itraconazole and also of the
anti-inflammatory drug Celecoxib at the tissue level. This is the first report of
the direct effect of Celecoxib on P. brasiliensis as well as in combination with Itraconazole.
Our results suggest that this combined treatment employing an antifungal and an anti-inflammatory
drug may constitute a new efficient therapeutic strategy. Grants: CNPq 305216/2016-3
and FAPEMIG APQ 012941-16. L.A. Santos and J.C. Grisolia are recipient of CAPES scholarships.
Thompson
G.
1
Angulo
D.
2
1Infectious Disease, University of California at Davis, Davis, United States of America
2SCYNEXIS, Inc., Jersey City, United States of America
P402. Successful Treatment of a Patient with Retroperitoneal Abscess caused by Candida
krusei with the Investigational Agent, Ibrexafungerp (formerly SCY-078): A Case Report
from the FURI study
Case Report: Objectives: Intraabdominal candidiasis is the second most common Candida
infection after candidemia. Candida albicans is the predominant cause of these infections,
with non-albicans Candida becoming more frequently observed in this clinical setting.
Echinocandins are the recommended treatment for intraabdominal Candida infections
but have varied clinical response. This is postulated to be based on poor drug levels
in tissue and abscesses. Ibrexafungerp (formerly SCY-078) is a novel IV/oral glucan
synthase inhibitor (triterpenoid) antifungal with activity against Candida, Aspergillus
and Pneumocystis. A Phase 3 open-label, single-arm study of oral ibrexafungerp (FURI;
NCT03059992) is ongoing for the treatment of patients intolerant of or with fungal
disease refractory to standard antifungal therapy. We present a patient case of retroperitoneal
abscess caused by Candida krusei from the FURI study. Methods: A 71 year old male
patient with ischemic stroke, pulmonary edema, and tracheostomy was being treated
for a retroperitoneal abscess, a complication of a perforated duodenal ulcer. Candida
krusei was isolated in peri-duodenal drain cultures and the patient was initiated
on micafungin therapy for 21 days. During micafungin therapy, cultures from the peri-duodenal
drain on three occasions remained positive for Candida krusei. The patient underwent
abscess drainage and was enrolled into the FURI study due to micafungin treatment
failure and was treated with oral ibrexafungerp 750mg BID for two days followed by
750mg daily. Results: The patient was treated with oral ibrexafungerp therapy for
21 days. Clinical improvement was observed during therapy. At End of Treatment visit,
the clinical signs and symptoms of fungal disease were considered by the investigator
to be resolved and the physical exam noted minimal discharge from the patient’s retroperitoneal
drain. As of the 6-week follow-up visit, clinical signs and symptoms of infection
were still resolved. No drug-related adverse events were reported. Conclusion: This
case report shows the potential of oral ibrexafungerp to treat intraabdominal infections
caused by Candida krusei in patients who fail echinocandin therapy. Continued enrollment
in the FURI study is warranted.
Lim
L.
Bioprocessing Technology Institute/National University of Singapore, Singapore, Singapore
P403. In vitro study of Antifungal Biopharmaceutical Dectin1-Fc
Objectives: Invasive fungal infections are a neglected category of disease that has
a high mortality and implicated in immunocompromised patients. Dectin-1 (CLEC7A) is
a type II transmembrane C-type lectin pattern recognition receptor expressed on cells
of the innate immune system like macrophages, monocytes and neutrophils that recognizes
β-glucans through its single C-type lectin-like domain at its extracellular region.
It functions by binding to cell walls of different fungal species, such as Saccharomyces
cerevisiae, Candida albicans, Pneumocystis carinii, Coccidioides posadasii, and Aspergillus
fumigatus; triggering immune cell responses like phagocytosis and cytokine production.
The absence of β-glucans in human cells presents the opportunity to exploit Dectin-1
as therapeutic vehicle to enhance immune response during a fungal infection. This
project aims to develop recombinant Dectin1-Immunoglobulin G1 Fc fusion protein as
a potential biopharmaceutical for passive immunization to aid the innate immune system
against Candida albicans fungal infection. The in vitro properties of the recombinantly
expressed fusion protein are examined in this study.
Methods: The fusion protein was expressed in DG44 Chinese Hamster Ovarian (CHO) cells
and amplified with methotrexate. The fusion protein was extracted from culture supernatant
via GE Histrap histag affinity and purified with GE Superdex 200 size exclusion chromatography
on a GE Akta Purifier FPLC. Immunofluorescence imaging with anti-Fc Alexa Fluor 568
conjugated antibody from ThermoFisher Scientific was used to assay binding of the
fusion protein to unicellular or hyphae Candida albicans. Binding affinity of the
Fc fusion protein versus a human IgG1 to Fcγ receptors from R&D Systems were screen
by surface plasmon resonance on a GE Biacore T200.
Results: Immunofluorescence imaging shows the fusion protein capable of binding to
Candida albicans both in the unicellular and hyphae morphology. Surface plasmon resonance
study of the Fc-fusion protein versus a IgG1 control reveal that the fusion protein
binds to FcγRI, FcγRIIIa and FcRn. However, the binding constants for the Fc-fusion
is generally weaker than a full fledge IgG1 antibody. Activity testing of the fusion
protein using THP-1 monocytes against unicellular Candida albicans in a 1 hr phagocytosis
assay shows a dose response. For the highest dose of 100μg/ml of fusion protein, a
reduction of greater than 50% of surviving Candida albicans cells compared to the
untreated control was observed.
Conclusion: The in vitro results reveal that the respective domains of the Fc-fusion
protein function as intended though binding to the Fc receptors are not as strong
as an antibody. This in turn may affect the efficacy of the drug. Further testing
with primary immune cells would be appropriate to study the full therapeutic potential
of this fusion protein. In addition, in vivo studies with an appropriate mouse model
will give a better picture of the performance of the Fc-fusion protein drug.
Oz
Y.
1
Onder
S.
2
Durmaz
G.
2
1Department of Microbiology, Division of Mycology, Eskisehir Osmangazi University
Medical Faculty, Eskisehir, Turkey
2Department of Microbiology, Eskisehir Osmangazi University Medical Faculty, Eskisehir,
Turkey
P404. The in vitro effect of farnesol on the activity of voriconazol and amphotericin
B against Aspergillus isolates
Objectives: Although new antifungal agents are being developed, there is an increasing
resistance to standard antifungal therapy, and no new classes of antifungal agents
have been approved for a long time. Currently, three antifungal drug classes including
triazoles, polyenes and echinocandins are available to use in treatment of IFIs. However,
treatment is often complicated due to their high toxicity, low tolerability, drug
interactions and limited spectrums of activities. Therefore, the requirement of new
drug or treatment alternatives especially those with a wider spectrum, lower toxicity
and cheaper are increasing day by day. Farnesol is an extracellular quorum-sensing
molecule producing by C.albicans and inhibits the yeast-to-hypha transition in C.albicans
and blocks biofilm formation. FAR is also a sesquiterpene alcohol existing in many
herbal products and exogenously FAR inhibits the conidiation in Aspergillus niger
and the germination of macroconidia in Fusarium graminearum. However, the number of
studies assessing the antifungal efficacy of FAR with standardised methods is limited.
In this study, we investigated the contribution of farnesol on the activity of antifungals
such as voriconazole and amphotericin B against clinical Aspergillus isolates.
Methods: Clinical Aspergillus isolates, A.fumigatus (n = 30) and A.flavus (n = 15),
were included in this study. The MIC values of antifungal drugs and farnesol were
determined against all Aspergillus isolates using reference broth microdilution method
(CLSI M38-A2). The interactions of farnesol with voriconazole and amphotericin B were
investigated by the checkerboard method and evaluated based on the fractional inhibitor
concentration index (FICI). The FICI was obtained by summing the FIC values of each
drug; the FIC was calculated for each agent by dividing the inhibitory concentration
of each antifungal or compound when used in combination by its MIC. Synergy was defined
as a FICI of ≤ 0.5; no interaction was defined as a FICI > 0.5 but < 4; and antagonism
was defined as a FICI ≥ 4.
Results: The MIC ranges of farnesol, voriconazole and amphotericin B were 1500-6000
µM, 0.125-1 µg/mL and 0.125-0.5 µg/mL against A.fumigatus isolates, 3000->6000 µM,
0.125-0.5 µg/mL and 0.25-2 µg/mL against A.flavus isolates, respectively. The most
common interaction in combination tests was “no interaction” and synergistic interaction
was not detected in any combination. The combination of farnesol with voriconazole
had antagonistic activity against 13 of 31 A.fumigatus isolates with FICI values of
4.01 to 8.5, and 4 of 14 A.flavus isolates with FICI values of 4.03 to 5. Similarly,
the combination of farnesol with amphotericin B had antagonistic activity against
7 of 31 A.fumigatus isolates with FICI values of 4.01 to 8.03, and 5 of 14 A.flavus
isolates with FICI values of 4.5 to 10.
Conclusion: Antifungal activity of farnesol and synergistic interaction when it was
combined with antifungal drugs have been reported against Candida spp and its biofilm.
However, farnesol did not produce any significant inhibitor activity alone or any
synergistic interaction in combination with voriconazole or amphotericin B against
Aspergillus isolates in this study. Conversely, considerably antagonistic interaction
was observed; 38% for farnesol with voriconazole combination, 27% for farnesol with
amphotericin B combination.
Lim
W.
1
Konings
M.
1
Rijnders
B.
1
Fahal
A.H.
2
Birch
M.
3
Sande
W. Van De
1
1Department of Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam,
Netherlands
2Mycetoma Research Center, University of Khartoum, Khartoum, Sudan
3F2G Ltd, Manchester, United Kingdom
P405. Olorofim is potent against Madurella mycetomatis – the most common causative
agent of Eumycetoma
Objectives:
Madurella mycetomatis is the main causative agent of eumycetoma, a chronic granulomatous
infection of the subcutaneous tissue. Currently, the only antifungal agents with activity
against M. mycetomatis are agents acting on ergosterol in the fungal cell membrane.
Itraconazole is currently the drug of choice, but the duration of treatment is long
and therapeutic failure is common. Therefore, there is an urgent need to identify
more potent antifungal agents with activity against M. mycetomatis. One of the novel
classes of antifungal agents are the orotomides and olorofim is the leading representative
of this class. It inhibits fungal pyrimidine biosynthesis and it is currently being
evaluated for invasive fungal infections in patients lacking treatment options. To
determine if olorofim has in vitro activity against M. mycetomatis, we carried out
MIC determinations against 21 M. mycetomatis clinical isolates with different genetic
and geographical background and compared the results to those obtained for itraconazole.
Methods: Minimal inhibitory concentrations (MIC) were determined for Olorofim and
Itraconazole against 21 M. mycetomatis clinical isolates from Sudan (14), Algeria
(1), Mali (1), India (1), Chad (1), the Netherlands (1) and also from an unknown origin
(2). The filamentous nature of M. mycetomatis necessitates the need for homogenization
of inoculum by sonication to obtain a standardized inoculum for testing. This creates
turbidity in the wells in an MIC test which complicates visual reading of the antifungal
activity, tests were therefore read using XTT as a colorometric agent to indicate
growth. MICs was performed using our CLSI-based in vitro susceptibility testing method
with XTT reading at 450nm at the end after a 7-day incubation period at 37˚C. As a
number of M. mycetomatis isolates produces pigments that influences colour intensity
and the endpoint reading, an 80% reduction in viable fungal mass was determined instead
of a 100%.
Results: We showed that olorofim was highly active against all tested M. mycetomatis
isolates. MICs obtained for olorofim ranged from <0.004 µg/ml to 0.125 µg/ml and 0.06
μg/ml olorofim was needed to inhibit 90% of the isolates (see results in Table). Olorofim
MICs were consistently one-dilution more potent than the MIC values for itraconazole.
For itraconazole, MICs ranged from 0.008 µg/ml to 0.25 µg/ml and 0.125 μg/ml was needed
to inhibit 90% of the isolates.
Conclusion: Our study showed that M. mycetomatis can also be inhibited with antifungal
agents with a different mode of action to the azoles. Olorofim from the novel antifungal
class of orotomides showed potent in vitro activity against all tested M. mycetomatis
isolates and had MICs similar to or slightly lower than those for itraconazole - the
current drug of choice for therapy. Further studies, including in vivo models are
warranted to determine if olorofim would be a suitable alternative to itraconazole
therapy.
Kennani
S. El
1
Picaud
S.
2
Champleboux
M.
1
Marais
J.
1
Courçon
M.
3
Arlotto
M.
1
Rabut
G.
4
Filippakopoulos
P.
2
Govin
J.
1
1Signaling Through Chromatin, INSTITUTE FOR ADVANCED BIOSCIENCES, GRENOBLE, France
2Ludwig Institute for Cancer Research, Nuffield Department of Clinical Medicine, University
of Oxford, Oxford, United Kingdom
3Biologie à Grande échelle, CEA, Grenoble, France
4Institute of Genetics and Development, Rennes, France
P407. Inhibiting fungal BET protein: a new hope to treat systemic fungal infections
Objectives: Invasive fungal infections kill an estimated 1.6 million people each year
– as many deaths as are caused by tuberculosis or malaria. Currently, only four drug
classes are available to treat these infections (polyenes, azoles, flucytosine and
echinocandins). This limited repertoire of antifungal drugs, combined with an alarming
rise in drug-resistant fungal strains, has created an urgent need for novel therapeutic
agents. In the developed world, the most common fungal disease among hospitalized
patients is invasive candidiasis. Among Candida species, C. albicans and C. glabrata
rank first and second in isolation frequency, respectively, accounting for ~70% of
all systemic candidiasis. This project investigates new antifungal strategies that
target the BET family of transcriptional regulators in these Candida species. The
fungal BET protein Bdf1 contains two bromodomains (BDs) and an extra-terminal domain
(ET). We recently established the proof of concept that small-molecule inhibition
of Bdf1 BDs compromises the viability and virulence of C. albicans (Mietton et al.,
Nature Communications 2017).
Methods: This project focuses on the ET domain and is based on a combination of genetics,
medicinal chemistry, structural biology and biochemistry studies.
Results: It shows that the ET domain is essential for the survival of C. albicans
and C. glabrata in vitro. Furthermore, we identified a specific groove on the ET domain
and demonstrate that it is also essential for growth, paving the way for the potential
discovery of selective inhibitors.
Conclusion: To conclude, epigenetic targets remain largely unexplored in the fungal
infection field. Our investigation of Bdf1 as a therapeutic target represents a novel
and highly promising area of exploration in the antifungal field.
Seyedmousavi
A.
1
Kwon-Chung
J.
2
Brüggemann
R.J.M.
3
Verweij
P.E.
4
1Department of Laboratory Medicine, Microbiology Service, National Institutes of Health
Clinical Center, Bethesda, United States of America
2Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy
and Infectious Diseases, Bethesda, Maryland, United States of America
3Pharmacy, RadboudUMC, Nijmegen, Netherlands
4Medical Microbiology, RadboudUMC, Nijmegen, Netherlands
P408. Efficacy of intravenous posaconazole for the treatment of azole-resistant invasive
aspergillosis
Objectives: Azole antifungals play a prominent role in the management of patients
with invasive aspergillosis (IA). However, azole resistance is a growing problem in
patients with aspergillus infection, which translates into treatment failure. Alternative
treatments with new formulations of azolesmay improve therapeutic outcome in IA even
for the clinical cases caused by strains with low susceptibility to currently available
azoles.
Methods: The in vivo efficacy of 0.25, 1, 4, 16 and 64 mg/kg/day recently introduced
intravenous formulation of posaconazole (POS)was assessed in mice (CD-1 strain) infected
with a wild type (POS MICEUCAST, 0.031mg/L) and azole-resistant Aspergillus fumigatus
(POS MICEUCAST, 0.5 mg/L) harboring TR34/L98H mutation in Cyp-51A gene. Efficacy of
POS treatment was assessed by tissue histopathology 3 days post infection and monitoring
of survival for 14 days following intravenous inoculation.
Results: The survival curves for all control groups receiving 0.9% saline intravenously
showed 100% mortality. The efficacy of POS treatment at the dosing regimens ≤4 mg/kg/day
depended on the MIC of each isolate. However, the maximum effect (100% survival at
day 14 post infection) was achieved with a POSdose of ≥ 16 mg/kg for both wild-type
and TR34/L98H mutant isolates, and histopathological slides revealed limited number
of inflammatory foci with or without detectable fungal elements in the Kidneys. The
Hill-type model with a variable slope fitted the relationship between the dose and
14-day survival well, with R2 values of 0.99 for the wild type, and 0.95 for the TR34/L98Hisolate.
The 50% effective dose (ED50) based on survival was 0.89 mg/kg (95% confidence interval
[CI], 0.59 to 1.4 mg/kg) for the wild type, and 4.5 (95% CI, 2.1 to 7.6 mg/kg) for
the TR34/L98Hisolate.
Conclusion: Overall, treatment with intravenous formulation of POS improved the survival
of the mice in a dose-dependent manner. A dose-response relationship was observed
regardless of the underlying azole-resistance mechanism. These results show intravenous
formulation of POS to be a promising therapeutic agent for treatment of azole-resistant
IA.
Miranda-Cadena
K.
1
Dias
M.
2
3
Costa-Barbosa
A.
2
3
Collins
T.
2
3
Marcos-Arias
C.
1
Eraso
E.
1
Quindós
G.
1
Sampaio
P.
2
3
1Inmunología, Microbiología Y Parasitología, Universidad del País Vasco/Euskal Herriko
Unibertsitatea UPV/EHU, Bilbao, Spain
2Centre of Molecular and Environmental Biology (cbma), Department of Biology, University
of Minho, Braga, Portugal
3Institute of Science and Innovation For Bio-sustainability (ib-s), University of
Minho, Braga, Portugal
P409. Anti-Candida activities of carvacrol, cinnamaldehyde, citral and thymol liposomes
Objectives: To develop and characterize carvacrol, cinnamaldehyde, citral and thymol-loaded
DODAB:MO nanoparticles, to test their cytotoxicity on murine macrophages and to evaluate
their anti-Candida activities in vitro.
Methods: Encapsulation was made using monoolein-based liposomes of DODAB:MO in a 1:2
molar ratio. DODAB:MO liposomes were prepared using thin lipid-film hydration method
Concentrations tested were 32, 64 and 128 mg/L of carvacrol and thymol; 16, 32 and
64 mg/L of cinnamaldehyde and 64, 128 and 256 mg/L of citral. Mean size (nm), polydispersity
(PDI) and surface charge (ζ-potential) were evaluated with Malvern ZetaSizer Nano
ZS particle analyser (Malvern, UK). Encapsulation efficiency (%EE) was determined
by HPLC-DAD, after separation by ultacentrigugation. Antifungal activities of encapsulated
and non-encapsulated phytocompounds (Sigma-Aldrich, USA) was evaluated by microdilution
against eight Candida albicans, one Candida auris, Candida dubliniensis, Candida tropicalis
and C. albicans reference strain SC5314. Cytotoxicity was assayed on murine macrophage-like
cell line RAW 264.7 by detection of cell metabolic activity by the reduction of MTT
to formazan and lactate dehydrogenase activity (LDH) assay.
Results: Nanoparticles of 128 mg/L of carvacrol and thymol presented high %EE (70.8
and 69.1%, respectively) without loss of anti-Candida activity. Geometric means of
MICs of carvacrol and thymol nanoparticles were 101.6 mg/L and 71.8 mg/L, respectively.
Nanoparticles of 256 mg/L of citral presented the best %EE (83.3%), but they did not
present antifungal activity. Cinnamaldehyde nanoparticles presented the lowest %EE
(20.6 to 44.1%) and maintained their antifungal activity. All nanoparticles were stable
during four weeks stocked at 4 ºC, with PDI values < 0.6 and ζ-potential among + 46.9
to + 55.6 mV. Thymol nanoparticles were the biggest with a particle size of 580.3
±17.4 nm, followed by cinnamaldehyde, carvacrol and citral nanoparticles (567.9 ±
13.9, 510 ± 9.6 and 497.6 ± 13.8 nm, respectively). Non-encapsulated 32 µg/mL of thymol
and carvacrol showed less cytotoxicity levels (76.5% and 74.8% cell viability by MTT
assay, respectively; and ≥ 80% by LDH assay), followed by citral 64 µg/mL with 68%
by MTT assay and 92.9% by LDH. Thymol and carvacrol nanoparticles were the best tolerated
by macrophages with a higher cell viability by MTT assay (83.5-112.8% for thymol nanoparticles
and 101-137% for carvacrol nanoparticles) when incubated with 18, 36 and 72 µg/mL
of thymol nanoparticles and 18 and 36 µg/mL of carvacrol nanoparticles.
Conclusion: Monoolein-based liposomes of DODAB:MO (1:2) loaded with 128 µg/mL of carvacrol
or 128 µg/mL of thymol can be a promising alternative of treatment of candidiasis,
due to their striking physicochemical properties, appropriate encapsulation efficiencies,
stability, reduced cytotoxicity and preserved anti-Candida activities. Work supported
by Gobierno Vasco-Eusko Jaurlaritza (GIC15/78 IT-990-16) and the Federation of European
Microbiological Societies by Research and Training grant (FEMS-GO-2017-020).
D’Agostino
M.
Laboratoire SIMPA, Vandoeuvre-lès-Nancy, France
P410. rIn vitro antifungal effect of plant-based compound CIN-102 on filamentous fungi
Objectives: Today the increase of invasive fungal infections due to the rise of immunosuppressive
therapies is a real problem especially in hospitals. Moreover, the emergence of resistant
strains induces a therapeutic failure. Face to this issues, new classes of antifungals
are expected. The plant kingdom thus represents an immense potential of natural resources
to exploit for these purposes. The SEPTEOS company has recently developed the CIN-102
compound, by using cinnamaldehyde and potentiating compounds from two synergic essential
oils of cinnamon. Cinnamaldehyde has already been describe to kill bacteria, yeast
and molds growth in both growing and non-growing state (not published). The objective
of this study is to determine the spectrum of activity of CIN-102 on the main genus
or species of filamentous fungi involved in human pathology.
Methods: The activity of CIN-102 product and its main constituent (cinnamaldehyde
/ CIN) are tested against a representative panel of strains of filamentous fungi (Aspergillus
(n = 39), Fusarium (n = 19) and Scedosporium n = 21)) of clinical importance. Clinical
and reference strains have been studied, comprising isolates characterized by innate
or acquired antifungal resistance patterns. A determination of MICs (minimum inhibitory
concentrations) was performed using the M38-A2 CLSI reference method. The observed
antifungal effect was then characterized by determining an inoculum effect.
Results: The MIC of CIN-102 determined for the different strains studied is mainly
between 62 (n = 28) and 125 (n = 30) ug / ml, similar to the MIC determined for main
active. Strains of the genus Aspergillus have on average a slightly higher MIC compared
to the genus Fusarium and Scedosporium: MIC mainly to 125 μg / ml for Aspergillus
(n = 23), and 62 μg / ml for Fusarium (n = 12) and Scedosporium (n = 12). Acquired
resistance against azole antifungals (Aspergillus fumigatus TR46, TR34 and G54E resistant
strains) or innate (naturally antifungals resistant Scedosporium strains) do not seem
to impact the effect of the compound CIN-102 and cinnamaldehyde. The inoculum as well
as the time and dose dependent effect of CIN-102 and cinnamaldehyde will also be characterized.
Conclusion: Although the MICs alone do not provide an opinion on the efficacy of CIN-102,
these results already show the antifungal effect of the compound. The current results
for CIN-102 are very close to those determined for cinnamaldehyde in our study but
also in the literature (Homa et al., 2015) (Essid et al., 2017). Further studies will
then be conducted, notably on the structural aspect and the mechanism of action, to
complete the knowledge on this new molecule. Moreover, an in vivo model of invasive
fungal infection is studied to develop our knowledge about this new molecule.
Chauvin
D.
1
Hust
M.
2
Schütte
M.
2
Chesnay
A.
1
3
Parent
C.
1
Moreira
G.M.S.G.
2
Arroyo
J.F.
4
Sanz
A.B.
4
Martineau
P.
5
Pugnière
M.
5
Bailly
E.
1
Chandenier
J.
1
3
Heuzé-VourcH
N.
1
Desoubeaux
G.
1
3
1Cepr Inserm U1100, Université de Tours, Tours, France
2Institut Für Biochemie, Biotechnologie und Bioinformatik, Technische Universität
Braunschweig, Braunschweig, Germany
3Parasitology - Mycology - Tropical Medicine, Hôpital Bretonneau, TOURS, France
4Microbiología Y Parasitología, Universidad Complutense, Madrid, Spain
5Institut De Recherche En Cancérologie De Montpellier, Université de Montpellier,
Montpellier, France
P411. New strategies based on antifungal antibodies for the treatement of aspergillosis
Objectives:
Aspergillus fumigatus is an airborne opportunistic fungal pathogen responsible for
severe infections. For instance, invasive pulmonary aspergillosis is a serious threat
for individuals suffering from severe immunosuppression, with mortality rates > 50%.
In parallel, allergic bronchopulmonary aspergillosis frequently encountered in cystic
fibrosis patients, is also a comorbidity factor. In addition to diagnostic means lacking
specificity, current treatments present a high toxicity which prevents their use in
weakened subjects, resulting in impaired prognostic. Because of their low toxicity
and high specificity, anti-infectious therapeutic antibodies could be a new alternative
to conventional therapeutics. In this study, we investigated the potential of Chitin
Ring Formation cell wall transglycosylases of A. fumigatus to be therapeutic targets
for therapeutic antibodies.
Methods:
In vitro neutralization test, in vitro cultures, fluorescence and confocal microscopy,
DNA sequencing, as well as flow cytometry and animal models were used.
Results: We demonstrated that the Crf target was highly conserved, regardless of the
pathophysiological context; whereas the CRF1 gene was found to be 100% conserved in
92% of the isolates studied, Crf proteins were expressed in 98% of the strains. In
addition, we highlighted the role of Crf proteins in fungal growth, using a deletion
mutant for CRF1 gene, for which a growth decrease of 23.6% was observed after 48 h.
It was demonstrated that anti-Crf antibodies neutralized the enzymatic activity of
recombinant Crf protein, and delayed fungal growth by 12.3% in vitro when added to
spores. In a neutropenic rat model of invasive pulmonary aspergillosis, anti-Crf antibodies
elicited a significant recruitment of neutrophils, macrophages and T CD4 lymphocytes
but it was not correlated with a decrease of fungal burden in lungs and improvement
in survival.
Conclusion: Overall, our study highlighted the potential relevance of targeting Crf
cell wall protein with therapeutic antibodies.
Arendrup
M.C.
1
2
3
Datcu
R.
1
Hare
R.
1
Jørgensen
K.M.
1
1Unit of Mycology, Statens Serum Institut, Copenhagen, Denmark
2Dept. of Clinical Microbiology, Rigshospitalet, Copenhagen, Denmark
3Dept. of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark
P412. Olorofim EUCAST susceptibility testing of contemporary moulds: in vitro activity
and improved reproducibility
Objectives: Olorofim is a novel antifungal orotomide compound for which we previously
demonstrated good in vitro activity against various moulds. This study sought to (a)
investigate whether MIC variation observed for A. fumigatus (unimodal range <0.004–0.25
mg/L) was due to inherent MIC variation or true differences in susceptibility and
(b) present EUCAST MICs for clinical mould isolates from 2018.
Methods: MICs (mg/L) of olorofim were determined by EUCAST E.Def 9.3 using A. fumigatus
ATCC 204305 for QC. MIC variability study: Fifteen A. fumigatus strains were selected
from the prior study, as follows: five low MIC isolates: MIC = ≤0.002 (n = 2) and
MIC = 0.004 (n = 3); five middle MIC isolates: MIC = 0.03 (n = 2) and MIC = 0.06 (n
= 3); and five high MIC isolates: MIC = 0.125 (n = 1) and MIC = 0.25 (n = 4). MICs
were determined visually by two-three observers blinded to the original MIC. MICs
against 365 clinical mould isolates were determined during 2018. For A. fumigatus
olorofim activity was evaluated individually for itraconazole susceptible (MIC≤1)
and non-susceptible organisms (MIC>1). The ECOFFinder programme was used for determining
statistical wildtype upper limits (highest MIC for organisms without phenotypically
detectable acquired resistance mechanisms, WT-ULs) using 95%, 97.5% and 99% subset
endpoints. For Fusarium isolates the 50%-MIC was also determined spectrophotometrically
using 50% growth inhibition.
Results: Repetitive testing of low, medium and high MIC A. fumigatus isolates resulted
in MICs, which for 14/15 isolates fell within two dilutions, whereas 1/10 MICs for
isolate #12 and 1/80 MICs for the control strain fell one dilution below the two-dilution
range when read by observer 1. The modal MIC for low/middle/high MIC isolates were
as follows for repeated testing: Observer 1 and 2: 0.03/0.03/0.016 and for observer
3: 0.03/0.03/0.03. Routine testing during 2018 for species represented by ≥15 isolates
generated uniform Gaussian MIC distributions spanning ≤5 dilutions with modal MICs
= 0.03-0.06 for all isolates except non-proliferatum Fusarium spp. (n = 10) (Table).
Modal MIC/WT-UL for A. fumigatus was 0.06/0.125 and was unaffected by itraconazole
susceptibility and choice of ECOFFinder endpoint. Olorofim displayed MICs ≤0.25 against
less common Aspergillus spp. except A. montevidensis (n = 1) as well as against dermatophytes
and other moulds including: Microsporum gypseum (n = 1), T. interdigitale (n = 1),
T. rubrum (n = 12), Rasamsonia aegroticola (n = 1), Rasamsonia argillacea (n = 1),
Scedosporium apiospermum (n = 3), Scedosporium boydii (n = 2). 50%-MICs for Fusarium
were as follows: F. dimerum >8 (n = 2), F. oxysporum 0.06 (n = 1), F. solani complex
MIC range 0.25-1, modal MIC 1 (n = 7), and F. proliferatum 0.03 (n = 1).
Conclusion: Upon repeated testing of A. fumigatus isolates with initially low/medium/high
MICs, no differences were observed. This suggests that the initial distribution observed
can be explained by technical variation and that olorofim has similar and uniform
activity against all isolates within the wild-type population. This was confirmed
when evaluating results for routine MIC testing during 2018. In conclusion, olorofim
displayed promising in vitro activity against most moulds included in this study independent
of azole susceptibility.
Arendrup
M.C.
1
2
3
Astvad
K.M.T.
1
Jørgensen
K.M.
1
1Unit of Mycology, Statens Serum Institut, Copenhagen, Denmark
2Dept. of Clinical Microbiology, Rigshospitalet, Copenhagen, Denmark
3Dept. of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark
P413. EUCAST susceptibility testing of manogepix and six comparative agents against
contemporary Danish mould isolates
Objectives: The new antifungal fosmanogepix (APX001) is a first-in-class drug candidate
and the methyl phosphate prodrug of the active moiety manogepix (APX001A). Manogepix
targets the conserved fungal inositol acyltransferase enzyme Gwt1, thereby preventing
GPI-anchored protein maturation and compromising fungal growth. Fosmanogepix is currently
in Phase 2 clinical trials. Here we compared the in vitro activity of manogepix and
six comparators against contemporary clinical mould isolates received for identification
and EUCAST susceptibility testing.
Methods: A total of 163 clinical mould isolates obtained Aug 2016 to Sep 2017 were
included. Aspergillus isolates were identified to the species complex level except
A. fumigatus that was identified sensu stricto using thermotolerance. EUCAST E.Def
9.3.1 reference method using cell-culture treated microtitre plates (Nunc, ThermoFisher
Scientific, cat. no. 167008) were prepared using serial two-fold dilutions of manogepix,
amphotericin B, isavuconazole, voriconazole, itraconazole and posaconazole. The plates
were stored at -80°C for ≥24 h prior to inoculation. Susceptibility endpoints were
determined visually as MECs for manogepix (the lowest concentration resulting in aberrant
growth) as complete visual or 50% spectrophotometric growth inhibition were not observed
(data not shown). For the comparators MICs were read visually according to the EUCAST
definition at the lowest concentration giving complete growth inhibition. The ECOFFinder
programme was used for determining a statistical single-centre manogepix wild-type
upper-limit for A. fumigatus.
Results: The manogepix MEC and comparator MICs are shown as modes (not done (ND) for
isolates represented by five or fewer isolates) and ranges in the Table. Manogepix
MECs were ≤0.125 mg/L against all Aspergillus and the two Fusarium isolates but no
activity could be detected against the dermatophyte and three mucorales isolates included
at the concentrations tested (MICs >0.5 mg/L). Manogepix in vitro activity remained
the same against A. fumigatus isolates resistant to itraconazole (Table). Using the
ECOFFinder programme, a single-centre wild-type upper-limit of 0.125 mg/L was determined
for A. fumigatus (applying a 97.5% cut-off).
Conclusion: Manogepix displayed uniform and potent in vitro activity against the different
Aspergillus species and Fusarium isolates included in the study as determined by MECs.
The in vitro activity was unaffected by acquired resistance in A. fumigatus. Thus,
fosmanogepix appears to be a promising therapeutic against these pathogens, many of
which are difficult to treat.
Jørgensen
K.M.
1
Hare
R.
1
Chowdhary
A.
2
Arendrup
M.C.
1
3
4
1Unit of Mycology, Statens Serum Institut, Copenhagen, Denmark
2Department of Medical Mycology, Vallabhbhai Patel Chest Institute, University of
Delhi, Delhi, India
3Dept. of Clinical Microbiology, Rigshospitalet, Copenhagen, Denmark
4Dept. of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark
P414. Candida auris is Highly In Vitro Susceptible to Ibrexafungerp (formerly SCY-078)
in EUCAST Antifungal Susceptibility Testing
Objectives:
Candida auris is a multidrug-resistant yeast rapidly emerging as a significant cause
of nosocomial infections. Ibrexafungerp is a novel oral synthetic enfumafungin derivative
inhibiting glucan synthase. It is active in vitro and in animal models against Candida,
Aspergillus and Pneumocystis and is currently in clinical development for mucocutaneous
and invasive fungal infections. Here, we investigate the susceptibility of C. auris
to ibrexafungerp (formerly SCY-078) compared to that of eight comparators and compared
to the activity against C. albicans and C. glabrata.
Methods: EUCAST AFST according to E.Def 7.3.1 was performed for ibrexafungerp (Scynexis,
Jersey City, NJ, USA) against 122 clinical C. auris isolates (from India (n = 120)
and Oman (n = 2)) and three C. auris control strains JCM15448, KCTC17809 and KCTC17810.
Sixteen Danish clinical C. albicans and 16 C. glabrata isolates and the control strains
C. albicans ATCC64548, C. krusei ATCC6258 and C. parapsilosis ATCC22019 were included
as comparators and controls. Cell-culture treated microtitre plates (Nunc, Thermo
Fisher Scientific, cat. no. 167008) were prepared using the ISO method. The ibrexafungerp
MICs were compared to previously published MICs for anidulafungin, micafungin, amphotericin
B, fluconazole, isavuconazole, itraconazole, posaconazole and voriconazole.
Results: The in vitro activity of ibrexafungerp (IBX) against C. auris was uniform
with MICs displaying a Gaussian distribution spanning 0.06-2 mg/L suggesting an equal
efficacy across the 122 isolates (Table). The modal MIC and MIC50 were 0.5 mg/L. For
the C. auris reference strains, the MICs were 0.06 mg/L, 0.125 mg/L and 0.5 mg/L,
respectively. The MIC ranges for nine repetitive MIC determinations of the control
strains MICs were as follows: C. albicans ATCC 64548: 0.06 mg/L, C. krusei ATCC 6258:
0.5-1 mg/L and C. parapsilosis ATCC 22019: 0.125-0.5 mg/L respectively. In contrast,
MIC distributions for anidulafungin (ANF), micafungin (MCF), isavuconazole (ISA),
voriconazole (VOR), itraconazole (ITR) and posaconazole (PRC) against C. auris were
wide (spanning 10-13 two-fold dilutions) suggesting differential activity against
the isolates. of note, ibrexafungerp MICs remained low (MICs of 0.25 mg/L and 0.5
mg/L) for the isolates resistant to anidulafungin and micafungin (MICs >32 mg/L).
Finally, fluconazole (FLU) MICs for all but one isolate were 16- >256 mg/L suggesting
almost universal fluconazole resistance whereas the amphotericin B (AMB) MICs clustered
close to the non-species specific breakpoint of 1 mg/L. Next, ibrexafungerp activity
was compared for C. albicans, C. glabrata and C. auris. MIC50, (range) were 0.06 mg/L
(0.03-0.125 mg/L), 0.25 mg/L (0.25-0.5 mg/L) and 0.5 mg/L (0.06-2 mg/L), respectively.
Conclusion: Ibrexafungerp shows promising in vitro activity against C. auris suggesting
it may be a welcomed therapeutic against this emerging threat with few treatment options.
Ibrexafungerp’s MICs were in general one step higher against C. auris than against
C. glabrata and, notably, remained unchanged against C. auris isolates with antifungal
resistance to the comparator drugs, including those highly echinocandin-resistant.
Seyedmousavi
A.
1
Chang
Y.C.
2
Law
D.
3
Birch
M.
3
Rex
J.
3
Kwon-Chung
J.
4
1Department of Laboratory Medicine, Microbiology Service, National Institutes of Health
Clinical Center, Bethesda, United States of America
2Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy
and Infectious Diseases, National Institutes of Health, Bethesda, United States of
America
3F2G Ltd, Manchester, United Kingdom
4Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy
and Infectious Diseases, Bethesda, Maryland, United States of America
P415. In vivo efficacy of olorofim against systemic infection caused by Scedosporium
apiospermum, Pseudallescheria boydii, and Lomentospora prolificans in neutropenic
CD-1 mice
Objectives: Clinically relevant memebrs of the Scedosporium/Pseudallescheria species
complex and Lomentospora prolificans are generally resistant to currently available
systemic antifungal agents and the infection due to these species is difficult to
treat. Alternative treatments with new classes of antifungals may improve therapeutic
outcomes of the disease caused by these difficult to treat moulds.
Methods: We studied the in vivo efficacy of olorofim (formerly F901318), a new fungicidal
agent that prevents growth of manyascomycetous mold speciesvia inhibition of de novo
pyrimidine biosynthesis, against scedosporiosis caused by three species in neutropenic
CD-1 mice. Cyclophosphamide immunosuppressed mice infected by Scedosporium apiospermum,
Pseudallescheria boydii and Lomentospora prolificans (tail vein infection) were treated
by intraperitoneal administration of olorofim (15 mg/kgevery 8 h for 9 days). The
efficacy of olorofimtreatment was assessed by survival rate 10 days post infection,
and tissue histopathology 3 days post infection.
Results: In the neutropenic CD-1 mice, olorofim therapy significantly improved survival
compared to the untreated controls; 80 %, 100 % and 100 % of treated mice survived
infection by Scedosporium apiospermum, Pseudallescheria boydii, Lomentospora prolificans,
respectively while less than 20% of the control mice (PBS-treated) survived at 10
days post infection. Furthermore, histopathological slides of kidneys revealed no
detectable fungal elements in the olorofim-treated mice, whilst numerous fungal hyphae
were present in control mice.
Conclusion: Overall, treatment with olorofimimproved the survival of the mice infected
with all three species causing scedosporiosis and showed rapid clearance of fungi
from the kidney by histological staining. These results show olorofim to be a promising
therapeutic agent for systemic scedosporiosis, a difficult disease to treat with currently
available antifngals.
Gebremariam
T.
1
Alkhazraji
S.
1
Gu
Y.
1
Alqarihi
A.
1
Mamouei
Z.
1
Shaw
K.J.
2
Ibrahim
A.S.
1
1Los Angeles Biomedical Research Institute, Harbor-UCLA Medical Center, Torrance,
United States of America
2Amplyx Pharmaceuticals, San Diego, United States of America
P416. APX001 (Fosmanogepix) is Effective in an Immunosuppressed Mouse Model of Fusariosis
Objectives: Fusariosis has high mortality rates. Owing to its rarity, comparative
clinical trials are hard to perform. Animal models are an appropriate complementary
avenue for evaluating antifungal therapy. Thus APX001 (fosmanogepix) was evaluated
in an immunosuppressed murine model of hematogenously disseminated fusariosis.
Methods: The minimum effective concentration (MEC) of APX001A (manogepix, the active
moiety of APX001) was determined against a Fusarium solani clinical isolate using
CLSI M38 method. ICR mice were immunosuppressed with cyclophosphamide and cortisone
acetate on days -2, and +3, relative to intravenous infection with 7.5×104 cells of
F. solani. For survival studies, treatment with placebo (diluent control), APX001
(78 or 104 mg/kg, PO), liposomal amphotericin B (LAmB, 15 mg/kg, IV), or voriconazole
(Vori, 40 mg/kg, PO) began 16 h post-infection and continued for 8 days for APX001
or Vori and 4 days for LAmB. To extend the half-life of APX001, mice were administered
50 mg/kg of the cytochrome P450 inhibitor 1-aminobenzotriazole (ABT) 2 h prior to
APX001 administration. Grapefruit juice in drinking water (50%) was given to Vori-treated
mice to enhance the drug’s half-life. To assess tissue fungal burden, mice were sacrificed
on Day +4 and organs processed for conidial equivalent (CE) by qPCR.
Results: The APX001A MEC value for the tested F. solani strain was 0.03 μg/mL. Treatment
with APX001 or LAmB enhanced median survival time vs. placebo (12, and 10 days for
78, and 104 mg/kg of APX001, respectively vs. 10 days for LAmB treatment, vs. 8 or
7 days for Vori or placebo, respectively, P <0.01). Further, APX001 and LAmB treatments
equally enhanced overall survival by day 21 when the experiment was terminated (40%
for LAmB or APX001 at 78 mg/kg, and 20% for APX001 at 104 mg/kg, vs. 0% for placebo
or Vori treatment). APX001 or LAmB treatments, but not Vori, resulted in ~2-3 log
reduction in kidney, and brain CE vs. placebo.
Conclusion: APX001A showed significant antifungal activity against F. solani in vitro
which translated to in vivo efficacy. APX001 was as effective as LAmB in this mouse
model of fusariosis. Continued investigation of APX001 as a novel antifungal agent
against this difficult to treat infection is warranted.
Wiederhold
N.
1
Patterson
H.
1
Birch
M.
2
Law
D.
2
Rex
J.
2
1Fungus Testing Laboratory, The University of Texas Health Science Center at San Antonio,
San Antonio, United States of America
2F2G Ltd, Manchester, United Kingdom
P417. Evaluation of the In vitro Activity of Olorofim Against Fusarium Species
Objectives: Invasive infections caused by Fusarium species are often associated with
significant morbidity and mortality in highly immunocompromised patients, and treatment
options are limited. Several species are often resistant to treatment with clinically
available antifungals. Olorofim (formerly F901318; F2G Ltd.) is a member of the orotomide
class of antifungal agents that inhibits fungal pyrimidine biosynthesis and has potent
activity against a variety of filamentous fungi. We evaluated the in vitro activity
of olorofim against a selection of various Fusarium isolates. The in vitro activity
was also measured for the clinically available antifungals amphotericin B, posaconazole,
and voriconazole.
Methods: Clinical isolates (n = 111) of Fusarium species in the collection of the
University of Texas Health Science Center Fungus Testing Laboratory were used. Susceptibility
testing was performed by broth microdilution according the CLSI M38 reference standard.
MICs for olorofim were determined after 48 hours of incubation using the 50% and 100%
inhibition endpoint, while those of the control agents (amphotericin B, posaconazole,
and voriconazole) were determined using the 100% inhibition endpoint.
Results: Olorofim demonstrated in vitro activity against F. oxysporum (MIC range 0.125-0.25
mg/L and 0.5 – 1 mg/L at the 50% and 100% endpoints, respectively), F. fujikuroi (≤0.015
mg/L and ≤0.015-0.125 mg/L), F. verticillioides (0.06-0.125 mg/L and 0.06-0.25 mg/L),
and F. proliferatum (≤0.015->8 mg/L and 0.06->8 mg/L) (Table). At the 50% inhibition
endpoint, olorofim demonstrated in vitro activity against some isolates of the F.
solani species complex (FSSC, now members of the genus Neocosmospora 0.5->8 mg/L)
and the F. incarnatum-equiseti species complex (FIESC, 0.25->8mg/L). In contrast,
when the 100% inhibition endpoint was used, limited to no in vitro activity was observed
against these species complexes (4->8 mg/L), nor was activity observed against F.
dimerum using either endpoint (>8 mg/L). Olorofim activity against some of the rarer
species of Fusarium was mixed, with the growth of some species being inhibited at
low MICs (i.e., F. brachygibbosum, F. decemcellulare, F. redolens, and F. thapsinum;
0.06-0.25 mg/L at 50% inhibition and 0.25-0.5 mg/L at 100% inhibition). In contrast,
limited to no activity was also observed against other rarer species (i.e., F. delphinoides,
F. nygamai, F. pallidoroseum, F. petroliphilum, and P. pseudensiforme; 0.25-8 mg/L
at 50% inhibition and ≥8 mg/L at 100% inhibition). The MIC ranges for the control
agents were also wide and differed across Fusarium species. The most consistent activity
was observed with amphotericin B.
Conclusion: Olorofim demonstrated in vitro activity against some clinical isolates
of Fusarium species. This activity appeared to be species-dependent, and was also
dependent on the endpoint used (50% vs. 100% inhibition of growth). Further worked
is needed to determine how the in vitro activity observed against Fusarium species
in this study may translate into in vivo efficacy, especially for more common species
for which there were differences between the activity of olorofim at the different
of growth inhibition endpoints.
Almeida
B. De
1
Lemes
T.
1
Almeida
M.
2
Volanti
D.
1
1Unesp, São José do Rio Preto, Brazil
2FAMERP, São José do Rio Preto, Brazil
P419. Nanoparticles as antimicrobial agent against Scytalidium hyalinum and Neoscytalidium
dimidiatum
Objectives: The present study aimed to evaluate the antifungal activity of silver-based
nanocomposites (NCs) deposited in hydroxyapatite (HAP), after synthesis by microwave,
against clinical strains of Scytalidium hyalinum and Neoscytalidium dimidiatum.
Methods: The analysis was performed with Ag/HAP NCs (4 and 8% Ag) and pure Ag to evaluate
the minimum inhibitory concentration to kill Scytalidium hyalinum and Neoscytalidium
dimidiatum, according to CLSI M27-S4 with modifications considering, in triplicate.
The fungi strains were maintained in RPMI broth culture media after overnight incubation
at 37°C, and each inoculum adjusted according to 0.5 MacFarland standard tube. In
96-well plates, 100 µL of RPMI with fungal inoculum was added and NCs were dispersed
into the 10% dimethyl sulfoxide (DMSO) - ranging from 1000 to 7.8 µg/mL. The plates
were incubated for 24 hours. After growth, the cell viability was conducted by enzymatic
reduction using triphenyl tetrazolium chloride (TTC).
Results: The highest antifungal activity was seen for 8% Ag/HAP NCs against Scytalidium
hyalinum and Neoscytalidium dimidiatum with MIC 250 µg/ml. In addition, the pure Ag
showed the worst antifungal activity with a MIC 1000 µg/mL for Scytalidium hyalinum
and MIC 250 µg/mL for Neoscytalidium dimidiatum.
Conclusion: This study contributes to the knowledge of interactions between nanocomposites
and fungal cell, as potential target of control infection caused by Scytalidium hyalinum
and Neoscytalidium dimidiatum. It is clear the occurrence of the synergistic effect
of Ag/HAP NCs in relation to pure Ag, event that must be considered, once HAP is biocompatible
and could reduce the toxicity of Ag.
Gebremariam
T.
1
Alkhazraji
S.
1
Gu
Y.
1
Alqarihi
A.
1
Mamouei
Z.
1
Shaw
K.J.
2
Ibrahim
A.S.
1
1Los Angeles Biomedical Research Institute, Harbor-UCLA Medical Center, Torrance,
United States of America
2Amplyx Pharmaceuticals, San Diego, United States of America
P420. rAPX001 (Fosmanogepix) is Effective in an Immunosuppressed Mouse Model of Rhizopus
oryzae Infection
Objectives: Mucormycosis is a life-threatening infection that predominantly occurs
in immunocompromised hosts. The antifungal APX001A (manogepix) inhibits Gwt1, an enzyme
required for the conserved glycosylphosphatidyl inositol (GPI) post-translational
modification in eukaryotes. We previously reported the activity of APX001 (fosmanogepix,
the prodrug of APX001A) against Rhizopus delemar (minimum effective concentration
[MEC] = 0.25 µg/mL). Here we assessed the activity against R. oryzae, which has an
elevated MEC value.
Methods:
R. oryzae 99-892 MIC and MEC values were 0.125 µg/mL and 4.0 µg/mL for isavuconazole
(ISAV) and APX001A, respectively. ICR mice were immunosuppressed with cyclophosphamide
(200 mg/kg) and cortisone acetate (500 mg/kg) on Days -2, +3, and +8 relative to intratracheal
infection with 2.5 x 105 cells of R. oryzae 99-892. For survival studies, treatment
with 104 mg/kg APX001 was compared to ISAV (110 mg/kg TID). Oral treatment started
on Day +1 through Day +7, relative to infection for survival studies, and through
Day +4 for tissue fungal burden studies (assessed by conidial equivalent [CE] using
qPCR). Placebo mice received vehicle control. To extend the half-life of APX001, mice
were administered 50 mg/kg of the cytochrome P450 inhibitor 1-aminobenzotriazole (ABT)
2 h prior to APX001 administration.
Results: APX001 and ISAV equally prolonged median survival time of mice (n = 20) versus
placebo (12 and 14 days for APX001 and ISAV, respectively, vs. 8 days for placebo).
Further, APX001 and ISAV treatment both resulted in 30% 21-day survival versus 0%
survival of placebo mice (P <0.05 by Log Rank test). Both drug treatments resulted
in ~1.5 log10 reduction in lung and brain CE vs. placebo-treated mice (n = 10, P <0.005
by Wilcoxon Rank Sum).
Conclusion: Despite a higher MEC value, APX001 showed significant efficacy against
R. oryzae that was as protective as ISAV in immunosuppressed mice. Given the previously
reported activity of APX001 against a strain of R. delemar with a lower MEC value,
APX001 has now been shown to be efficacious against both species of Rhizopus, which
together are responsible for ~60-70% of isolates causing lethal mucormycosis. Thus,
continued investigation of APX001 against mucormycosis is warranted.
Alkhazraji
S.
1
Gebremariam
T.
1
Alqarihi
A.
1
Gu
Y.
1
Shaw
K.J.
2
Ibrahim
A.S.
1
1Los Angeles Biomedical Research Institute, Harbor-UCLA Medical Center, Torrance,
United States of America
2Amplyx Pharmaceuticals, San Diego, United States of America
P421. APX001 Protects Immunosuppressed Mice from Scedosporiosis
Objectives: Scedosporiosis has high mortality rates. Owing to its rarity, comparative
clinical trials are hard to perform. Animal models are an appropriate complementary
avenue for evaluating antifungal therapy. Thus, APX001 was evaluated in an immunosuppressed
murine model of invasive scedosporiosis.
Methods: The minimum effective concentration (MEC) of APX001A (the active moiety of
APX001) was determined against 9 clinical isolates of Scedosporium apiospermum, S.
boydii and Lomentospora prolificans using CLSI M38 methodology. ICR mice were immunosuppressed
with cyclophosphamide and cortisone acetate on days -2, and +3, relative to intratracheal
infection with 3.0×107 cells of S. apiospermum. For survival studies, treatment with
placebo (diluent control), APX001 (26, 52, 104, or 156 mg/kg, po), or liposomal amphotericin
B (LAmB, 10 mg/kg, iv) began 16 h postinfection and continued for 7 or 11 days for
APX and 4 days for LAmB. To extend the half-life of APX001, mice were administered
50 mg/kg of the cytochrome P450 inhibitor 1-aminobenzotriazole (ABT) 2 h prior to
APX001 administration. To assess tissue fungal burden, mice were sacrificed on Day
+4 and organs processed for conidial equivalent (CE) by qPCR.
Results: The APX001A MEC value for all tested isolates was 0.03 μg/mL. Treatment with
ABT alone or LAmB, did not enhance survival of mice infected with S. apiospermum vs.
placebo mice (n = 20 or 30). Treatment with APX001 at 26, 52, 104 or 156 mg/kg for
7 days enhanced median survival time vs. placebo (10, 12, 11, and 15 days for 26,
52, 104, and 156 mg/kg arms, respectively vs. 7 days for placebo, P<0.001). Further,
APX001 treatment enhanced overall survival by day 21 when the experiment was terminated
(30%, 40%, and 40%, respectively for 26, 52 or 156 mg/kg vs 10% for placebo). Survival
after treatment with APX001 for 7 days or 11 days was not different. All APX001 treatments
resulted in ~2 log reduction in lung, brain and kidney CE.
Conclusion: APX001A is cidal against S. apiospermum, S. boydii and L. prolificans
in vitro. APX001 protected immunosuppressed mice from scedosporiosis due to S. apiospermum.
Continued investigation of APX001 as a novel antifungal agent against scedosporiosis
is warranted.
Sastoque
A.
1
2
3
Ehemann
K.
2
Triana
S.
2
3
Fernandez-Niño
M.
3
González
A.
3
Restrepo
S.
2
Ramírez
A.M. Celis
2
1Biotechnology Institute, Universidad Nacional de Colombia, Bogotá D.C., Colombia
2Biological Sciences, Universidad de los Andes, Bogotá D.C., Colombia
3Chemical Engineering, Universidad de los Andes, Bogotá D.C., Colombia
P422. Evaluation of new therapeutic candidates for the treatment of Malassezia pachydermatis
Objectives:
Malassezia pachydermatis is a lipophilic and lipid-dependent yeast, that is part of
the microbiota in domestic and wild animals’ skin. However, it could be associated
with otitis in canines and bloodstream infections in humans. Diseases caused by M.
pachydermatis frequently exhibit chronic and recurrent clinical course, and the current
antifungal therapy based on azoles is associated with increased resistance as well
as significant side effects. Genome sequencing, metabolic network reconstruction,
and gene essentiality analyses have been performed, allowing to identify fifteen candidates
as therapeutic targets such as homoserine dehydrogenase (HSD), homocitrate synthase
(HCS) and saccharopine dehydrogenase (SDH). These enzymes participate in the pathways
of L-lysine or L-threonine biosynthesis, which have been reported as antibacterials
and with antifungal effect. To assess the impact of these amino acids over the growth
of Malassezia pachydermatis we evaluated the inhibition of HCS and SDH by L-lysine
and HSD by L-threonine based on the feedback control, we additionally conducted in
vitro susceptibility tests.
Methods: Recombinant proteins were expressed in Escherichia coli and purified to determine
enzymatic activity through spectrophotometry detection of corresponding cofactors.
Moreover, in vitro susceptibility tests using a modified CLSI M27-A3 method and agar
diffusion assays were performed. The concentrations tested were (μg/ml) from 1500
to 4000 using Sabouraud dextrose broth (SDB) with tween 40 and tween 60.
Results: Therefore, the results of our study showed L-lysine 1 mM and 75 mM were able
to inhibit the enzymatic activity of HCS and SDH, respectively while L-threonine 1
mM inhibited the activity of HSD. Lysine and threonine showed to be competitive inhibitors
affecting the substrate-enzyme complex (SE) formation. For L-lysine reduction in turbidity
was observed at 3100 µg/mL by microdilution method. In addition, agar diffusion showed
a diameter of inhibition of 13 mm using 50 mg/mL. On the contrary, L-threonine did
not display inhibitory activity in M. pachydermatis at 50 mg/mL as the maximum allowed
concentration to be evaluated.
Conclusion: This study provides evidence that these three enzymes are efficiently
regulated by the feedback of the final product of their corresponding metabolic pathway.
L-lysine is a potent inhibitor for M. pachydermatis, possible for saturation of metabolic
pathways and accumulation of high levels of L-lysine that generate toxicity and amino
acids starvation in the cell. L- lysine will be considered as a potential antifungal/fungal
static candidate. For L-threonine it is necessary to evaluate higher concentrations.
Pré
S. Du
1
Law
D.
1
Sibley
G.
1
Read
N.
2
Bromley
M.
2
Oliver
J.
1
Birch
M.
1
1F2G Limited, Manchester, United Kingdom
2Manchester Fungal Infection Group, University of Manchester, Manchester, United Kingdom
P423. The effect of the orotomide antifungal olorofim on the growth and viability
of Scedosporium and Lomentospora species.
Objectives: Olorofim is the first member of the new orotomide class of systemic antifungals
acting through inhibition of fungal DHODH. Olorofim is active against all species
of Aspergillus tested to date including cryptic species. In addition, there is activity
against a wide range of filamentous fungi including Scedosporium, Paecilomyces and
endemic moulds. Scedosporium and Lomentospora species represent some of the most clinically
challenging fungal infections often showing resistance to currently available antifungal
agents. Previously olorofim has been shown to be highly active in vitro against a
range of Scedosporium species and the closely related multidrug-resistant species
Lomentospora prolificans (Wiederhold et al 2017, Biwas et al 2018). In this study
we examined the effects of olorofim on isolates from the Scedosporium complex at a
cellular level using microscopy and viability dyes.
Methods:
In vitro MICs were undertaken using standard CLSI methodology. Images of the bottom
of each well were obtained using a Leica SP8X confocal microscope. Studies on viability
were carried out by allowing conidia to germinate for 8h and then exposing to olorofim
at the MIC concentration for up to 120h. The viability dye DiBAC was used to determine
cell death following exposure to olorofim over time.
Results: Olorofim MICs for S. apiospermum 451 and L. prolificans 206 were both determined
to be 0.25 µg/mL. Imaging of microtitre wells above and below this MIC value showed
that olorofim dramatically reduced growth of both S. apiospermum 451 and L. prolificans
206 at concentrations several dilutions below the MIC value. DiBAC staining was conducted
with one of the study strains (L. prolificans 206) and showed that hyphae began to
take up the dye after 24h and that the uptake increased steadily over time. When observations
were stopped at 120h, >90% of hyphae had taken up DiBAC and were non-viable.
Conclusion: Olorofim demonstrated time-dependent killing of L. prolificans hyphae,
consistent with previous observations with Aspergillus spp. Microscopic analysis of
olorofim treated S. apiospermum and L. prolificans cultures, demonstrated profound
effects on growth even at concentrations below the MIC level.
Huband
M.D.
1
Pfaller
M.A.
1
Flamm
R.K.
1
Bien
P.
2
Hodges
M.
2
Castanheira
M.
1
1JMI Laboratories, North Liberty, United States of America
2Amplyx Pharmaceuticals, San Diego, United States of America
P425. In Vitro Activity of APX001A (Manogepix) and Comparator Agents against 1,706
Fungal Isolates Collected During an International Surveillance Program (2017)
Objectives: Current antifungal agents cover a majority of opportunistic fungal pathogens;
however, breakthrough invasive fungal infections continue to occur and increasingly
involve relatively uncommon yeasts and/or moulds that tend to exhibit decreased susceptibility
to current agents. APX001A (manogepix) is a first-in-class small molecule inhibitor
of the fungal Gwt1 enzyme that is required for acylation of inositol during glycosylphosphatidylinositol
(GPI) anchor biosynthesis. It is active against the major fungal pathogens: Candida
(except C. krusei), Aspergillus, and hard-to-treat moulds, including Fusarium and
Scedosporium. In this study, we tested APX001A, anidulafungin (ANF), micafungin (MCF),
fluconazole (FLU), and others against 1,706 contemporary clinical fungal isolates
collected worldwide during 2017.
Methods: A total of 1,706 non-duplicate fungal isolates were collected from 68 medical
centers in North America (37.3%), Europe (43.4%), Asia-Pacific (12.7%), and Latin
America (6.6%). Among the isolates tested, 78.5% were Candida spp, 3.9% were non-Candida
yeasts, including 30 Cryptococcus neoformans var. grubii (1.8%), 14.7% were Aspergillus,
and 2.9% were other moulds. All isolates were tested by CLSI reference broth microdilution
methods.
Results: APX001A (MIC50/90, 0.008/0.06 µg/mL) was the most active agent tested against
Candida isolates (Figure); corresponding ANF, MCF, and FLU MIC90 values were 16- to
64-fold higher. Similarly, APX001A (MIC50/90, 0.25/0.5 µg/mL) was ≥8-fold more active
than ANF, MCF, and FLU against C. neoformans var. grubii. Against Aspergillus, AXP001A
(MIC50/90, 0.015/0.03 µg/mL) was comparable in activity to ANF and MCF. APX001A (MIC90,
0.06 µg/mL) was ≥128-fold more active than ANF and MCF against Scedosporium isolates.
Conclusion: APX001A demonstrated potent in vitro activity against 1,706 fungal isolates,
including echinocandin- and fluconazole-resistant strains. The extended spectrum of
APX001A was also notable for its potency against many less common, yet antifungal-resistant,
strains such as Candida auris, A. lentulus, A. ustus, Fusarium solani species complex,
and Scedosporium. Further studies are needed to demonstrate the utility of APX001A
in difficult-to-treat resistant fungal infections.
Helleberg
M.
1
Jørgensen
K.M.
2
Datcu
R.
2
Hare
R.
2
Arendrup
M.C.
2
3
4
1Dept. of Infectious Diseases, Rigshospitalet, Copenhagen, Denmark
2Unit of Mycology, Statens Serum Institut, Copenhagen, Denmark
3Rigshospitalet, Dept. of Clinical microbiology, Copenhagen, Denmark
4University of Copenhagen, Dept. of Clinical Medicine, Copenhagen, Denmark
P426. Rezafungin In Vitro Activity against Phase 2 STRIVE Part A and Contemporary
Nordic Clinical Candida Isolates Determined by the EUCAST Reference Method
Objectives: Rezafungin is a novel echinocandin with favourable safety and pharmacokinetic
characteristics, which allows for high drug exposures and once-weekly dosing. Epidemiological
cut-off values (ECOFFs) of rezafungin have not yet been established. We determined
the in vitro activity of rezafungin and four comparators against contemporary Nordic
clinical isolates of Candida and other yeasts and established single centre wildtype
upper limits (WT-UL). Subsequently, these WT-UL were used to evaluate rezafungin susceptibility
for clinical isolates from the completed part A of STRIVE, the phase 2 trial of rezafungin
for treatment of candidaemia and invasive candidiasis (NCT02734862).
Methods: 1,249 clinical isolates (19 Candida, 13 other yeast species) from Denmark,
Norway and Sweden received at the Danish mycology reference laboratory in 2017-18
were identified using CHROMagar, matrix-assisted laser desorption ionization–time
of flight mass spectrometry and, when needed, internal transcribed spacer sequencing.
EUCAST E.Def 7.3.1 susceptibility testing included rezafungin, anidulafungin, micafungin,
amphotericin B and fluconazole. WT-UL were established following EUCAST principles
for visual and statistical ECOFF value setting for rezafungin and for comparators
against species without EUCAST ECOFFs, allowing classification as wildtype or non-wildtype.
The FKS target genes were sequenced for echinocandin non-wildtype isolates.
Results: Rezafungin MIC distributions for the most common Candida species and modal
MICs for rezafungin and comparators are shown in Table 1. Rezafungin had species-specific
in vitro activity similar to that of anidulafungin and micafungin. At a mg/L basis
rezafungin was overall less active (modal MICs ≥ 2 two-fold dilutions higher) than
anidulafungin and micafungin, but equally or more active than fluconazole and amphotericin
B, except against C. parapsilosis, other Candida (amphotericin B only) and other yeasts.
Rates of rezafungin non-wildtype isolates among the most common Candida species were
<3%, only marginally higher than for amphotericin B and lower than for fluconazole,
anidulafungin and micafungin (Table 2). We identified 27 (2.0%) echinocandin non-wildtype
isolates: C. albicans (n = 11, 1.9%), C. glabrata (n = 11, 3.3%), C. tropicalis (n
= 2, 2.7%), C. dubliniensis (n = 2, 2.9%) and C. krusei (n = 1, 1.2%). Alterations
in Fks1 and/or Fks2 hot-spots (or within ±3 amino acids) were found in 20 of 25 sequenced
non-wildtype isolates: Fks1: C. albicans: D648Y, P1354P/S (2), P1354S (3), R1361R/S,
R1361G; C. tropicalis: F650S and S654P; C. dubliniensis: S645P (2); C. krusei: S659F.
Fks2: C. glabrata: S663P (2), S663F (3). Fks1+Fks2: C. glabrata: S663F/L630Q and L664Q+Y658N+premature
stop-codon. The rezafungin MICs for non-wildtype isolates were 0.25–2 mg/L. Rezafungin
susceptibility testing of 95 Candida blood isolates from the STRIVE trial yielded
MIC distributions comparable to those for the Nordic clinical isolates (Table 1).
One C. rugosa isolate had an MIC of >8 mg/L, suggesting intrinsic resistance, and
one C. glabrata isolate had an MIC of 0.5 mg/L, but no mutations in FKS target genes.
Conclusion: Rezafungin displayed broad in vitro activity with a species-specific susceptibility
pattern similar to that of other echinocandins. WT-UL were suggested for the most
common species. Few non-wildtype strains with alterations in FKS target genes or reduced
susceptibility to rezafungin were identified among clinical Candida isolates from
Nordic surveillance and the STRIVE trial.
Langridge
P.
Otu
A.
Denning
D.
National Aspergillosis Centre, Manchester University NHS Foundation Trust, Manchester,
United Kingdom
P427. An evaluation of the safety and efficacy of nebulised amphotericin B deoxycholate
(Fungizone) for treatment of pulmonary aspergillosis
Objectives: We evaluated the safety and efficacy of nebulised Fungizone in the long-term
treatment of various forms of pulmonary aspergillosis
Methods: This was a retrospective service evaluation of 177 patients with various
forms of pulmonary aspergillosis attending the National Aspergillosis Centre in Manchester.
Due to risk of intolerance/bronchospasm, patients first received a challenge test
with nebulised Fungizone in the hospital setting with spirometry pre/ post Fungizone.
To improve tolerability, nebulised short acting beta agonist was given prior to administration
of Fungizone (10mg in 4ml water for injection).
Results: The baseline FEV1 and FVC of the patients ranged from 0.94-2.97L and 13.3-5.15L
respectively. 66% (117) were able to tolerate their first test dose, of whom 26 (21%)
stopped using the nebulised therapy before their next clinic visit. Reasons cited
for Fungizone intolerance included increased breathlessness (8), wheeze (7), bronchospasm
(6), sore throat/voice (4), coughing (4), chest tightness (3), light-headedness/dizziness
(2), diarrhoea (2), fatigue (2), mouth blisters/ulcers (2), headaches (2), nausea/vomiting
(2) in addition to other symptoms. Eighteen continued nebulisation of Fungizone 10mg
twice a day for >3 months. Eleven had allergic bronchopulmonary aspergillosis (ABPA),
4 had severe asthma with fugal sensitization (SAFS), 2 had Aspergillus bronchitis
and 1 had Aspergillus sensitisation with cavitating nodules. Treatment courses ranged
from 4months to 4years. At their first clinic follow-up, there were reported benefits
with sputum production (7), sinus symptoms (2), dyspnoea (2), exercise tolerance (2),
reduction in prednisolone maintenance dose (1) and one demonstrated radiologic improvement.
Three had interruptions to their Fungizone treatment which had to be restarted following
clinical deterioration off therapy (2 of whom had dose change to once daily 12.5mg).
Five patients have remained on treatment with sustained benefit (e.g. clinical stability,
marked reduction in frequency of chest infections, reduction in Aspergillus specific
IgE and increased exercise tolerance). Four patients discontinued therapy as there
was no perceived clinical benefit. Aspergillus PCR on sputum at first repeated measure
following initiation of Fungizone treatment changed from positive to negative in 7
and stayed negative in 7. No pre-treatment PCR tests were done in 5 patients and no
follow-up PCR tests were performed in 6 patients. Fungal cultures of sputum went from
positive to negative after >3 months treatment in 6 patients, remained positive in
3 patients, remained negative in 4 patients and became positive in 2 patients.
Conclusion: Nebulised Fungizone is a poorly tolerated treatment for pulmonary Aspergillosis
with high dropout rates. There appears to be both clinical and serologic benefits
following sustained treatment with nebulised Fungizone in some patients
Uttam
G.
1
Singh
K.
1
Katiyar
D.
2
1Animal Mycology Laboratory, zoology, Mmv, Banaras Hindu University, Varanasi, India
2Chemistry, Mmv, Banaras Hindu University, Varanasi, India
P428. In-Silico and In-Vitro Evaluation of Anticandidal Activity of Cinnamaldehyde
Objectives:
Candida albicans is a common commensal which resides in human body and causes invasive
fungal infections especially in immunocompromised hosts. This study aims to evaluate
the anticandidal activity of cinnamaldehyde through in-silico approach and in-vitro
methods.
Methods: Interactions between cinnamaldehyde and potential drug targets of C. albicans
was elucidated using in-silico approach which was further validated by determination
of minimum inhibitory concentration (MIC) and the minimum fungicidal concentration
(MFC) of cinnamaldehyde using broth microdilution method recommended by CLSI 2009
for yeast (M27-A2). The effect of cinnamaldehyde on micromorphology of C. albicans
was studied. Sorbitol Assay (effect on cell wall) and Ergosterol Binding Assay of
C. albicans were also performed. Time-kill curve was conducted using cell viability
testing.
Results:
In-silico studies revealed that cinnamaldehyde showed good binding affinity with potential
targets of C. albicans such as ergosterol, N-myristoyl transferase and Secreted aspartic
proteinase 5. MIC and MFC of cinnamaldehyde were found to be 8.2 μg/ml and 16.4 μg/ml
respectively. In the morphological interference assays, it was observed that the cinnamaldehyde
inhibited pseudohyphae, blastospores and chlamydospores formation. The time-kill curve
of cinnamaldehyde showed that it required only few hours of exposure to effectively
kill >90% of the inoculum.
Conclusion: Cinnamaldehyde showed good in-vitro antifungal potential against C. albicans.
However, evaluation of pharmacodynamics and pharmacokinetics studies is desirous for
complete elucidation of its action as a therapeutic which will be helpful for mankind.
Singh
K.
Kumari
A.
Uttam
G.
Srivastava
N.
Animal Mycology Laboratory, Department of Zoology, Mmv, Banaras Hindu University,
Varanasi, India
P429. Antifungal Activity of Cinnamaldehyde against Some Human Pathogenic Fungi
Objectives: Despite the availability of effective antifungals, the morbidity and mortality
of fungal infections has remained high especially in individuals with impaired immunity.
The limited targets of existing antifungals may be one of the reasons for the emergence
of drug-resistant strains. In this regard, the anti-fungal potential of cinnamaldehyde,
a plant product and active constituent of cinnamon was tested through in-silico approach
which was further validated by in-vitro methods.
Methods: Antifungal activity of cinnamaldehyde was tested against Cryptococcus neoformans
(ATCC 6352), Candida albicans (MTCC 3017), Aureobasidium pullulans var. pullulans
(CBS 577.93), Aspergillus terreus, Alternaria alternata and Fusarium oxysporum (MTCC
2773) using broth micro-dilution methods recommended by CLSI (2009) for yeasts and
moulds. Further, PDB structures of drug targets of said fungus species were downloaded
or molleded (if not available) and their interactions with cinnamaldehyde were elucidated
in-silico using RCSB Protein databank, PHYRE2 and PatchDock servers.
Results: Cinnamaldehyde showed potent anti-fungal activity against all the fungal
pathogens screened. However, Cryptococcus neoformans (ATCC 6352) was found highly
susceptible against it with the MIC value 1.367±0.260 mg/ml. In-silico interaction
between targets of Cryptococcus neoformans (lanosterol 14-alpha demethylase), Candida
albicans (ergosterol, N-myristoyl transferase and Secreted aspartic proteinase 5),
Aureobasidium pullulans (chitinase), Aspergillus terreus (chitinase), Alternaria alternata
(ornithine descarboxylase) and Fusarium oxysporum (Thiamine thiazole synthase) with
cinnamaldehyde exhibited high binding energies and hydrogen bonds.
Conclusion: Cinnamaldehyde possesses broad spectrum and potential anti-fungal activity.
Being a plant product, it also has advantage for structural diversity and therefore,
could be a potential drug candidate for the treatment of fungal diseases. However,
its drug likeness properties should be evaluated by ex-vivo and in-vivo studies.
Carvalho-Pereira
J.I.
1
2
Gonçalves
M.S.
3
Sousa
M.J.
1
2
Sampaio
P.
1
2
1Centre of Molecular and Environmental Biology (cbma), Department of Biology, University
of Minho, Braga, Portugal
2Institute of Science and Innovation For Bio-sustainability (ib-s), University of
Minho, Braga, Portugal
3Centre of Chemistry, University of Minho, Braga, Portugal
P430. rAssessment of anti-Candida activity of Benzo(a)phenoxazines
Objectives: Fungal infections represent a major health problem due to their prevalence,
increasing number of cases and high complexity of the etiological agents involved.
Candida species are the most common and clinically important being able to cause from
less-severe superficial lesions to life-threatening disseminated mycosis. C. albicans
remains the most common species isolated, however, infections caused by non-albicans
species, such as C. auris, are increasing. Current treatments for fungal infections
are not 100% effective due to the drug toxicity, pathogen resistance, low drug bioavailability
and late diagnosis. At the moment, few groups of antimycotics are available to deal
with these infections. The loss of clinical efficacy of these drugs creates a potential
risk of spreading of resistant strains through the emergence of clones with high pathogenic
potential. This illustrates that the development of new antimicrobials is crucial.
Phenoxazine derivatives, namely benzo(a)phenoxazines have been shown to present good
antifungal properties. Thus, in this work we evaluated the antifungal activity of
five benzo(α)phenoxazin derivatives, against distinct Candida species and selected
the most promising to develop an effective antifungal nanoformulation.
Methods: The revised European Committee on Antimicrobial Susceptibility Testing (EUCAST)
protocol was used to determine the minimum inhibitory concentrations (MICs) of five
benzo(α)phenoxazines against seven Candida species, namely C.albicans, C.glabrata,
C.parapsilosis, C.tropicalis, C.krusei, C.bracarensis and C.auris. The most effective
compounds were selected for the development of formulations based on DODAB:MO liposomes.
All formulations were evaluated regarding the mean size, polydispersity index, surface
charge, liposome stability and encapsulation efficiency. The most stable formulations
were selected for antifungal activity re-evaluation against all seven Candida species
and for cytotoxicity assessment with different cell lines and using both the MTT and
the lactate dehydrogenase (LDH) release assays.
Results: demonstrated that all five benzo(α)phenoxazin derivatives tested presented
antifungal activity against all seven Candida species, with MICs between 3.75 to 60
μM. Two of these benzo(a)phenoxazin derivatives presented MICs of 3.75-15 μM against
all species tested, being selected to develop DODAB:MO formulations. The liposomal
formulations were positively charged, presenting mean sizes between 300-500nm and
polydispersity indexes of 0.4-0.5. The encapsulation efficiency was between 80-95%
for both benzo(α)phenoxazin derivatives tested. The activity of formulation against
all seven Candida species were evaluate with the liposomal formulations. Results demonstrated
that empty liposomes did not affect Candida growth and that the encapsulation of benzo(α)phenoxazins
increased their activity against Candida species. The cytotoxicity of the promising
formulations was tested using different cell lines and the non-toxic formulations
were selected.
Conclusion: The results obtained demonstrated that benzo(α)phenoxazins derivatives
could be potential antifungal drugs since present activity against all Candida species
tested, including the multidrug resistant C. auris. Moreover, the encapsulation of
the benzo(α)phenoxazin derivatives increase the antifungal activity and reduce the
cytotoxicity of compounds.
Pattini
V. Camilo
1
Polaquini
C.R.
1
Lemes
T.
1
Brizzotti
N.
2
Siqueira
J.P.
2
Almeida
M.
2
Regasini
L.O.
3
1São Paulo State University - Institute of Biosciences, Humanities and Exact Sciences
(UNESP - IBILCE), São José do Rio Preto, Brazil
2FAMERP, São José do Rio Preto, Brazil
3Chemistry and Environmental Sciences, UNESP (Sao Paulo State University), São José
do Rio Preto, Brazil
P431. Inhibitory effect of a curcumin-based compound against Epidermophyton floccosum
clinical isolates
Objectives: The present study aimed to evaluate the antifungal activity of a curcumin-based
compound against clinical strains of Epidermophyton floccosum isolated from dermatomycoses.
Methods: For this study, the curcumin-based compound was obtained from the Laboratory
of Antibiotics and Chemotherapeutics – LAQ, of the Institute of Biosciences, Letters
and Exact Sciences (IBILCE, Unesp São José do Rio Preto). This new compound is a hybrid
curcumin compound with substitutions to make the substance more stable and active.
The clinical strains were from the culture collection of the Laboratory of Microbiology
of the Medical School in São José do Rio Preto (FAMERP), Brazil. Susceptibility tests
were performed for two E.floccosum strains (6069 and 6113), using the Clinical and
Laboratory Standards Institute M38-A2 guidelines as reference. Percentage of inhibition
was calculated by spectrophotometry using the: I = 1 - (AbsT-AbsCT / AbsCC) × 100;
where: I = percentage of inhibition; AbsT = absorbance of the inoculum plus the compound
solution; AbsCT absorbance of sterility control; AbsCC = absorbance of growth control.
The curcumin (Merck®) was used as a control.
Results: The minimum inhibitory concentration (MIC) obtained for the curcumin-based
compound against E.floccosum 6069 and 6113 strains was 15.62μg/mL, inhibiting 80 and
87% the growth of the strains, respectively. On the other hand, pure curcumin (Merck®)
exhibited no activity at the concentrations tested.
Conclusion: The curcuminoid showed potent antifungal activity against E. floccosum
strains, higher than pure curcumin (Merck®), showing a potential alternative against
E. floccosum. In the future, toxicity tests will be performed allowing new therapeutic
approaches in the treatment of dermatomycoses.
Pattini
V. Camilo
1
Brizzotti
N.
2
Lemes
T.
1
Siqueira
J.P.
2
Marques
M.
2
Caetano
M.
3
Maschio-Lima
T.
3
Almeida
B. De
3
Bianco
L.
1
Silva
C.
2
Polaquini
C.R.
1
Regasini
L.O.
4
Castilho
E.
2
Almeida
M.
2
1São Paulo State University - Institute of Biosciences, Humanities and Exact Sciences
(UNESP - IBILCE), São José do Rio Preto, Brazil
2FAMERP, São José do Rio Preto, Brazil
3São Paulo State University - Institute of Biosciences, Humanities and Exact Sciences
(UNESP - IBILCE), São José do Rio Preto, Brazil
4Chemistry and Environmental Sciences, UNESP (Sao Paulo State University), São José
do Rio Preto, Brazil
P432. Antifungal activity of Kombucha Hibiscus sabdariffa infusion extract against
Trichophyton mentagrophytes and Trichophyton rubrum.
Objectives: The present study aimed to explore the antifungal potential of Kombucha
infusion extracts on planktonic cells of potentially pathogenic fungal isolates, as
an alternative to synthetic drugs.
Methods: Firstly, a SCOBY (Symbiotic Culture of Bacteria and Yeast), obtained from
a local natural products store, was placed in 1 liter of distilled water with 20g
of sucrose weekly for 30 days to remove any previous residues. The Hibiscus sabdariffa
infusion (HI) was prepared with four grams of leaves/flowers boiled in 450 mL of sterile
mineral water for 5 minutes and filtered on filter paper. The initial fermentation
was obtained by mixing 20 g of SCOBY and 80 g of sucrose to the infusion, and the
final volume was adjusted to 800 mL with sterile mineral water. The culture was then
incubated at 25 °C for 20 days. The experiment was carried out in triplicate. After
incubation, the solution was filtered in the MILLIPORE® System with 0.2 μm membrane.
The chemical compounds of the filtrate were extracted using ethyl acetate. To obtain
the dry and purified extract of the chemical compounds, the acetate phase was placed
on a rotary evaporator. Minimal inhibitory concentrations (MIC) and minimal fungicide
concentrations (MFC) of Kombucha HI extract against Trichophyton rubrum and Trichophyton
mentagrophytes clinical isolates were investigated by the microdilution method, using
as reference the document M38-A2 of the Clinical and Laboratory Standards Institute.
Results: The results obtained for the MIC of Kombucha HI extract against the T. mentagrophytes
and T. rubrum strain were 125 μg/mL and 62.5 μg/mL, respectively. For the MFC, values
observed were 250 μg/mL for T. mentagrophytes and 125 Oμg/mL for T. rubrum.
Conclusion: Kombucha HI extract showed antifungal activity against T. mentagrophytes
and T. rubrum. In the future, isolation and identification of the extract compounds
may allow new therapeutic approaches in the control of fungal infections.
Antoran
A.
1
Aparicio-Fernandez
L.
1
Buldain
I.
1
Martin-Souto
L.
1
Areitio
M.
1
Rementeria
A.
1
Ghannoum
M.A.
2
Fuchs
B.B.
3
Mylonakis
E.
3
Hernando
F.L.
1
Ramirez-Garcia
A.
1
1Immunology, Microbiology and Parasitology, University of the Basque Country (UPV/EHU),
Leioa, Spain
2Dermatology, Case Western Reserve University, Cleveland, United States of America
3Division of Infectious Diseases, Rhode Island Hospital and Alpert Medical School
of Brown University, Providence, United States of America
P433. Characterization of the specificity of the Ca37 monoclonal antibody and its
protective role against Candida albicans infection
Objectives:
Candida albicans is a commensal yeast of mucosal surfaces of human body. However,
when the host is immunocompromised, this yeast can cause invasive infections, that
present an unacceptably high mortality rate (up to 50%). Therefore, looking for alternative
treatments, we have focused on monoclonal antibodies. Alcohol dehydrogenase (Adh)
protein was selected for antibody development, due to its role in carcinogenic acetaldehyde
production, its surface location and its immunogenicity potential. The objective of
this work was to characterize the specificity of this Ca37 monoclonal antibody against
Candida albicans and its ability to interfere with yeast infection.
Methods: Adh1 protein was cloned in Escherichia coli. This recombinant protein was
used for mice immunization and Ca37 monoclonal antibody development. Proteomic analysis
by two dimensional (2-DE) western blotting and LC-MS/MS identification, in yeast and
hyphal fractions, were performed in order to identify the proteins detected by Ca37
and to determine its specificity against Adh1 protein. Indirect immunofluorescence
was carried out to study surface location of the protein. In this case, sodium metaperiodate
treatment was used for surface carbohydrate removal. Effect of Ca37 on yeast germination,
growth and interaction with antifungals was studied. Galleria mellonella in vivo model
infection, using an ΔADH1 mutant and parental strains was used to study the effect
of ADH1 gene loss on virulence, and for testing Ca37 antibody role as therapeutic
agent against C. albicans infection.
Results: The 2-DE western blot performed on cell wall-associated protein fraction
showed that Ca37 detected Adh1 protein in yeast and Adh2 in hyphal cells. Immunofluorescence
results indicated that the epitope recognized by the Ca37 was partially covered by
carbohydrates, as antibody only bound to fungal cells after sodium metaperiodate treatment,
or after long incubation times. Yeast germination was slightly inhibited, but growth
inhibition reached up to 90%. The antibody also showed an additive effect with amphotericin
B and fluconazole antifungals. Finally, in Galleria mellonella infection, we detected
a higher survival when using the ΔADH1 strain, and the same level of survival using
fluconazole or Ca37 treatment.
Conclusion: The Ca37 monoclonal antibody is specific for Adh proteins, which are located
at fungal cell surface, partially hidden by carbohydrates, and seem relevant for virulence
in vivo. Moreover, Ca37 is effective against yeast growth, provides an additive effect
with antifungal drugs, and confers protection in vivo. Altogether, Adh proteins seem
interesting targets against Candida albicans infections. Financial support: This study
was founded by a grant of UPV/EHU (PPG17/41). LA-F is recipient of a PhD grant of
UPV/EHU and LM-S of the Basque Government.
Thompson
G.R.
1
Honore
P.M.
2
Horcajada
J.P.
3
Fortun
J.
4
Hites
M.
5
Bassetti
M.
6
Mena
K.
7
Navalta
L.
7
Viani
R.
7
Sandison
T.
7
Pappas
P.
8
1University of Davis Medical Center, Davis, United States of America
2Brugmann University Hospital, Brussels, Belgium
3Hospital del Mar, Barcelona, Spain
4University Hospital Ramon y Cajal, Madrid, Spain
5Erasme Hospital, Brussels, Belgium
6Dept of Medicine, University of Udine, Udine, Italy
7Cidara Therapeutics, San Diego, United States of America
8University of Alabama at Birmingham, Birmingham, United States of America
P436. Rezafungin Clinical Safety and Efficacy in the Treatment of Candidemia and/or
Invasive Candidiasis: Combined Results from the STRIVE Phase 2 Trial Parts A and B
Objectives: Rezafungin (RZF) is a novel echinocandin designed to have next-generation
properties, such as increased stability and safety and novel pharmacokinetics/pharmacodynamics
(PK/PD), distinct from other systemic antifungals. The PK profile of RZF includes
a prolonged half-life and front-loaded plasma exposure that allow for once-weekly
therapy, as well as maximized pharmacometrics of antifungal efficacy. STRIVE is a
global, double-blind, randomized, clinical trial that compared the safety and efficacy
of RZF once-weekly to caspofungin once-daily in patients with candidemia and/or invasive
candidiasis (IC). After successful completion of STRIVE Part A (Thompson et al, 2018),
a second randomization (Part B) was conducted. Combined results from Parts A and B
are presented herein.
Methods: Adults (≥18 y) with systemic signs and mycological confirmation of candidemia
and/or IC were randomized to RZF once-weekly or standard of care (SOC) with caspofungin
for ≥14 days (up to 4 weeks) (Figure 1). Efficacy was assessed by overall response
(resolution of clinical signs of infection + mycological eradication) at day 14 (primary
endpoint) and PI assessment of clinical response at day 14 and all-cause mortality
at day 30 (secondary endpoints). Safety was evaluated by adverse events (AEs) and
mortality.
Results: of 207 patients enrolled, 183 patients were included in the microbiological
intent-to-treat population. Treatment groups were well balanced and matched in demographics
and baseline characteristics. Patients with IC comprised ~21% of the combined population.
RZF 400 mg/200 mg demonstrated greater efficacy than SOC, with the highest rates of
overall success and clinical cure and the lowest rate of 30-day all-cause mortality
of all 3 groups (Table 1). The rate of all-cause mortality through follow-up in the
pooled RZF group was 15.6% (19/122) and 19.7% (12/61) for SOC. No concerning safety
trends were observed. The incidence of ≥1 treatment-emergent adverse event (TEAE)
related to study drug was 13/134 (9.7%) among RZF-treated patients (pooled) and 9/68
(13.2%) with SOC. Between the pooled RZF and SOC groups, rates of study drug–related
TEAEs leading to study discontinuation (2.2% and 1.5%, respectively) and serious AEs
(1.5% and 2.9%, respectively) were comparable.
Conclusion: RZF was safe and efficacious in the Phase 2 trial of RZF treatment in
patients with candidemia and/or IC. RZF 400 mg/200 mg, the dosing regimen used in
the ongoing Ph 3 study, demonstrated greater efficacy than SOC and the most favorable
efficacy of all groups. No concerning trends in TEAEs were observed and, again, the
RZF 400 mg/200 mg once weekly group demonstrated the most favorable safety outcomes
in terms of TEAEs related to study drug. The recently completed Part B of STRIVE,
combined with the previous success of STRIVE Part A, demonstrates the safety and efficacy
of RZF treatment and supports its Phase 3 development.
Shadrivova
O.
1
Tonkoshkur
M.
1
Desyatik
E.
2
Volkova
A.
3
Popova
M.
3
Ermolova
S.
4
Bogomolova
T.
2
Ignatyeva
S.
2
Zubarovskaya
L.
3
Afanasyev
B.
3
Klimko
N.
1
1Department of Clinical Mycology, Allergy and Immunology, North-Western State Medical
University named after I.I.Mechnikov, St. Petersburg, Russian Federation
2Kashkin Research Institute of Medical Mycology, North-Western State Medical University
named after I.I.Mechnikov, St. Petersburg, Russian Federation
3I.Pavlov First Saint Petersburg State Medical University, St. Petersburg, Russian
Federation
4Department of Pulmonology, 3Leningrad Regional Clinical Hospital, St. Petersburg,
Russian Federation
P440. rInvasive aspergillosis in adult non-hematologic patients
Objectives: To investigate the features of invasive aspergillosis (IA) in non-hematologic
patients.
Methods: Retrospective analysis of clinical data of adult non-hematologicpatients
with IA. The EORTS/MSG 2008 criteria were used for IA diagnosis and assessment of
response of therapy.
Results: In the study were included 46 non-hematologic patients with IA, median age
– 48 years (19 - 99), females – 57%. The background conditions were autoimmune diseases
– 24%, kidney diseases with acute or chronic renal failure – 20%, oncology – 15%,
heart and vascular diseases – 11%, severe viral-bacterial pneumonia – 8%, HIV infection
– 7%, lung pathology – 7%, severe influenza H3N2 – 4%, other – 4%. The risk factors
of IA were glucocorticosteroid therapy – 50%, prolonged lymphocytopenia (<1,0 ×109/l,
median – 18 days) – 48%, immunosuppressive therapy – 35%, ICU stay – 30%, precede
surgical treatment – 28%, organs transplantation – 20%, severe neutropenia (median
– 10 days) – 13%, diabetes – 11%, allogeneic hematopoetic stem cells transplantation
(HSCT) – 2%, autologous HSCT – 2%. The main sites of infection were lungs – 85%, central
nervous system (CNS) – 11%, and paranasal sinuses – 9%. Disseminated (≥2 organs) IA
was in 17% patients. Clinical signs of IA were fever (≥ 38,5 °C) – 79%, cough – 76%,
respiratory failure – 59%, chest pain – 26%, and hemoptysis – 22%. CT scan signs were
bilateral lesions – 56%, air crescent sign – 12%. The main etiological agents were
A.fumigatus (62%), A.niger (14%), A.flavus(14%), A.ustus (5%) and A.ochraceus (5%).
«Proven» IA was diagnosed in 28% patients, «probable» – 72%. Antifungal therapy was
used in 93% patients, surgical treatment – 7%. Overall 12-weeks survival of patients
was 76%.
Conclusion: Invasive aspergillosisdeveloped in non-hematologic patientsusually with
autoimmune (24%), renal (20%) or oncology diseases (15%). The main risk factors were
glucocorticosteroid therapy (50%), lymphocytopenia (48%), immunosuppressive therapy
(35%), organs and hematopoetic stem cells transplantation (24%). The main etiological
agents were A.fumigatus (62%), A.niger (14%), and A.flavus(14%). The main sites of
infection were lungs (85%), disseminated (≥2 organs) infection was in 17% patients.
Overall 12-weeks survival of patients was 76%.
Stubbe
D.
1
Hendrickx
M.
2
Becker
P.
2
1Mycology & Aerobiology, Bccm/ihem Collection, Sciensano, Brussels, Belgium
2Mycology, Sciensano, BCCM/IHEM, Brussels, Belgium
P441. A plea to preserve fungal pathogens in public microbial resource centres
Objectives: Open science aims at sharing scientific outputs in order to maximize the
impact of research. This allows follow-on studies, facilitates new discoveries, improves
reproducibility of experiments and favours transparency of results. Although open
data is becoming a well-known concept, less attention is given to the availability
of research materials. In medical sciences, public culture collections represent an
historical example of open science, thanks to their longstanding experience in the
preservation of living microorganisms and their distribution for further scientific
investigations or development. These microbial resource centres provide well-characterized,
quality-controlled and authenticated strains and associated data. In medical mycology,
the diversity of fungal pathogens is important and needs to be secured following (inter)national
legislations for future basic and applied health questions. We wish to promote the
deposit of isolates in public collections. However, the responsibility to make fungal
strains available is shared by researchers, funding agencies and publishers.
Methods: We made a random selection of articles in the field of medical mycology to
check whether or not studied isolates were deposited and made available in public
collections.
Results: A random selection was made of 90 articles issued between 2017 and 2019 in
seven journals specialized in medical mycology. Out of them, only 13 (less than 15%)
mentioned the deposit of the strains.
Conclusion: Medical mycologists need to be more aware towards strain conservation.
Governmental funding policies should request the deposit of strains isolated during
financed projects. Regarding publishers, most journals encourage authors to make biological
material available but few specifically require deposit and most isolates investigated
in publications are not preserved. Editors should therefore implement mechanisms for
active agreement by authors to deposit strains when submitting an article. Such mechanisms
could follow Transparency and Openness Promotion guidelines1 for journals that include
a standard for research materials. Two level of stringency are proposed for this standard:
(1) the article states whether strains are available and, if so, where to access them
and (2) the mandatory deposit of the strains in a trusted repository.
Loeffert-Frémiot
S.
Microbiology Laboratory, Member of Wg 1 of The Cen, Laboratoires ANIOS, LILLE, France
P442. Fungicidal and Yeasticidal activity of chemical disinfectant and antiseptic
products: European standards & Real hospital life - How to be successful?
Objectives: Chemical disinfectants and antiseptic products are a crucial part of the
infection control strategy in hospital and are upregulated by European standards.
If infection control fails, the consequences could be severe especially for immunocompromised.
This is why it is important that testing procedures of chemical chemical disinfectants
are sufficient and well understand to ensure that disinfectants for the medical area
are reliable. The aim of this work is to help users to understand where, when and
how to use these products and to make and update of the conception and evolution of
these standards following what is happening in real work.
Methods: Explain the European Standards to which products have to conform in order
to support the claims for Fungicidal and yeasticidal activity. This include the description
of EN 14885 “Chemical disinfectants and antiseptics – Application of European Standards
for chemical disinfectants and antiseptics” (2018) requirements and the tests conditions
of the EN 13624 “Chemical disinfectants and antiseptics – Quantitative suspension
test for the evaluation of fungicidal or yeasticidal activity in the medical area
-Test method and requirements (phase 2, step 1)” (2013). In order to adapt the European
standards to what happen in medical area, standards are systematically revised by
group of expert members of the Working Group 1 of the European Comission of standardization
(Commission Europeenne de Normalisation: CEN).
Results: It is known that some strains could be challenging for healthcare workers
by their emerging character or by the apparition/ evolution of their resistance in
different part of European country. Therefore, European standards need to continuously
follow and adapt to the field situation. The choice of test organisms and conditions
of EN 13624 is under discussion to see their relevance. It is a critical point that
need to be raised and share with expert people of the field.
Conclusion: European Standards requirements must be continuously revised to be suitable
for effective infection strategy in medical area and particularly in healthcare settings.
These standards are more often not really known by healthcare people working on the
field, fighting everyday against resitant pathogens. They need to have all the keys
to ensure a reliable startegy against these pathogens.
Mgbeahuruike
A.
Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine,
University of Nigeria, Nsukka, Nsukka, Enugu, Nigeria
P443. Comparing the efficacy of three adsorbents, bentonite, activated charcoal and
fuller’s earth on the detoxification of aflatoxin-contaminated feeds.
Objectives: To test the ability of three low-cost and locally available adsorbents
(activated charcoal, bentonite and fuller’s earth) to detoxify poultry feeds contaminated
with aflatoxin.
Methods: Mould growth was induced on poultry feeds by sprinkling water on the feeds
until the feed was fully wet, the feed was packed in dark nylon bags and covered tightly.
The bags were stored in dark environments for 3 weeks. The feeds were divided into
4 groups, Contaminated but untreated, Contaminated but treated with bentonite, Contaminated
but treated with Activated charcoal and contaminated but treated with Fuller’s earth.
Aflatotoxin concentration in the feeds was measured using reverse phase HPLC. Fungal
contaminants in the feeds were isolated and molecularly identified. Phylogentic analysis
was reconstructed using MEGA 5.0. Measurement of performance indices of the birds
like heamatological and serum biochemical parameters were carried out and analysed
statistically using Minitab.
Results: Bentonite was the most effective adsorbent and lowered the total aflatoxin
concentration from 120 ± 38 µg/kg to 15 ± 5.0 µg/kg, while the concentration was reduced
to 20 ± 6.5 µg/kg in feed treated with fuller’s earth. The three adsorbent treatments
resulted in 63-100% weight increase of the birds, compared with birds fed the untreated,
aflatoxin-containing feed. Based on sequencing of the internal transcribed spacer
and phylogenetic analysis, 86% of the identified fungal contaminants were Aspergillus
species, with Aspergillus tamarii and A. nominus being the most prevalent with a frequency
of occurrence of 21% each. The number of white blood cells in blood samples was increased
by 13-17% in birds that consumed adsorbent-treated feed, compared with the untreated,
aflatoxin-contaminated feed. Serum biochemical analysis further showed that aspartate
amino transferase activity was 2.3 to 2.6 fold higher in birds that consumed the contaminated
but untreated feed, compared with feed treated with adsorbents. Hepatic lesions were
also prominent in the liver of the birds fed contaminated but untreated feed but were
reduced in the group that were fed the adsorbent-treated feeds, especially in the
bentonite-treated feed.
Conclusion: Conclusively, the adsorbents were able to reduce the concentration of
aflatoxin in the feed and this reflected positively on the general performance of
the birds.
Scroferneker
M.L.
1
Hellwig
A.H. Da Silva
2
Abreu
H. Klein De
2
Ribeiro
A. Carvalho
1
Oliveira
H. Tedesco De
1
Pezzi
E. Heidrich
1
Zanette
R. Adriel
3
1Department of Microbiology, Immunology and Parasitology, Universidade Federal do
Rio Grande do Sul, Porto Alegre, Brazil
2Postgraduate Program In Medicine: Medical Sciences, Universidade Federal do Rio Grande
do Sul, Porto Alegre, Brazil
3Postgraduate Program In Biological Sciences: Pharmacology and Therapeutics, Universidade
Federal do Rio Grande do Sul, Porto Alegre, Brazil
P444. Evaluation of the growth of Sporothrix schenckii and Sporothrix brasiliensis
when exposed to the iron element
Objectives: The aim is to evaluate the viability of Sporothrix spp. when exposed to
iron, using 11 isolates of S. schenckii and 10 isolates from S. brasiliensis.
Methods: All the samples used were previously analyzed by molecular methods. Conidia
were collected from cultures with seven days of growth in potato dextrose agar medium
and suspended in 0.85% saline solution. Each suspension was standardized in a Neubauer
chamber, adjusted to the concentration of 106 conidia / ml, and inoculated at the
center of petri plates containing potato dextrose agar plus the following iron concentrations
(FeCl3): 0.0625%; 0.125%; 0.25%. The isolates were also inoculated into plates containing
potato dextrose agar pure or added with 1 mM ascorbic acid and 1 mM ferrozine to eliminate
traces of iron present in the medium. Plates were incubated in triplicates at 35 °
C for 28 days to measure the mean diameter of colonies (mm) and perform analysis of
variance followed by the Tukey post-hoc test in the GraphPad Prism statistical program,
with significance p < 0.05.
Results: It has been found that high iron concentrations are toxic to Sporothrix spp.,
as seen in the concentration of 0.25% FeCl3, where there was no growth of the isolates.
In addition, medium with absence of iron also had to lower growth of Sporothrix spp.
when compared to the potato dextrose agar medium and the medium plus 0.0625% and 0.125%
FeCl3.
Conclusion: It is possible to conclude that the viability of Sporothrix schenckii
and Sporothrix brasiliensis is affected by iron, which is an important element for
its biological activity.
Scroferneker
M.L.
1
Tavares
P. Lemos
2
Berte
F. Kercher
1
Ribeiro
A. Carvalho
1
Rott
M. Brittes
1
1Department of Microbiology, Immunology and Parasitology, Universidade Federal do
Rio Grande do Sul, Porto Alegre, Brazil
2Postgraduate Program In Medicine: Medical Sciences, Universidade Federal do Rio Grande
do Sul, Porto Alegre, Brazil
P445. The interaction between Sporothrix schenckii sensu sctricto and Sporothrix brasiliensis
with Acanthamoeba castellanii
Objectives: The aim of our study was to verify the profile of interaction between
S. schenckii sensu stricto and S. brasiliensis and A. castellanii by an in vitro model
of coculture as a modulator factor of both microorganisms intrinsic characteristics.
Methods: For this purpose the phagocytosis index of S. schenckii sensu stricto and
S. brasiliensis by A. castellanii was evaluated; the S. schenckii sensu stricto and
S. brasiliensis viability after contact with A. castellanii; the amoeba viability
after contact with the fungus; and the influence of S. schenckii sensu stricto and
S. brasiliensis in encystment process of A. castellanii were performed. The analyses
indicated that S. schenckii and S. brasiliensis have suffered phagocytic events by
A. castellanii and this index is significantly higher for S. schenckii in the first
two hours compared to S. brasiliensis.
Results: Our results showed a significant increase in conidia and hyphae count after
72h of coculture of S. brasiliensis and lysis of amoebae occurred after fungal internalization.
Conclusion:
S. schenckii sensu stricto and S. brasiliensis probably used amoebae as nutritional
source. Our results were obtained in vitro and may not demonstrate the same behavior
in vivo, being required in vivo studies with co-infection in order to have a thorough
understanding of this relationship.
Delhaes
L.
1
Francis
F.
2
Enaud
R.
3
Soret
P.
4
Consortia
M.
5
Bui
S.
3
Fayon
M.
3
Thiebaut
R.
2
1Parasitology Mycology, Univeristy of Bordeaux, Centre de Recherche Cardio-Thoracique
de Bordeaux, U1045, F-33000, Bordeaux, France, Bordeaux, France
2CHU Bordeaux, Department of Public Health, Bordeaux, France
3University of Bordeaux, Centre de Recherche Cardio-Thoracique de Bordeaux, U1045,
CHU de Bordeaux, CRCM Pédiatrique, CIC 1401, Bordeaux, France
4University of Bordeaux, Inserm, Bordeaux Population Health Research Center, UMR 1219,
INRIA SISTM Team, Bordeaux, France
5The Mucofong Investigation Group, Bordeaux, France
P446. Estimation of fungal risk in cystic fibrosis: lessons from the MucoFong prospective
project
Objectives: Beside the main objectives of the multicentric project "MucoFong" (PHRC-19021906)
that evaluated the respiratory fungal composition in CF and provided the first French
guidelines for mycological analysis of CF sputum, data collected during this two-year
follow-up of 299 CF patients (3 visits per patient including biological, radiological,
and clinical data) were analyzed to assess associations between the microorganisms
identified in the sputum and the clinical evolution.
Methods: Relations between microorganisms identified in the sputum and clinical course
of patients were longitudinally analyzed taking as output variable FEV1 (Forced Expiratory
Volume in 1s expressed as percent of predicted value). As data were collected several
times throughout the follow-up, a mixed-model analysis was performed to evaluate the
effect of a sporadic or chronic colonization with bacteria and/or fungi at the inclusion
on the evolution of lung function over two years. Univariate plus multivariate analysis
were achieved. A sensitivity analysis was performed to identify interactions between
microbes.
Results:
P. aeruginosa, A. xylosoxidans, S. maltophilia, and C. albicans were associated to
a more significant decrease in FEV1 during the two-year follow-up, adjusted on age,
gender, hospital centre, BMI, and gastrooesophageal reflux disease. Unexpectedly,
FEV1 evolution was lower of 11.26% in patients presenting an intermittent colonisation
with non-pneumoniae Streptococcus than others.
Conclusion: To conclude, these results were in agreement with data recently published,
and provided new insights into the bacterial but also the fungal colonization as a
key factor in assessing and predicting lung function evolution in CF patients. The
Mucofong Investigation Group: Marc Pihet, Emilie Fréalle, Yolande Lemeille, Claudine
Pinel, Hervé Pelloux, Gilles Gargala, Loic Favennec, Isabelle Accoceberry, Isabelle
Durand-Joly, Frédéric Dalle, Frédéric Huet, Annlyse Fanton, Amale Boldron, Guy-André
Loeuille, Philippe Domblides, Bérengère Coltey, Isabelle Pin, Catherine Llerena, Françoise
Troussier, Christine Person, Christophe Marguet, Nathalie Wizla, Caroline Thumerelle,
Dominique Turck, Anne Prévotat, Benoit Wallaert, Sylvie Leroy, Jean-Philippe Bouchara
Grant: Project MucoFng 2.0 (RF20160501626/1/1/275) from Vaincre la Mucoviscidose (VLM)
Scroferneker
M.L.
1
Koehler
A.
2
Corbellini
V.
3
1Department of Microbiology, Immunology and Parasitology, Universidade Federal do
Rio Grande do Sul, Porto Alegre, Brazil
2Postgraduate Program In Medicine: Medical Sciences, Universidade Federal do Rio Grande
do Sul, Porto Alegre, Brazil
3Department of Chemistry and Physics, Universidade de Santa Cruz do Sul, Santa Cruz
do Sul, Brazil
P447. Evaluation of metabolic adaptations of Fonsecaea pedrosoi using tricyclazole
and FTIR
Objectives: Evaluating the metabolic adaptations of Fonsecaea pedrosoi when exposed
to tricyclazole using Fourier Transform Infrared Spectroscopy (FTIR).
Methods: Twelve isolates of F. pedrosoi from the collection of the Pathogenic Fungi
Laboratory, Department of Microbiology, ICBS, UFRGS, were cultivated for 14 days at
30ºC in Petri dishes with Sabouraud agar. For each isolate were used two plates with
4 µg/mL or 16 µg/mL of tricyclazole. The spectra were acquired through attenuated
total reflection (ATR) using fragments of the cultures on dehydrated agar. Five spectra
were recorded from 4000 to 650 cm-1 for each isolate. The data set was preprocessed
and normalized by amplitude. The Pirouette® software was used to perform the Principal
Component Analysis (PCA) and the Partial Least Squares Discriminant Analysis (PLS-DA)
using the first derivative and an orthogonal signal correction. These analyzes were
perfomed to verify if it was possible to separate the classes (growth with 4 or 16
µg/mL) through the spectra, showing if there are metabolic adaptations of F. pedrosoi
when exposed to tricyclazole.
Results: The PCA didn’t allowed class differentiation due to irrelevante background
information. With PLS-DA, using only one component, the minimum error of class prediction
was about 0.001, with Bayes limit between classes close to 0.5 and 100% of accuracy
in the identification of the cultures in 4 or 16 µg/mL of tricyclazole. The results
show that the metabolic adpatations are reproducible, but discrete, and the regions
of the spectra that most contributed to the differentiation were the bands of 989
cm-1 and 1086 cm-1 (sugars); 1508 cm-1 (proteins) and 1617 cm-1 (unsaturated carbon
bonds, evidence of the formation of the 1,8-dihydroxynaphtalene (DHN) melanin precursors).
Conclusion: There are metabolic adaptations of F. pedrosoi with the exposition to
tricyclazole. These adaptations can be evaluated through FTIR, which is relevant for
the development of techniques to make correlations with models of virulence and pathogenicity
of these fungi.
Oladele
R.
1
Akase
I.
2
Fahal
A.
3
Govender
N.
4
Hoenigl
M.
5
Gangneux
J.-P.
6
Chiller
T.
7
Cornely
O.A.
8
Denning
D.
9
Chakrabarti
A.
10
1Medical Microbiology & Parasitology, College of Medicine of University of Lagos,
Lagos, Nigeria
2Internal Medicine, Lagos University Teaching Hospital, Lagos, Nigeria
3Mycetoma Research Centre, Kartoum, Sudan
4National Institute for Communicable Diseases, Johannesburg, South Africa
5Division of Infectious Diseases, Department of Medicine, UCSD, San Diego, United
States of America, Austria, United States of America
6Universite de Rennes, Rennes, France
7Mycotic Division, Centre for Disease Control, Atlanta, United States of America
8Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany,
Cologne, Germany
9Global Action Fund for Fungal Infections, Geneva, Switzerland
10Medical Microbiology, Postgraduate Institute of Medical Education and Research,
Chandigarh, India
P449. Bridging the knowledge gap on mycoses in Africa; setting up a Pan-African Mycology
Working Group
Objectives: The African continent has an estimated population of 1.3 billion people
accounting for roughly about a quarter of the world’s population and has an estimated
700,000 fungal related deaths in the setting of HIV infections. From the data generated
by GAFFI; an estimated 47,643,919 Africans suffer from fungal diseases, of which 1,728,657
suffer from a serious fungal infection annually (GAFFI Factsheet). This is a course
for concern considering the fact that this is data from only 15 (out of 57) African
countries (Table 1). Almost all African countries lack a surveillance system for fungal
infections; the only exception is South Africa. Interestingly, only South Africa has
a reference mycology laboratory. Also, there is a pervasive picture of inadequate/poor
diagnostic capacity, low level of awareness/ index of suspicion among health care
workers and policymakers, unavailability, and non-accessibility to antifungal medications.
Most African countries have poorly funded and overburdened health systems. Additionally,
a high prevalence of HIV in Sub-Saharan Africa contributes to a high burden of opportunistic
fungal infections. Recently, the African sub-region developed a network of mycology
experts whose goal is to provide a common platform for a compressive discussion and
collaboration on fungal infections.
Methods: Recent outreach efforts of ISHAM and also the European Confederation of Medical
Mycology (ECMM) aimed to increase the involvement of African countries and experts
in e.g. the “One World One Guideline” initiative and also the ECMM Academy. The Medical
Mycology Society of Nigeria with support from ISHAM and ECMM held its first international
conference on March 2019, which cumulated with the establishment of the PanAfrican
Mycology Working Group (PAMWG).
Results: PAMWG will organize and engage African leaders in the field of mycology in
the African sub-region linked to ISHAM. The aim is is to provide better interaction&synergy
among regional leaders in order to develop educational programs for capacity building
to aid in the diagnosis and care of patients with fungal infections. It will also
encourage countries’ initiatives to develop clinical guidelines for the clinical management
of fungal infections and support the need for the establishment of reference mycology
laboratories. Objectives The ISHAM-PAMWG aims to provide a platform for all the leaders
in the field on mycology from different African countries in order to initiate epidemiological
studies, regional guidelines, and educational programs to increase the capacity/ expertise
of African specialists in the early detection and treatment of fungal infections.
These activities will be achieved via the following: Education and training programs
for health care workers involved in the care and evaluation of patients with fungal
infections. South-south, and north-south collaborations and partnership. Organization
of regional collaborative studies. Participation in the ECMM One World One Guideline
program. Encourage all PAWG members to affiliate with ISHAM Organize local meetings
in African countries in collaboration with ISHAM and ECMM Participation of leading
centers in Africa in the ECMM Excellence Center program To advocate the recognition
of neglected fungal diseases in Africa.
Conclusion: Meeting the objectives listed above will generate the needed data for
advocacy needed to drive government commitment to allocate the resources required.
Pinheiro
R. Rollin
1
Rochetti
V. Pereira
1
Xisto
M.I.D.S.
1
Bastos
B.
2
Rella
A.
3
Rozental
S.
2
Poeta
M. Del
3
Barreto-Bergter
E.
1
1Instituto De Microbiologia Paulo De Góes, Universidade Federal do Rio de Janeiro,
Rio de Janeiro, Brazil
2Instituto De Biofísica Carlos Chagas, Universidade Federal do Rio de Janeiro, Rio
de Janeiro, Brazil
3Department of Molecular Genetics and Microbiology, Stony Brook University, Stony
Brook/New York, United States of America
P450. rImportance of Sphingolipid Biosynthetic Pathway for Growth, Biofilm Formation
and Membrane Integrity of the Pathogenic Fungus Scedosporium boydii
Objectives:
Scedosporium boydii is a worldwide emerging pathogen able to cause a wide-spectrum
infection ranging from mycetoma to invasive infections in immunocompromised patients.
It is also considered the second more frequent pathogenic fungus associated to cystic
fibrosis patients. Sphingolipids are abundant components of membranes in fungal cells,
playing a variety of roles such as heat stress response, signal transduction, hyphal
growth, endocytosis and apoptosis. Particularly, glucosylceramides (GlcCer) have been
studying by our group during the last decades in Scedosporium/Lomentospora complex,
being associated to fungal growth and pathogenesis. This study aims to evaluate the
sphingolipid synthesis during the germination process, as well as the importance of
different sphingolipids for fungal growth, membrane integrity using some inhibitors
of the biosynthetic pathway.
Methods:
S. boydii conidia were treated with Myriocin, a serine palmitoyltransferase inhibitor,
or PPMP (1-phenyl-2-palmitoylamino-3-morpholino-1-propanol), a glucosylceramide synthase
inhibitor, and the results were compared to the non-treated control. Sphingolipid
synthesis was evaluated by mass spectrometry of lipids extracted by fungal cells treated
or not with the inhibitors. The effect of the inhibitors on fungal growth was analyzed
by germination assay, colony forming units (CFU) quantification and biofilm formation.
Filipin staining, checked by fluorescence microscopy, was performed to evaluate the
accumulation of membrane microdomains on hyphal tips. Membrane integrity was analyzed
by transmission electron microscopy (TEM) and fungal susceptibility to membrane stressors,
such as SDS and NaCl. Susceptibility to antifungal agents in the presence of Myriocin
and PPMP was evaluated through the checkerboard method.
Results:
S. boydii treated with Myriocin and PPMP displayed decreased sphingolipid production
and showed a reduced growth, forming less CFU and biofilms compared to the control.
Additionally, the germination process was impaired, since treated cells formed short
germ tubes compared to mature hyphae in the control. It was confirmed with filipin
staining, in which lipid raft accumulation in hyphal tips, essential for hyphae elongation,
was not observed in treated cells. Analysis by TEM revealed the presence of regions
with loss of membrane integrity, what was confirmed through the increase in membrane
susceptibility to SDS and NaCl in treated cells. Myriocin-treated cells became more
susceptible to different classes of antifungal drugs, suggesting that sphingolipids
on the membrane could interfere in drug susceptibility.
Conclusion: Sphingolipids have been considering a potent target for the develop of
new antifungal drugs. Thus, the study of these molecules in pathogenic fungal becomes
a useful approach in medical mycology. The present data highlight the participation
of sphingolipids in different fungal cell events that are crucial for fungal growth,
virulence and drug resistance. Hyphae elongation and biofilm formation are important
processes for mold proliferation and host tissue invasion. In addition, membrane integrity,
especially regarding lipid raft areas, is essential for signal transduction, stress
resistance and a variety of other physiological processes in fungal cells. For these
reasons, the use of sphingolipid inhibitors helped to show how these molecules are
promising targets for new drugs against Scedosporium/Lomentospora infections.
Mantilla
M.
1
Ariza
G.
1
Cabrera
C.
2
Restrepo
S.
1
Ramírez
A.M. Celis
1
1Biological Sciences, Universidad de los Andes, Bogotá D.C., Colombia
2Chemistry, Universidad de los Andes, Bogotá D.C., Colombia
P451. Studying the unknown: implementation of two recognized protocols to characterize
lipid droplets in Malassezia pachydermatis
Objectives:
Malassezia pachydermatis is a lipid-dependent yeast that is part of animal skin mycobiota
and is associated with otitis externa and dermatitis in animals, as well as fungemia
in humans. The lipid metabolism of this yeast is of high relevance because it may
play an essential role during pathogenesis; however, these processes are yet to be
well understood. Lipid droplets (LD) have become a subject of study in Malassezia,
as they are known to store lipids and proteins in other biological models such as
Mycobacteria, Hepatitis B virus, Saccharomyces cerevisiae and, mammal eukaryotic cells.
Therefore, this study aims to standardize for the first time two protocols for the
analysis of LD in Malassezia pachydermatis: The first one to characterize their structure
by observation under a laser scanning confocal fluorescence microscopy; and the second
one for the extraction and purification of LD for future lipidomic analysis.
Methods: Here we standardized two protocols to describe LD: the first one to characterize
the structure is based on co-staining yeast cells and LDs with different fluorochromes:
Nile Red (Sigma-Aldrich), LipidTox red neutral (Invitrogen) and BODIPY 493/503 (Invitrogen),
in combination with calcofluor white (Sigma-Aldrich). The first three fluorochromes
bind to neutral lipids present in the LD and calcofluor binds to the cellulose and
chitin in the fungal cell wall. The second protocol to extract and purify LD induces
yeast cell lysis by a combination of chemical and physical techniques, followed by
the modification of the density of the solution with different buffers to allow the
extraction and purification of LD.
Results: We standardized a protocol to optimize LD fluorescent staining to characterize
their structure under confocal microscopy. We found that the optimal steps are as
follows: 1) Preparation of the staining solution with a mixture of 4% formaldehyde
and the three neutral lipid fluorophores. 2) Incubation at room temperature of LipidTox
and Nile Red for 2 hours, and BODIPY for 30 minutes. 3) Double washing with PBS, resuspension
in 4% formaldehyde and mounting on microscope slides. 4) Addition of calcofluor white,
and 0.01% and 10%(v/v) KOH to the slide. We also optimized the protocol to get the
of rupture M. pachydermatis cells for the extraction and purification of LDs. We found
that the optimal conditions are: 1) Enzymatic digestion with Trichoderma harzianum
enzymes (Sigma-Aldrich) for 2 hours at 47ºC. 2) Mechanical disruption by adding 0.5
mm zirconium pearls to the sample and ten cycles of 1 minute of a vortex and 30 seconds
on ice. In addition, cell wall removal by stroking the sample 10 times with a Dounce
homogenizer. 3) Finally, several ultracentrifugation cycles in buffers containing
Ficoll 400 (Sigma-Aldrich) to separate the LDs in the supernatant from the organelles
that stay in the pellet.
Conclusion: Improving and standardizing these protocols can contribute to the study
of the morphology, structure and, in the future, the lipid composition of LD in Malassezia
pachydermatis. This study also could disclose clues about the lipid metabolism field
in this yeast.
Speth
C.
1
2
Marcabruni
J.
1
Parth
N.
1
Koci
F.
1
Lass-Flörl
C.
2
Maier
H.
3
Rambach
G.
4
1Div Hygiene & Med. Microbiology, Med. Univ. Innsbruck, Innsbruck, Austria
2Christian Doppler Laboratory for Invasive Fungal Infections, Innsbruck, Austria
3Institute of Pathology, INNPATH, Innsbruck, Austria
4Div Hygiene & Med. Microbiology, Med. Univ. Innsbruck, Medical University of Innsbruck,
Austria
P452. Characterization of the local inflammatory reaction after inhalative infection
of immunocompromised mice with the mucormycete Rhizopus arrhizus
Objectives: The mucormycete species Rhizopus arrhizus mainly enters the body of immunosuppressed
patients via inhalation of the spores. The local pathogenesis of inflammatory processes
in the lung is poorly understood. For that reason we investigated the inflammatory
reaction and the antifungal immune reaction in the lung of Rhizopus-infected mice.
Methods: Mice treated with cortisone or cyclophosphamide were intravenously infected
with a high dose of Rhizopus arrhizus spores. For comparison mice with selective deficiency
in neutrophils, alveolar macrophages or complement were used. Lung parameters were
investigated by histology and by isolation of bronchoalveolar lavage (BAL). Immune
processes were quantified in blood samples taken from the animals.
Results: Lack of neutrophils as well as treatment with cyclophosphamide were the most
prominent risk factors for a lethal outcome after Rhizopus infection; in contrast,
depletion of alveolar macrophages or cortisone treatment represented only moderate
risk factors. Interestingly, complement deficiency even ameliorated the outcome, presumably
due to a reduced inflammatory process. Lack of macroscopic organ abnormalities beside
the lung indicated that fungal infection was mainly limited to the lung. BAL analyses
revealed clear signs of excessive inflammation such as presence of erythrocytes, massive
infiltration of granulocytes and high levels of the marker haptoglobin. Furthermore,
macrophages in the lung were significantly augmented and these immune cells were highly
activated. Most spores found in BAL were bound to or phagocytosed by neutrophils and
macrophages and only few fungal hyphae were visible.
Conclusion: Inflammatory processes in the lung after inhalation of Rhizopus arrhizus
are the main reason for fatal outcome; fungal growth and/or dissemination plays a
minor role.
Rambach
G.
1
2
Marcabruni
J.
1
Parth
N.
1
Lass-Flörl
C.
1
2
Speth
C.
1
2
1Div Hygiene & Med. Microbiology, Med. Univ. Innsbruck, Innsbruck, Austria
2Christian Doppler Laboratory for Invasive Fungal Infections, Innsbruck, Austria
P453. Pathogenesis and amphotericin B treatment of Lichtheimia corymbifera infection
in a murine model
Objectives: Immunocompromised individuals are the main risk group for invasive mucormycosis,
but the relevance of the precise nature of immunodeficiency is insufficiently investigated.
Furthermore, only limited information exists about differences in the efficiency of
amphotericin B (AmB) therapy in various patient groups. For that purpose we aimed
to study pathogenesis and AmB treatment of inhalative Lichtheimia corymbifera infection
and to compare the susceptibility of pancytopenic mice versus mice with selective
defects in innate immunity.
Methods: Mice treated with cyclophosphamide or cortisone or selectively deficient
in neutrophils, alveolar macrophages or complement, were inhalatively infected with
Lichtheimia corymbifera. Half of the animals received therapy with liposomal AmB.
Survival, clinical status and immunological processes were monitored.
Results: Immunosuppression of the animals with cyclophosphamide represented the most
relevant risk factor for fatal outcome of Lichtheimia infection, followed by treatment
with cortisone. Although neutropenia is assumed to be the correlate for susceptibility
against fungal infection, selective depletion of neutrophils predisposed only moderately
for severe disease and mortality. Interestingly, neither complement deficiency nor
depletion of alveolar macrophages represented major risk factors for the animals.
Various clinical parameters such as body temperature and weight loss as well as immunological
parameters confirmed these results. For those animals dying from invasive Lichtheimia
infection, neurological symptoms were the second most reason for scarifying the mice.
Treatment with liposomal AmB successfully improved the outcome in the animal groups
with neutropenia, cortisone acetate and cyclophosphamide with the most prominent effect
in the cortisone group.
Conclusion: Affectation of several immune cell types is necessary to make the animals
susceptible for invasive mucormycosis by Lichtheimia corymbifera, whereas selective
loss of neutrophils or alveolar macrophages have only minor effects.
Guruceaga
X.
1
Perez-Cuesta
U.
1
Pellon
A.
2
Uribe
B.
3
Gonzalez
O.
3
Keller
N. P.
4
Hernando
F.L.
1
Anguita
J.
2
Alonso
R.M.
3
Ramirez-Garcia
A.
1
Rementeria
A.
1
1Immunology, Microbiology and Parasitology, University of the Basque Country (UPV/EHU),
Leioa, Spain
2Inflammation and Macrophage Plasticity Laboratory, CIC bioGUNE, Derio, Spain
3Department of Analytical Chemistry, University of the Basque Country (UPV/EHU), Leioa,
Spain
4Department of Medical Microbiology and Immunology, University of Wisconsin, Madison,
United States of America
P454. The role of Aspergillus fumigatus fumagillin in fungal virulence.
Objectives:
Aspergillus fumigatus is a saprophytic fungus capable to cause pulmonary infections
in immunosuppressed patients. We have previously demonstrated an overexpression of
67% of the genes involved in the fumagillin metabolic pathway during experimental
infection of immunosuppressed mice. The aim of this work was to demonstrate the implication
of this molecule in fungal pathogenesis.
Methods: We performed a set of in vitro experiments using commercial toxin preparations
to first standardize the UHPLC-PDA analytical method and then identify its toxic effects
on macrophages (RAW 264.7), melanome (B16-F10), and the lung epithelial (A549) cell
lines. Moreover, we carried out fungus-host cell co-incubation assays using murine
primary bone marrow derived macrophages (BMMs). Finally, in vivo assays were performed
by intranasal infection of immunosuppressed Swiss mice treated with 100 mg/kg of cyclophosphamide
intraperitoneally every 72 h, starting four days before the infection. The mice, in
group on 10, were infected with, the non-fumagillin producer mutant strain, ΔfumR,
as well as the wild type (WT), ΔakuBku80
, and the complemented strains. All the experiments were repeated at least thrice.
Results: The UHPLC-PDA method assay showed that A. fumigatus WT strain is able to
produce the toxin at a 1.31 μg/ml growing in RPMI during 24 h at 37°C. Furthermore,
when we incubated the cell lines in the presence of 1 μg/ml of commercial toxin for
24 h, eighty percent of the toxin disappeared from the media of A549 cultures, 58%
in the case of B16-F10 melanome and only 4% in cultures with RAW 264.7 cells. However,
the viability of the A549 cells was not significantly affected, while B16-F10 and
RAW264.7 cells showed a significant reduction in their viability compared to. untreated
cells. Assays in vitro showed that the null strain, ΔfumR, was significantly more
susceptible to phagocytosis by the BMMs than the complemented or WT control strains.
On the other hand, no differences in germination were observed during the co-incubation
with BMMs, but ΔfumR showed a significant increase in the percentage of double branches
conidia compared to the other two strains. Indeed around 25% of the germinated ΔfumR
conidia showed two branches after 8 hours of co-incubation. The in vivo study did
not show any significant differences in mice survival between the groups infected
with the ΔfumR and the WT strains. In contrast, mice infected with the complemented
strain showed a higher mortality rate, maybe due to the increase secretion of fumagillin
by complemented strain, as we showed in the in vitro assays.
Conclusion: This study shows that A. fumigatus is able to secrete the mycotoxin fumagillin,
a toxin that penetrates inside the cells causing cell death. Furthermore, this toxin
could be a fungal defense factor against phagocytosis and may play an important role
during A. fumigatus pulmonary infection. Acknowledgement. This study was funded by
a grant of UPV/EHU (PPG17/41). Pre-doctoral grants of the Basque Government and UPV/EHU
have supported XG, and BU and UPC, respectively.
Buccheri
R.
1
2
Neto
A. Nunes Duarte
1
Silva
F.L. Batista Da
2
Carvalho
G.O. Marques Haddad
2
Silva
L. Buffoni Roque Da
3
Neto
R. Soares De Azevedo
1
Taborda
C.P.
3
Benard
G.
4
1Department of Pathology, Medical School, University of São Paulo, São Paulo, Brazil
2Laboratório De Investigação Médica Lim53, Insituto de Medicina Tropical, São Paulo,
Brazil
3Laboratório De Fungos Dimórficos Patogêncios, Biomedical Sciences Institute, University
of São Paulo, São Paulo, Brazil
4Laboratório De Investigação Médica Lim53, Instituto de Medicina Tropical/Faculdade
de Medicina da Universidade de São Paulo, São Paulo, Brazil
P456. Establishment of an experimental protocol of chronic tobacco exposure to study
its effects on a mice model of pulmonary paracoccidioidomycosis
Objectives: Paracoccidioidomycosis (PCM) is caused by Paracoccidioides spp, and the
most important systemic mycosis in non-immunosuppressed patents in Latin America.
It has two major clinical presentations, the acute/subacute, more rare (≤5% of the
cases) form (AF), and the more prevalent, chronic form (CF). In the AF, the phagocytic-mononuclear
system is predominantly affected, while the lung is the target organ in the CF. The
AF in general develops some time (weeks to months) after exposure to Paracoccidioides
spp. and the CF develops from reactivated quiescent foci many years or decades after
the initial exposure. However, we still do not know the factors that trigger the reactivation
of the fungus and the development of the CF. of note is the observation that most
(>90%) CF patients refer tobacco smoking. It is well known that chronic tobacco exposure
leads to imbalance of the pulmonary immune responses, and has a role in the development
of other chronic pulmonary infections such as tuberculosis. We therefore aimed to
develop a protocol of chronic tobacco exposure to study its effects on an experimental
mice model of pulmonary PCM. We present here the preliminary results of this protocol.
Methods: C57BL/6 animals were exposed to cigarette smoke for 120 minutes (equivalent
to ~16 cigarettes) twice/day, 5 days/week, for a total of 20 weeks, using a previously
described device [Biselli PJC et al. Braz J Med Biol Res. 2011;44:460-468]. Three
groups of animals were studied: an infected/exposed group, inoculated intra-tracheally
with 102
P. brasiliensis yeast cells 2 weeks prior to initiation of tobacco exposure, an infected
only group, and an exposed only group. The tobacco exposure protocol was adjusted
to result in carboxyhemoglobin (COHb) levels of 10-15% after the daily first exposure,
and 15-20% after the second exposure, to simulate the levels observed in chronic moderate
smokers [Stewart RD et al. JAMA. 1974;26:1187-95]. Animals were sacrificed at weeks
4, 12 and 20 and their lungs examined for colony forming units, area involved by the
granulomatous inflammation and histological assessment of the effects of tobacco exposure.
Results: The tobacco exposure protocol was successful in mimicking the effect of chronic
tobacco smoking in humans: a) the mean level of COHb after the first tobacco exposure
(measured monthly) was 15,5% (range 10,9-16,1%), and of 20,5% (range 15,9-21,1%) after
the second exposure, while in the no-exposed infected group it was 0,1% (range 0-0,3%);
b) By week 20 there were moderate dysplasia of the columnar epithelium, increased
mucous production, alveolar septum destruction and focal areas of bronchiolitis. Preliminary
data showed that tobacco exposure increased both the CFU and the area involved by
the granulomatous inflammation as shown below.
Conclusion: These preliminary results indicate that: 1) We were able to establish
a protocol of tobacco exposure mimicking that observed in patients with chronic form
paracoccidioidomycosis. 2) Tobacco exposure impacts the pulmonary disease in a mouse
model of PCM. Financial support: Fapesp #2016/08730-6 and #2017/12374-3. CPT and GB
receive a senior researcher fellowship from CNPq
Guruceaga
X.
1
Perez-Cuesta
U.
1
Martin-Vicente
A.
2
Souza
A.C.O.
2
Nywening
A.V.
2
Abdallah
Q. Al
2
Ge
W.
2
Hernando
F.L.
1
Fortwendel
J.
2
Ramirez-Garcia
A.
1
Rementeria
A.
1
1Immunology, Microbiology and Parasitology, University of the Basque Country (UPV/EHU),
Leioa, Spain
2Department of Clinical Pharmacy and Translational Science, University of Tennessee
Health Science Center (UTHSC), Memphis, United States of America
P457. Phenotypic characterization of Aspergillus fumigatus maiA deficient strain.
Objectives: Invasive aspergillosis is the most dangerous disease produced by Aspergillus
fumigatus. To identify genes important for host-pathogen interactions, we analyzed
fungal gene expression in contact with macrophages and lung epithelium cell lines,
as well as during an in vivo infection in immunosuppressed mice and detected multiple
genes overexpressed in all the situations. One of these genes, maiA, is involved in
the Tyrosine metabolic pathway and could be an important gene for energy production
during host-pathogen interactions. For that, the aim of this work was to characterize
a mutant strain deficient in maiA gene.
Methods: The loss of function mutant strain was built by CRISPR-Cas9 technology using
the A. fumigatus genetic background Af-293. The transformation method was carried
out using a standard protocol. For the phenotypic spot characterization assays, we
used Glucose minimal medium (GMM) agar plates supplemented with Congo red (80 µg/ml),
Calcofluor white (80 µg/ml), Voriconazole (0,5 µg/ml), Caspofungin (4 µg/ml) or Anidulafungin
(4 µg/ml) and minimal medium supplemented with L-tyrosine (10 mM) or Phenylalanine
(10 mM) as the sole carbon source. Antifungal susceptibility tests using Voriconazole,
Itraconazole, Isavuconazole, Posaconazole, Caspofungin and Anidulafungin were carried
out following the EUCAST method. Finally, glucan quantification, following the Aniline
blue assay, and pyomelanin quantification, using GMM broth and GMM broth supplemented
with L-Tyrosine (10mM) or Phenylalanine (10mM), were also performed. All the assays
were performed in triplicate.
Results: The CRISPR-Cas9 technique provided a gene-targeting efficiency of 60% (3
out of 5 colonies) using the A. fumigatus genetic background Af-293. After spot dilution
assays analysis, differences in the fungal growth between the wild type and the mutant
strain were not found in presence of Calcofluor white, Voriconazole, Caspofungin or
Anidulafungin. In contrast, ΔmaiA was hypersusceptible to Congo red despite a lack
of significantly different amounts of glucan per milligram of mycelia. Furthermore,
no significant differences were observed in the MIC or MEC for the triazoles or the
echinocandins, respectively. However, ΔmaiA was unable to use either L-tyrosine or
Phenylalanine as the sole carbon source and pyomelanin production by the mutant strain
was significantly higher than the wild type when GMM broth was supplemented with L-tyrosine
or Phenylalanine. In GMM broth non-supplemented, both the wild type and ΔmaiA showed
the same pyomelanin production pattern.
Conclusion: CRISPR-Cas9 is an accurate technique that allowed us to generate the mutant
strain ΔmaiA using the A. fumigatus genetic background Af-293 with a great efficiency.
This mutant was not able to grow in presence of Congo red or to use L-Tyrosine or
Phenylalanine as a sole carbon source. Pyomelanin production was higher for ΔmaiA
in the presence of both amino acids, which is consistent with results published using
other mutants of the Tyrosine degradation pathway. Future studies will explore in
deep the role of maiA in animal models of IA. Acknowledgement. This study was funded
by a grant of UPV/EHU (PPG17/41). EMBO Short-Term Fellowships and pre-doctoral grants
of the UPV/EHU have supported XG and UPC respectively.
Govic
Y. Le
1
2
Havlicek
V.
3
Papon
N.
1
Gal
S. Le
1
Bouchara
J.-P.
1
4
Vandeputte
P.
1
4
1Host-Pathogen Interaction Study Group, SFR ICAT 4208, Univ Angers, Univ Brest, Institute
of Biology in Health, IRIS, University Hospital Center, Angers, France, Angers Cedex,
France
2Laboratory of Parasitology-Mycology, University Hospital Center, Fort-de-France,
Martinique, Fort-de-France, Martinique
3Institute of Microbiology, Academy of Science, Prague, Czech Republic
4Laboratory of Parasitology-Mycology, University Hospital Center, Angers, France,
Angers, France
P458. Scedosporium apiospermum sidD gene is responsible for the biosynthesis of the
hydroxamate-type siderophore Nα-methylcoprogen B, and is essential for fungal growth
Objectives:
Scedosporium species are filamentous fungi which commonly colonize the airways of
patients with cystic fibrosis (CF). Nevertheless, there is only scarce information
on their virulence mechanisms. Iron acquisition is crucial for the survival of nearly
all microorganisms and is implicated in virulence of numerous pathogens that chronically
inhabit the CF lung. Several genes relevant for siderophore-mediated iron uptake were
previously identified in Scedosporium apiospermum genome, notably an orthologue of
sidD, a non-ribosomal peptide synthetase (NRPS) gene that was demonstrated to drive
the production of the extracellular hydroxamate-type siderophore fusarinine C (FsC)
in Aspergillus fumigatus. Here, we aimed to determine the chemical structure of the
siderophore produced by S. apiospermum sidD gene and to assess its role in fungal
growth.
Methods: First, sidD gene was disrupted in a wild-type (WT) strain after previous
disruption of the gene ku70 encoding one of the components of the DNA repair system.
Next, siderophore analysis was performed on lyophilized filtrates from WT and ΔsidD
strains grown in minimal Yeast Nitrogen Base (YNB) medium for 48 h at 37°C. WT strain
was cultured in YNB supplemented or not with 100 µM bathophenantroline disulfonate
(BPS), while the mutants (n = 3) were grown in YNB without BPS only. The impact of
sidD deletion was then studied by cultivating the fungus on yeast extract-peptone-dextrose-agar
(YPDA) plates containing BPS and supplemented with culture filtrates from either WT
or mutant strains. Finally, the ability of S. apiospermum to assimilate iron from
xenosiderophores was assessed by using iron-saturated ferrichrome, ferrioxamine or
pyoverdine (20 µM each) as sole source of iron.
Results: Analysis of the extracellular siderophores by high-resolution mass spectrometry
performed on culture filtrate from WT strain yielded a molecular mass of m/z (M-2H
+ Fe)+ = 794.314 for the iron-saturated siderophore, perfectly matching Nα-methylcoprogen
B. This compound was absent in all ΔsidD mutant strains, indicating that sidD gene
is essential for its synthesis. Other siderophores like FsC or TAFC were not found
in any sample. Moreover, the disruption of sidD resulted in the lack of growth under
iron-restricted conditions, and growth could be restored by supplementation of the
culture medium (YPDA+BPS) with a culture filtrate from the WT strain. Conversely,
incorporation of lyophilized filtrates from mutant strains in the culture medium did
not rescue growth, corroborating the biochemical analyses. Interestingly, the use
of iron-saturated ferrichrome or ferrioxamine as sole source of iron permitted a normal
growth of all strains, indicating their direct assimilation by the fungus independently
of siderophore production. Conversely, pyoverdine supported growth of the WT strain
only, suggesting that N-methylcoprogen B is important for iron acquisition from pyoverdine.
Conclusion: Our results revealed that the S. apiospermum sidD gene drives the synthesis
of a unique extracellular siderophore, N
α-methylcoprogen B, that is essential for fungal growth. This compound seems important
for iron acquisition from pyoverdine, which might explain why S. apiospermum and Pseudomonas
aeruginosa are rarely found concomitantly in the CF lungs. Works are in progress to
determine the contribution of sidD in the virulence of S. apiospermum.
Aparicio-Fernandez
L.
Antoran
A.
Martin-Souto
L.
Buldain
I.
Areitio
M.
Rementeria
A.
Hernando
F.L.
Ramirez-Garcia
A.
Immunology, Microbiology and Parasitology, University of the Basque Country (UPV/EHU),
Leioa, Spain
P459. Effect of Candida albicans on the malignant characteristic of tumor cells
Objectives:
Candida albicans is an opportunistic pathogenic fungus usually present in different
areas of the human body such as mouth and gut. Under certain circumstances, this yeast
is able to reach the bloodstream causing disseminated candidiasis. Therefore, C. albicans
is in close relationship with human cells, being in constant contact with them. This
microorganism has been related to cancer and metastasis through different mechanisms
such as carcinogenic product synthesis and induction of pro-metastatic microenvironment.
Among the types of cancer studied in relation to C. albicans are oral malignancies
and melanoma metastasis. Hence, this work aims to determinate whether the exposure
to C. albicans is able to change tumor cells phenotype, increasing their aggressiveness.
Methods: Three tumor cell lines were used for this study: murine melanoma cells (B16M),
human oral squamous cell carcinoma (H357) and murine colon carcinoma cells (C26).
First, an adhesion assay was performed to test the effect of Candida albicans on tumor
cells. Then, cancer cell lines were stimulated with antibiotic-inactivated fungus
and conditioned media of the fungus, and wound healing assay was performed to measure
migration activity of tumor cells. Afterwards, RNA was extracted from tumor cells
to examine the expression of cancer-related genes by RT-qPCR and the supernatant was
collected to measure the level of pro-inflamatory and cancer-related cytokines by
ELISA.
Results: First, we observed that C. albicans was able to adhere to the three tumor
cell lines. Then, wound healing assays with both antibiotic-inactivated C. albicans
and conditioned media of C. albicans showed an increase in migration capacity of the
different cell lines in comparison with the control, which indicates that the exposure
to this fungus can induce a phenotypical change in tumor cells. The pro-tumor microenvironment
was also altered, being the expression of different studied cytokines and cancer-related
genes increased in presence of the fungus.
Conclusion: These results suggest that exposure to C. albicans induce alteration or
changes in tumor cells and the related microenvironment that can lead to an increased
malignancy. Acknowledgement: This study was funded by a grant of UPV/EHU (PPG17/41).
Pre-doctoral Grants of Basque Government and UPV/EHU have supported LMS and LAF respectively.
Willeman
T.
1
Tonini
J.
2
Cécile
G.
3
Bailly
S.
4
Gandia
P.
5
Stanke-Labesque
F.
4
Maubon
D.
6
Gautier-Veyret
E.
4
1Laboratoire de Pharmacologie, Pharmacogénétique et Toxicologie, CHU Grenoble Alpes,
France, GRENOBLE Cedex, France
2Laboratoire de Pharmacologie, Pharmacogénétique et Toxicologie, CHU Grenoble Alpes,
France, GRENOBLE Cedex, France
3Univ. Grenoble Alpes, CNRS, CHU Grenoble Alpes, Grenoble INP*, TIMC-IMAG, 38000 Grenoble,
France * Institute of Engineering Univ. Grenoble Alpes, GRENOBLE, France
4Univ. Grenoble Alpes, Inserm, CHU Grenoble Alpes, HP2, 38000 Grenoble, France, GRENOBLE,
France
5UMR1436-INTHERES, 31076 Toulouse, France, Toulouse, France
6Univ. Grenoble Alpes, CNRS, CHU Grenoble Alpes, Grenoble INP*, TIMC-IMAG, 38000 Grenoble,
France * Institute of Engineering Univ. Grenoble Alpes, GRENOBLE Cedex, France
P465. Refining the therapeutic range of posaconazole and isavuconazole for efficient
therapeutic drug monitoring using a bioassay approach
Objectives: Therapeutic drug monitoring for posaconazole (POS) tablet and isavuconazole
(ISA) is still under debate and target trough concentrations (Cmin) need to be specified.
We aimed to investigate the pharmacokinetic-pharmacodynamic relationship of POS and
ISA, using a bioassay approach as surrogate marker of antifungal activity, in order
to refine the therapeutic Cmin of both antifungals.
Methods: A bioassay using a cellulose disk diffusion method was performed to determine
the growth inhibition zone (GIZ) of POS and ISA on A.fumigatus (POS and ISA) and C.
parapsilosis (ISA only). GIZs of plasma from patients undergoing routine TDM for POS
(n = 136) or ISA (n = 40) were determined.
Results: GIZs of plasma patients and antifungal Cmin were highly correlated both for
ISA (A fumigatus: rho = 0.942, p<0.0001; C. parapsilosis: rho = 0.949, p<0.0001) and
POS (rho = 0.922, p<0.0001), and these relationships were represented with a Michaelis-Menten
model. Based on this modeling, the recommended thresholds of 0.7, 1, and 1.25 mg/L
for the POS Cmin corresponded to 50.1, 55.2, and 59.1% of the maximal GIZ, respectively.
We propose an upper threshold of 4.8 mg/L for the POS Cmin and a lower threshold of
2.0 mg/L for the Cmin of ISA, as they respectively corresponded to concentrations
leading to 90 % and 50 % of the maximal GIZ on A.fumigatus.
Conclusion: The determination of antifungal activity using this bioassay allowed refining
target Cmin for both POS and ISA, especially the upper threshold of POS (4.8 mg/L)
and the lower threshold of ISA (2.0 mg/L).
Gautier-Veyret
E.
1
Thiebaut-Bertrand
A.
2
Roustit
M.
1
Fonrose
X.
3
Bolcato
L.
4
Depeisses
J.
2
Lefebvre
C.
5
Masson
D.
6
Schummer
G.
7
Cahn
J.-Y.
8
Stanke-Labesque
F.
1
1Univ. Grenoble Alpes, Inserm, CHU Grenoble Alpes, HP2, 38000 Grenoble, France, GRENOBLE,
France
2Hématologie Clinique CHU Grenoble Alpes, Grenoble, France
3Chu Grenoble Alpes, Laboratoire de Pharmacologie, Pharmacogénétique et Toxicologie,
GRENOBLE, France
4Laboratoire de Pharmacologie, Pharmacogénétique et Toxicologie, GRENOBLE, France
5Laboratoire d’Hématologie, CHU Grenoble Alpes, Grenoble, France
6Etablissement Français du Sang, La Tronche, France
7Centre de ressources biologiques, CHU Grenoble Alpes, GRENOBLE Cedex, France
8Univ. Grenoble Alpes, CHU Grenoble Alpes, TIMC-Therex, Grenoble, France
P466. Prospective evaluation of a combined genetic score for the individualization
of voriconazole dose
Objectives: Voriconazole (VRC) exhibits a highly variable pharmacokinetics which is
partly related to genetic polymorphisms affecting activities of cytochromes (CYP)
2C19 and 3A. This prospective observational study aimed to evaluate the impact of
a previously described genetic score combining CYP2C19 and CYP3A genotypes (Gautier-Veyret,
AAC 2015) to predict infratherapeutic plasma VRC trough concentration (Cmin) (defined
as VRC Cmin < 1mg/L) in order to individualize VRC dose.
Methods: CYP2C19, CYP3A4 and CYP3A5 genotypes were determined in hematological patients
treated by VRC and who benefited from longitudinal therapeutic drug monitoring in
Grenoble University Hospital between January 2016 and December 2018. The genetic score
was determined for each patient and patients were classified according to their genetic
score ≤ or > 2. The higher the genetic score, the faster the metabolism of the patient.
Statistical analyzes were performed on VRC Cmin adjusted on dose (VRC Cmin/D), considering
either the initial VRC Cmin/D or all VRC Cmin.
Results: Forty-three patients (43 % of female, median age [Q1-Q3]: 57 [45-67] years)
were included and 127 VRC Cmin analyzed. Ninety five percent of patients (41/43) received
VRC for curative treatment of invasive aspergillosis (22 possible, 17 probable and
2 proven). Median initial VRC Cmin was 2.2 [1.1-4.3] mg/L and 21 % of patients (n
= 9/43) had infratherapeutic initial VRC Cmin. Sixty-three percent of patients had
at least one genetic variant for CYP2C19, 3A4 or 3A5, with 8 patients with a genetic
score <2. Among 9 patients with infratherapeutic initial VRC Cmin, 8 (89%) had a genetic
score ≥ 2. In univariate analysis, initial VRC Cmin/D, as well as all VRC Cmin/D was
not influenced by genetic score, but significantly associated with advanced age, increased
CRP and bilirubin levels. Multivariate analysis performed in all VRC Cmin/D identified
CRP (p<0.0001), bilirubin (p = 0.01) and age (p = 0.007) as independent determinants
of VRC Cmin/D, while genetic score had no impact. Genetic score was significantly
associated with VRC Cmin/D only in the subgroup of VRC Cmin (n = 51) determined in
absence of severe inflammation (defined by CRP level < median CRP of 65 mg/L).
Conclusion: Our genetic score that combined genetic variants of CYP2C19, 3A4 and 3A5
seems interesting to predict subtherapeutic VRC Cmin, but its impact is suppressed
by severe inflammation. Strategies for individualization of VRC dose must integrate
pharmacogenetics variants, but also the inflammatory status of patient.
Daele
R. Van
1
2
Brüggemann
R.J.M.
3
4
Holtappels
M.
5
Depuydt
P.
6
Rijnders
B.
7
Cotton
F.
8
Fage
D.
8
Dreesen
E.
2
Wauters
J.
9
10
Spriet
I.
1
2
1Pharmacy Department, University Hospitals Leuven, Leuven, Belgium
2Department of Pharmaceutical and Pharmacological Sciences, KU Leuven, Leuven, Belgium
3Radboud University Medical Center, Department of Pharmacy and Radboud Institute for
Health Sciences, Nijmegen, Netherlands
4Center of Expertise In Mycology Radboudumc /cwz, Radboud University Medical Center,
Nijmegen, Netherlands
5Laboratory For Clinical Infectious and Inflammatory Disorders, KU Leuven, Leuven,
Belgium
6Department of Intensive Care, Ghent University Hospital, Ghent, Belgium
7Department of Infectious Diseases, Erasmus University Medical Center Rotterdam, Rotterdam,
Netherlands
8Department of Clinical Chemistry, LHUB-ULB, Erasme Hospital and Université Libre
de Bruxelles, Bruxelles, Belgium
9Department of Microbiology and Immunology, KU Leuven, Leuven, Belgium
10Medical Intensive Care Unit, University Hospitals Leuven, Leuven, Belgium
P467. Pharmacokinetics of posaconazole in critically ill patients undergoing extracorporeal
membrane oxygenation (ECMO)
Objectives: The use of posaconazole as prophylaxis for influenza-associated aspergillosis
is currently investigated in critically ill patients. An important subset of patients
admitted to the intensive care unit (ICU) for influenza is undergoing extracorporeal
membrane oxygenation (ECMO) for severe acute hypoxemic respiratory failure. Little
is known on the exposure to posaconazole in this population. This study presents the
pharmacokinetics (PK) of seven ICU patients treated with intravenous posaconazole
while on ICU undergoing ECMO.
Methods: All but one of the included patients were participating in the posaconazole-arm
of the ongoing multicenter Posa-Flu study (NCT03378479), assessing the efficacy of
a 7-day prophylactic intravenous treatment with posaconazole in ICU patients admitted
for severe influenza. The additional patient was a lung transplant recipient treated
with posaconazole for invasive aspergillosis. Blood samples were collected at an early
(day 2-3) and late (day 5-7) time point, using a full (predose, 1.5,2,3,4,6,8,10,12,18,24h)
or limited (predose, 1.5-3, 4-8, 8-12, 12-24h) sampling scheme. Trough concentrations
were collected on all other days. All patients received an intravenous loading dose
of 300 mg BID followed by a maintenance dose of 300 mg QD. Written informed consent
was obtained from the patients or their relatives. PK parameters were estimated in
Excel®.
Results: All patients received veno-venous ECMO and two patients were supported by
continuous veno-venous hemodialysis (CVVH). The median area under the curve (AUC0-24),
clearance (CL) and volume of distribution (Vd) were 35.69 mg.h/L, 8.42 L/h and 421.0
L, respectively. Exposure documented in the ECMO patients is shown in Table 1, along
with the results previously documented in healthy volunteers, patients with hematological
disease and critically ill patients. As shown in Figure 1, all trough concentrations
(Cmin) were above 0.7 mg/L, which is the suggested threshold for prophylaxis in patients
with hematological disease, and 71% of the Cmin were also ≥ 1 mg/L, which is the suggested
hematological threshold for treatment of invasive aspergillosis (ECIL-6 and ESCMID-ECMM-ERS
2017). The median average concentration (Cavg) of both exposure days, i.e. 1.49 mg/L,
was within the European Medicines Agency (EMA) target range (0.5-2.5 mg/L). The targeted
posaconazole AUC0-24 of 20.87-22.5 mg.h/L for a MIC value of 0.125 mg/L (DRU 2014),
was also achieved.
Conclusion: Posaconazole exposure was similar compared to that documented in hematology
patients but higher than in ICU patients without ECMO. The lower limit for prophylaxis,
as recommended in patients with hematological malignancies, is attained in all patients.
This should be interpreted with caution as the optimal exposure to ensure prophylactic
efficacy might be different in critically ill patients admitted for severe influenza.
This optimal exposure is currently being investigated in the Posa-Flu trial. Based
on this limited dataset, ECMO does not seem to influence posaconazole exposure. An
a priori dose adjustment does not seem to be necessary but therapeutic drug monitoring
is recommended to confirm the exposure. Despite the fact that this observation should
be established in a larger number of patients, these results suggest that posaconazole
exposure is maintained in critically ill patients undergoing ECMO. Hence we recommend
the standard dosing regimen.
Wasmann
R.E.
1
2
Smit
C.
3
4
Donselaar
M. Van
1
Dongen
E. Van
5
Wiezer
R.
6
Burger
D.
1
Knibbe
C.
3
4
Brüggemann
R.J.M.
1
2
1Pharmacy, Radboudumc, Nijmegen, Netherlands
2Center of Expertise in Mycology Radboudumc / CWZ, Nijmegen, Netherlands
3Clinical Pharmacy, St. Antonius Hospital, Nieuwegein, Netherlands
4Division of Systems Biomedicine and Pharmacology, Leiden Academic Centre For Drug
Research, Leiden University, Leiden, Netherlands
5Department of Anesthesiology, Intensive Care and Pain Management, St. Antonius Hospital,
Nieuwegein, Netherlands
6Department of Surgery, St. Antonius Hospital, Nieuwegein, Netherlands
P468. Pharmacokinetics of posaconazole in normal-weight and morbidly obese adults
Objectives: One in five individuals will be obese in 2025. Obesity is associated with
underdosing of antimicrobial drugs for prophylaxis and treatment of (life-threatening)
infections. Posaconazole is a broad-spectrum triazole antifungal drug frequently used
for prophylaxis and treatment of mold infections. There is anecdotal evidence that
posaconazole exposure is impacted by weight resulting in suboptimal target attainment.
We performed a prospective clinical trial to investigate the effect of body size in
obese subjects and determined the probability of reaching the target trough concentration
for prophylaxis (>0.7mg/L) and therapy (>1.0mg/L).
Methods: Obese subjects (BMI above 35 kg/m2) undergoing bariatric surgery and normal-weight
subjects (BMI 18.5-25 kg/m2) were included in an open-label, single-dose, multicenter,
pharmacokinetic study. Obese subjects were randomized to receive either 300 or 400
mg posaconazole IV while normal-weight subjects all received 300 mg posaconazole IV.
Blood samples were collected at nine time points up to 24 hours after start of infusion.
Statistical analysis and population pharmacokinetic modeling was performed using R
and NONMEM 7.3.
Results: 8 obese subjects on 300 mg, 8 obese subjects on 400 mg and 8 normal-weight
subjects on 300 mg were included. Weight [range] was 129 kg [109-190]), 144 kg [107-175]),
72.3 kg [61.4-85.4] respectively. The observed geometric mean [range] AUC0-24h in
normal-weight versus obese subjects receiving 300 mg posaconazole was 21.4 mg*h/L
[15.6-29.1] versus 13.1 mg*h/L [9.1-18.5] (p<0.05). Obese subjects receiving 400 mg
posaconazole had an AUC0-24h of 16.8 mg*h/L [12.2-25.6].A two-compartment model with
first-order elimination, a proportional residual error model and inter-individual
variability on the central compartment best described the data. Total body weight
was the best predictor of variability of clearance and the central and peripheral
compartment. Simulations demonstrated that a 300 mg dose results in a >90% PTA in
patients up to 190kg for prophylaxis. For treatment, a 300 mg dose is sufficient up
to 120 kg after which a dose increase to 400 mg daily should result in a >90% PTA
up to 170 kg.
Conclusion: We found that obese individuals have a significant lower exposure to posaconazole
compared to normal-weight individuals even when using a 400 mg dose. Nevertheless,
PTA (at 0.7 mg/L) was high using the IV formulation. In the treatment setting higher
dosages are warranted.
Chong
L.L.
1
Ling
L.M.
2
1Haematology, Tan Tock Seng Hospital, Singapore, Singapore
2Infectious Diseases, Tan Tock Seng Hospital, Singapore, Singapore
P469. The effects of Cytochrome P450 2C19 profile on voriconazole serum trough concentrations
and hepatotoxicity in Haematology patients
Objectives: Cytochrome P450 (CYP) 2C19 genetic polymorphism contributes to variability
of voriconazole pharmacokinetics. Supra-therapeutic voriconazole trough concentrations
have been correlated with hepatotoxicity. This study aims to establish the effects
of CYP2C19 profile on voriconazole troughs and hepatotoxicity.
Methods: A prospective, observational study was done to determine voriconazole trough
concentrations at pre-steady state (day 2 to 4 of therapy) and steady state (day 6
onwards). Blood sampling was repeated weekly until week 12 of recruitment or death.
The CYP2C19 genotype was also identified for patients on voriconazole.
Results: 11 patients were analyzed for CYP2C19 polymorphism: 1*/1* normal metabolizers
(n = 5, 45 %), 1*/2* intermediate metabolizers (n = 3, 27 %) and 2*/2* poor metabolizers
(n = 3, 27 %), respectively. 4 out of 6 patients were intermediate or poor metabolizers
whom had supra-therapeutic voriconazole concentrations (trough > 5.5 mg/L). All poor
metabolizers developed abnormal liver function tests (range of ALP: 230 to 630).
Conclusion: Knowledge of CYP2C19 profile would allow pre-emptive dose adjustments
during the first week of voriconazole therapy and thus reduce the incidence of hepatotoxicity.
Wasmann
R.E.
1
2
Smit
C.
3
Dongen
E. Van
5
Wiezer
R.
6
Adler-Moore
J.
7
Beer
Y. De
8
Burger
D.
1
Knibbe
C.
3
4
Brüggemann
R.J.M.
9
10
1Pharmacy, Radboudumc, Nijmegen, Netherlands
2Center of Expertise in Mycology Radboudumc / CWZ, Nijmegen, Netherlands
3Clinical Pharmacy, St. Antonius Hospital, Nieuwegein, Netherlands
4Division of Systems Biomedicine and Pharmacology, Leiden Academic Centre For Drug
Research, Leiden University, Leiden, Netherlands
5Department of Anesthesiology, Intensive Care and Pain Management, St. Antonius Hospital,
Nieuwegein, Netherlands
6Department of Surgery, St. Antonius Hospital, Nieuwegein, Netherlands
7Biology, Cal Poly Pomona, Pomona, United States of America
8Department of Clinical Pharmacy & Toxicology, Maastricht University Medical Center+,
Maastricht, Netherlands
9Pharmacy, RadboudUMC, Nijmegen, Netherlands
10Center of Expertise In Mycology Radboudumc /cwz, Radboud University Medical Center,
Nijmegen, Netherlands
P470. rObese patients require a fixed dose of liposomal amphotericin B
Objectives: In 2014, approximately 11% of adult men and 15% of adult women were obese
worldwide, a number that is increasing. Like other patients, obese patients may be
confronted with invasive fungal disease and require treatment with antifungal agents
such as liposomal amphotericin B (L-AmB, Ambisome®). We performed a pharmacokinetic
study in morbidly obese individuals to define the optimal dose of L-Amb by quantifying
the impact of obesity on the pharmacokinetics of L-AmB.
Methods: In this open-label study, morbidly obese but otherwise healthy subjects (BMI
> 40 kg/m2) received L-AmB received a single dose of 1 or 2 mg/kg (n = 8+8) the day
before undergoing laparoscopic bariatric surgery. Each subject was intensively sampled
(n = 9) up to 48 hours to determine amphotericin B plasma concentrations. The following
information was collected: body size descriptors (e.g. total body weight [TBW], lean
body weight, BMI, ideal body weight calculated according to Janmahasatian et al.,
body surface area), age and sex. Population pharmacokinetic analyses was performed
in NONMEM 7.3 with PsN 4.7. For the covariate analyses the relationships between empirical
Bayes estimates and covariates were investigated in scatter plots. The final model
was used to simulated AUC0-24h and Cmax in steady state conditions in 10.000 subjects
with body weights uniformly distributed between 60 and 180 kg. Each virtual subject
< 100 kg received a daily 3 mg/kg L-AmB infusion in one hour, subjects ≥100 kg received
either 3 mg/kg or a fixed 300 mg dose as a one hour infusion.
Results: 16 morbidly obese subjects with median [range] BMI of 45.9 [40.2-52.1] kg/m2
and a total body weight of 137 [104-177] kg were included. A two-compartment model
best explained the data. There was no relationship between clearance of L-AmB and
any of the body size descriptors. A linear relationship was found between the central
volume of distribution (Vc) and TBW. Simulations show that the AUC0-24 increases with
weight when patients are dosed on a mg per kg basis). In parallel, Cmax also increases
with body weight when L-AmB is dosed on a per kg basis. This phenomenon is primarily
driven by the absolute increase in the dose with a clearance that does not change
with weight. When using a dose cap of 300 mg for patients > 100 kg the AUC would remain
similar across weight while a similar Cmax (13% lower) in a 140 kg patient compared
to a 70 kg patient will be obtained.
Conclusion: Based on our results we see limitations to using a mg per kg dose regime
for adult patients as clearance and therefore exposure to AmB is not affected by body
weight. In addition, body weight derived dosing might lead to an increased risk of
toxicity in heavy patients since toxicity is associated with AUC. A fixed dose regimen
would not only be more appropriate but also more practical for clinicians. In obese
patients specifically, we recommend using the licensed 3 mg/kg dose and cap the dose
at a maximum weight of 100 kg resulting in a 300 mg fixed dose.
Caballero
U.
1
Nuñez-Marcos
S.
1
2
Eraso
E.
2
Pemán
J.
3
Quindós
G.
2
Jauregizar
N.
1
1Pharmacology, University of the Basque Country, Leioa, Spain
2Inmunology, Microbiology, Parasitology, University of the Basque Country (UVP/EHU),
Bilbao, Spain
3Servicio De Microbiología, Hospital Universitario y Politécnico La Fe, Valencia,
Spain
P471. Evaluation of the in vitro antifungal activities of combinations of amphotericin
B with anidulafungin or caspofungin against Candida auris by time-kill curves
Objectives:
Candida auris has recently emerged as a cause of nosocomial outbreaks. The health
alert caused by this pathogen is related to the difficulty of a proper identification,
its persistence in the hospital environment and multidrug resistance. Antifungal combination
therapy may allow to overcome this multiresistance. Despite their relevance, research
about the activity of antifungal drug combinations against C. auris is still scarce.
The objective of this study was to evaluate the activity of the combinations of amphotericin
B with anidulafungin or caspofungin against C. auris clinical isolates by time-kill
curves.
Methods:
In vitro static time-kill curves experiments were performed in duplicate in microtitre
plates in RPMI-1640 medium with six C. auris clinical blood isolates (Hospital La
Fe, Valencia, Spain). Inoculum size ranged from 1 to 5 x 105 CFU/mL and concentrations
assayed in combination experiments were 0.5 and 1 mg/L for amphotericin B and 0.25,
0.5, 1 and 2 mg/L for anidulafungin and caspofungin. Samples for viable counts were
taken at 0, 2, 4, 6, 8, 24 and 48 h. Plots of averaged colony counts (log CFU/mL)
versus time were constructed for data analysis. The first-order killing rate constant
K (h-1) obtained after fitting an exponential equation to the data and time to achieve
the fungicidal endpoint t99.9% (h) were calculated to assess antifungal activity and
to compare different combinations between them. Fungistatic or fungicidal activities,
and synergistic, antagonist or indifferent interactions between these drugs were considered
according to published definitions [1].
Results: Combinations of 0.5 mg/mL of amphotericin B with 0.5, 1 and 2 mg/mL of anidulafungin
or caspofungin were synergistic (>2 log reduction compared to the drug with greater
activity) and fungistatic. However, combinations of 1 mg/mL of amphotericin B with
0.25 and 0.5 mg/mL of anidulafungin or caspofungin were additive (<2 log reduction)
and fungicidal. There were not significant differences between the use of anidulafungin
or caspofungin in combinations with amphotericin B.
Conclusion: Combination of amphotericin B with anidulafungin and caspofungin showed
promising results. Combinations of 0.5 mg/L of amphotericin B with low doses of echinocandins
were synergistic, whereas the interaction with 1 mg/mL of amphotericin B was additive
but exerted fungicidal activity. This is a remarkable finding, specially if we take
into account that echinocandins do not show fungicidal behaviour against C. auris.
The results could support the use of these antifungal combinations for treating C.
auris deep infections. Funding: This study was partially financed by GIC 15/78 IT-990-16
(Gobierno Vasco-Eusko Jaurlaritza), PI17/01538 (FIS, Spain) and SAF2017-86188-P (MINECO,
Spain). Unai Caballero has received a grant by the University of the Basque Country
(UPV/EHU) [1] Serena C et al., In vitro interactions of micafungin with amphotericin
B against clinical isolates of Candida spp. Antimicrob Agents Chemother. 2008; 52(4):1529-32
Kurland
S.
1
Furebring
M.
1
Shams
A.
1
Chryssanthou
E.
2
Sjölin
J.
1
Löwdin
E.
1
1Section of Infectious Diseases, Department of Medical Sciences, Uppsala University,
Uppsala, Sweden
2Clinical Microbiology, Karolinska University Hospital, Stockholm, Sweden
P472. Time-kill Studies of Caspofungin in an Ex vivo model in Relation to Plasma Protein
Levels: Is the Protein Binding Less Than Expected?
Objectives: The echinocandin caspofungin is used as first-line therapy in candidemia,
the most common invasive candida infection. The protein bound fraction of caspofungin
is 96.5% (in healthy volunteers). In our recent study on critically ill patients treated
with caspofungin, a median AUC in plasma of 58 (range 44-113) mg*h/L was reported,
corresponding to an average plasma concentration of 2.4 (1.8-4.7) mg/L. This would
result in a free unbound concentration of 0.08 (0.06-0.16) mg/L, concentrations equivalent
to the MIC distribution for wild type Candida glabrata. For fungicidal effect higher
free concentrations would be needed. In critically ill patients the plasma protein
level is habitually reduced. The objectives of this study were to determine the killing
effect of caspofungin on C. glabrata with clinically relevant concentrations of caspofungin
in an ex vivo model using human plasma and furthermore to study the efficacy in relation
to different plasma protein levels.
Methods: The efficacy of caspofungin on four blood isolates of C. glabrata was determined
by time-kill studies performed in plasma from healthy blood donors. In order to investigate
the significance of the protein level on the killing effect of caspofungin, C. glabrata
with an inoculum of 5x104 CFU/ml was added to plasma, plasma diluted with 50% Ringer´s
Acetate (Ri-Ac) and plasma diluted with 75% Ri-Ac. The MIC values for the isolates
were determined by Sensititre Yeast One method and were 0.06, 0.12 and 1 mg/L, respectively.
The caspofungin concentrations investigated were 1, 2, 5 and 9 mg/L. CFU/ml were determined
at 0, 2, 6 and 24 hours.
Results: The results showed a higher reduction in CFU/ml than expected based on MIC
and the calculated unbound caspofungin concentration. There was a ≥ 2 log reduction
in log CFU/ml with a concentration of 1 mg/L (corresponding to 0.035 mg/L free drug/unbound
concentration) for the two strains with MIC 0.06 mg/L. For the strain with MIC 0.12
mg/Lthe corresponding reduction was approximately 1 log CFU/ml. Furthermore, there
was a limited but significant decrease in CFU/ml in the time-kill studies performed
in plasma with reduced levels of protein in comparison with plasma with normal protein
level. For the strain with MIC 1 mg/L there was no reduction in CFU/ml in plasma.
Although, there was a killing with the concentrations 5 and 9 mg/L in the experiments
in plasma diluted with 75% Ri-Ac.
Conclusion: The killing of C. glabrata with caspofungin in plasma was higher than
expected based on MIC and the reported amount of unbound caspofungin in normal plasma.
At lower protein levels this killing was augmented which is consistent with an increased
concentration of free caspofungin.
Colman
S.
Cattoir
L.
Boel
A.
Microbiology, OLV Hospital, Aalst, Belgium
P475. Subcutaneous nodule caused by Phaeoacremonium fuscum in a non-immunocompromised
patient.
Case Report: Introduction Phaeohyphomycosis (PHM) is a chronic infection caused by
dematiaceous fungi which usually involves the skin and subcutaneous tissue. As a result
of the use of molecular diagnostic techniques, previously unrecognized causative agents
of phaeohyphomycosis have been discovered. Here we report the first case of phaeohyphomycosis
caused by Phaeoacremonium fuscum in an immunocompetent patient. Case presentation
A 60 year old Filipino male presented with rotator cuff pathology to the orthopedics
consultation. On physical examination a painless, soft, subcutaneous mass above the
right patella was noted, which didn’t cause the patient any complaints. The suprapatellar
nodule had been growing slowly for 3 years. The patient denied any apparent antecedent
trauma, arthropod bite, family history or contact with a person with similar lesions.
The man still visits the Philippines on an infrequent basis. He had no fever, weight
loss or other symptoms. Detailed physical examination further revealed a 4x3 cm, immobile,
non-pulsatile light red mass. There were no other signs of inflammation of the skin.
A serous effusion was aspirated from the mass. Fungal culture of the aspirated fluid
showed growth of a filamentous fungus on chocolate blood agar and Sabouraud’s dextrose
agar. Molecular identification was performed by sequence analysis of the internal
transcribed spacer region 2 (ITS2). BLAST search revealed a similarity of 100% with
Phaeoacremonium fuscum. The antifungal susceptibility profile was established by EUCAST
broth microdilution: itraconazole >16 mg/L, posaconazole 0.25 mg/L, amphotericin B
0.50 mg/L, voriconazole 0.50 mg/L. This susceptibility profile is similar to those
previously published. A wide local excision of the lesion was performed a couple of
weeks later. The patient had an uneventful postoperative recovery. No antifungal therapy
was started postoperatively since the patient was not immunocompromised and there
was lack of reimbursement. Histopathological examination of the specimen showed fibrous
tissue with a mixed cellular infiltrate including lymphocytes, plasma cells, macrophages
and neutrophils. Periodic acid-schiff stain and Grocott stain showed evidence of branching
septate hyphae. Discussion PHM is caused by brown pigmented fungi containing melanin
in their cell wall. Phaeoacremonium species are known as vascular plant pathogens,
responsible for wilting and dieback of woody plants. They are also opportunistic pathogens
in humans, mainly causing cutaneous or subcutaneous PHM. However, sporadic cases of
respiratory tract infection, septic arthritis and disseminated infection are described.
The true prevalence of Phaeoacremonium species is probably underestimated due to the
asymptomatic nature of most subcutaneous lesions and the difficult identification.
There is no standard antifungal regimen or evidence regarding the necessity for antifungal
treatment as concomitant with surgery for localized infections. Long courses of antifungals,
such as voriconazole treatment, are often employed to reduce the risk of recurrence.
Case reports suggest that surgery can be curative if the infection is localized and
adequately removed, as was the case in our patient. Regardless of the immune status
of the patient, clinicians should consider the possibility of a fungal infection when
they encounter chronic subcutaneous nodules without major symptoms.
Sacheli
R.
1
Menatong
X.
1
Labarbe
C.
1
Crutzen
C.
1
Harag
S.
2
André
J.
2
Evrard
S.
3
Lagrou
K.
4
Laffineur
K.
5
Rousseaux
D.
6
Detollenaere
L.
1
Adjetey
C.
1
Seidel
L.
7
Hayette
M.-P.
1
1Clinical Microbiology- Nrc For Mycoses, University Hospital of Liège, Liège, Belgium
2Dermatology, CHU St Pierre, Brussels, Belgium
3Clinical Microbiology, CHR Citadelle, Liège, Belgium
4Clinical Bacteriology and Mycology, Katholieke Universiteit Leuven, Leuven, Belgium
5Clinical Biology, Clinique St Luc Bouge, Bouge, Belgium
6Clinical Biology, Centre Hospitalier Chretien Heusy, Verviers, Belgium
7Statistics, University Hospital of Liège, Liège, Belgium
P476. Belgian national survey on tinea capitis: epidemiology and molecular investigations.
Objectives: Tinea capitis (TC) is a superficial infection of the scalp caused by dermatophyte
fungi which affects mainly prepubescent children. This last decade, a huge increase
of African anthropophilic strains causing tinea capitis, has been observed in Europe,
probably due to immigration waves from African countries. The Belgian National Reference
Center for Mycosis (NRC) has conducted a surveillance study about TC in 2018. This
work presents final results of the study for the epidemiological part and preliminary
results for the molecular part.
Methods: Belgian laboratories were invited to send all dermatophytes strains isolated
from the scalp from January to December 2018. Dermatologists were involved and were
asked to fill a form containing several epidemiological information about the patient.
Strains identification was confirmed by ITS sequencing. A multiplex pan-dermatophyte
real time PCR assay (DermaGenius®, PathoNostics) was applied if necessary. Typing
of the M.audouinii strains was done using rep-PCR method (Diversilab, BioMérieux).
Results: A total of 337 strains have been collected from 337 patients. The main population
concerned by TC was children from 5-9 years (165/337, 49,01%). Males (214/337, 63,5%)
were more affected than females (123/337, 36,5%), the sex ratio M/F was of 1,74. The
majority of the strains was collected in Brussels area (181/337, 53,8%), followed
by Liege area (73/337; 21,7%). Other Belgian cities were less concerned by TC. Among
known ethnical origins (n = 119), African people (114/119, 96,2%) were more concerned
by TC than European people (5/119, 3,8%), (p<0,0001). The majority of patients were
from Guinea (26/119, 21,8%), followed by Cameroun (14/119, 11,8%) and RDC (14/119,
11,8%), many other African nationalities were represented (12 different countries,
all over Africa). The main transmission mode of TC was the familial way (83,3% among
known cases n = 126, 105/126). The major etiological agent was Microsporum audouinii
(118/337, 35%) followed by Trichophyton soudanense (83/337, 24,6%), T. tonsurans (27/337,
17%), M. canis (36/337, 10,7%), T. violaceum (28/337, 8,3%), T. benhamiae (7/337,
2,1%), T. mentagrophytes (5/337, 1,48%) and M. incurvatum (1/337, 0,3%). This last
rare dermatophyte has never been reported as responsible for TC in Belgium before.
M. audouinii strains have been genotyped by rep-PCR and three genotypic variants have
been characterized, one of them circulating mainly in Brussels area. No link with
a particular ethnical origin could be found among genotypic groups.
Conclusion: African anthropophilic dermatophytes such as M.audouinii and T. soudanense
are mainly responsible for tinea capitis in Belgium. Large cosmopolitan cities like
Brussels and Liege are the most concerned. People from African origin are mostly affected
by TC. Among the M. audouinii strains circulating in Belgium, a genotypic diversity
has been characterized.
Lecerf
P.
1
Jazaeri
Y.
1
Hendrickx
M.
2
Richert
B.
1
Packeu
A.
3
1Faculty of Medecine, Université Libre de Bruxelles, Brussels, Belgium
2Mycology, Sciensano, BCCM/IHEM, Brussels, Belgium
3Mycology & Aerobiology, Sciensano, Brussels, Belgium
P477. Identification of dermatophytes isolated from tinea capitis infections using
a liquid media MALDI-TOF MS protocol
Objectives:
Tinea capitis is a common childhood superficial infection of the scalp caused by dermatophytes.
Conventional methods based on morphological characteristics to identify theses fungi
are time-consuming and complex, requiring expert mycological knowledge. Recently,
MALDI-TOF MS has become a powerful tool in clinical microbiology for rapid identification
of these microorganisms. In the present study, a liquid media MALDI-TOF MS protocol
was developed and validated for the fast and accurate identification of dermatophytes
species responsible for tinea capitis infection, using both dermatophytes from reference
strains and clinical isolates. The main purpose was to work with primary isolates
in order to avoid the culture step and lead to a faster diagnosis. Identification
techniques (conventional identification and MALDI-TOF MS based on liquid and solid
culture) were evaluated and compared for their rate of correct identification and
turnaround time.for the fast and accurate identification of dermatophytes species
responsible for tinea capitis infection, using both dermatophytes from reference strains
and clinical isolates. The main purpose was to work with primary isolates in order
to avoid the culture step and lead to a faster diagnosis. Identification techniques
(conventional identification and MALDI-TOF MS based on liquid and solid culture) were
evaluated and compared for their rate of correct identification and turnaround time.
Methods: The in-house BCCM/IHEM database made from references strains on solid culture
was used. First, the liquid media MALDI-TOF MS protocol was validated using references
strains. Secondly, the same protocol was applied on clinical isolates collected from
Saint-Pierre University Hospital and paralleled with solid media MALDI-TOF MS protocol.
Results: The use of the liquid media MALDI-TOF MS protocol resulted in a rate of 100%
of correct identification at the species level for reference strains and 78,8% for
clinical isolates. The protocol was statistically significantly faster than the conventional
method. The results of the different methods disagreed for 17 isolates. The complete
extraction from the solid media MALDI-TOF MS gave the highest correct species identification
with the highest mean of log-scores.
Conclusion: The liquid media MALDI-TOF MS technique is an accurate method for the
correct identification of dermatophytes at the species level and is much faster than
the conventional technique. The main advantage lies in not needing a culture step
for primary isolates, thus leading to a faster diagnosis. For the identification of
closely related species, the complete extraction from solid media MALDI-TOF MS protocol
remains the most reliable technique.
Verissimo
C.
Simões
H.
Sabino
R.
Simões
D.
Infectious Disease, National Health Institute, Lisbon, Portugal
P478. Dermatophytes’ identification by Matrix-assisted laser desorption ionization-time
of flight mass spectrometry (MALDI-TOF MS) - the experience of a clinical laboratory
Objectives: Dermatophytes are a challenging group of fungi that infect the keratinized
tissues. The taxonomy of these fungi has changed recently with the reclassification
of some species and description of new ones. However, many clinical laboratories still
base the identification of dermatophytes on their phenotype. Since dermatophytes are
very pleomorphic, macro and micromorphology are often insufficient to reach a correct
classification and may lead to misidentifications. The identification based on MALDI-TOF
relies on the protein profile of the microorganism. Thus, this study aims to summarize
our current laboratorial experience of dermatophyte identification using MALDI-TOF
MS.
Methods: From january to april 2018, 95 dermatophytes isolates, collected from human
keratinized samples and also from quality control programs were characterized by phenotypic
analysis, and by VITEK MS V3.2 bioMerieux. Before identification procedure, isolates
were inoculated on Sabouraud Dextrose agar plates and incubated at 27°C during 5 to
10 days. Species were identified taking into account clinical features, as well as
cultural, microscopic and physiological characteristics. Prior to MALDI-TOF MS analysis,
the samples were pre-treated according to the manufacturer’s protocol for filamentous
fungi. Molecular identification by sequencing of the internal transcribed spacer 1
(ITS1) was performed in 34 of those isolates
Results: Through phenotypic analysis eight different species were identified (54 Trichophyton
rubrum; 4 T.soudanense; 22 T.interdigitale; 1 T.mentagrophytes; 3 T.tonsurans; 7 Microsporum
canis; 3 M.audouinii; 1 Microsporum spp.- (non canis or audouinii). MALDI-TOF analysis
showed an identification agreement in 80 cases (84,2%) with a confidence level of
99,9%. Eight isolates showed divergent identification results: three T.rubrum were
identified as T.violaceum, three T.soudanense were identified as T.rubrum, one T.mentagrophytes
was identified as T.interdigitale and one T.tonsurans was identified as T.rubrum.
In four cases MALDI-TOF analysis did not get a profile. The ITS sequencing analysis
of discrepant results corroborated the MALDI-TOF identification in five of them. On
the other hand, T.soudanense was only identified by phenotypic analysis since MALDI-TOF
and ITS sequencing result was T.rubrum. MALDITOF identification of T.violaceum was
not confirmed by ITS sequencing that identified T. rubrum instead, in accordance with
the phenotypic identification.
Conclusion: Correct identification of dermatophytes to species level requires sequencing
of the ITS, LSU, and/or beta-tubulin regions. The implementation of this methodology
in a clinical laboratory is expensive and time consuming. MALDI-TOF identification
is a good option for dermatophytes’ identification performed in laboratory routine,
since costs of consumables as well as time of sample preparation are lower than for
PCR analysis and doesn’t require long training period as phenotypic identification
does. In this study, however, both methods failed to identify some species variants
like Trichophyton soudanense or T. violaceum. The combined use of both MALDI-TOF and
phenotypic methods seems to be the better approach for dermatophytes’ identification
since some species show significant phenotypic and clinical differences.
Vargas
L.
Alvarado
E.
Stenström
C.
Forsblom
S.
Girestam
B.
Loko
G.
Chryssanthou
E.
Clinical Microbiology, Karolinska University Hospital, Stockholm, Sweden
P479. A retrospective study of dermatophyte infections caused by Tricophyton rubrum
and T. interdigitale in the County of Stockholm, Sweden.
Objectives: Dermatophytosis is a common fungal infection caused by keratinophilic
fungi that are capable of invading nail, hair and superficial layers of the skin of
humans and animals. The aim of this study was to determine the epidemiologic profile
of the most frequent dermatophytosis such as Tinea unguium and T. pedis caused by
Tricophyton rubrum or T. interdigitale in Stockholm, Sweden.
Methods: In 2015 we developed and validated a rapid and sensitive real-time PCR method
for detection of the two most prevalent dermatophytes in Northern Europe. Fungal DNA
was extracted directly from clinical samples (toe or finger nail and skin scrapings
from the feet) by using proteinase K and heat as pre-lysis step, followed by automated
DNA extraction on the PSH/MagNA Pure 96 Compact. Real-time PCR was performed using
pan-dermatophyte primers for detection of all dermatophytes and specific primers for
identification of T. rubrum and T. interdigitale. Specimens from finger nails were
also cultured on Chrom agar plates for detection of onychomycosis caused by yeasts.
Results: Laboratory records comprising PCR results of 13,683 specimens (3,304 skin
respective 10,379 nail fragments) collected from May 2015 through March 2019 were
retrospectively analyzed. Tricophyton rubrum (42%) was the predominant pathogen identified
from these cases, followed by T. interdigitale (4,9%). In contrast, 2% of the samples
were positive for pan-Derm indicating the prevalence of infections caused by other
pathogens distinct to T. rubrum or T. interdigitale. Sex distribution analysis of
the patients showed that males (40%) were more susceptible to suffer dermatophyte
infections compared to females (21,9%). (Furthermore, the prevalence of onychomycosis
in finger nails caused by yeasts were also evaluated in this study.) Culture analysis
from total 941 finger nails showed that 25% were positive for yeast infection. Candida
parapsilosis (52%), followed by C. albicans (35%) and C. guilliermondii (6%) were
the predominant species isolated in such cultures.
Conclusion: The findings presented in our study are in concordance with previous studies
where T. rubrum is the most frequent dermatophyte species isolated in the general
population of industrialized countries. Detection of dermatophytes using real-time
PCR represents a faster and sensitive method to evaluate large volume of clinical
samples.
Rupin
E.
Pelloux
H.
Fricker-Hidalgo
H.
Leccia
M.-T.
Cognet
O.
Parasitology-mycology, Grenoble Alps University Hospital, GRENOBLE, France
P480. rEvaluation of Diafactory tinea unguium®, a new rapid immunochromatographic
test for the diagnostic of onychomycosis
Objectives: Suspected nail onychomycosis remains a frequent and still increasing problem
despite therapeutic advances. Based on clinical features the diagnosis of onychomycosis
is confirmed by direct examination and culture. Direct examination can be done on
the same day but cultures required several weeks what delays diagnosis and therapy.
Furthermore, direct examination and culture have low sensitivity. Diafactory tinea
unguium®, a new rapid immunochromatography test was recently developed. The aim of
this study was to compare this new test to conventional methods (direct examination
and culture) in suspected tinea unguium.
Methods: Diafactory tinea unguium® (Biosynex, France) detects dermatophytes by using
monoclonal antibodies that react specifically with polysaccharide present in the cell
wall of dermatophytes. Direct microscopic examination was performed using KOH enhanced
with chlorazol black E and dimethyl sulfoxide. Cultures were carried out on Sabouraud
medium with or without cycloheximide (bioMérieux, France), incubated at 25°C. The
three methods were evaluated on nails samples (n = 70) collected from patients (n
= 50) with suspected nail mycosis infection in the Grenoble Alps University Hospital.
Results: Among the 70 samples, cultures were positive in 53 nails. Dermatophytes were
isolated in 40 samples (Trichophyton rubrum n = 35, Trichophyton interdigitale n =
3, Trichophyton tonsurans n = 2), yeasts in 8 samples (Candida parapsilosis n = 6,
Candida albicans n = 2), and moulds in 5 samples (Scopulariopsis spp. n = 4, Fusarium
spp. n = 1). The results were positive with Diafactory tinea unguium® in 48 samples,
correlated with direct microscopy (i.e. presence of filamentous fungal elements) in
36 samples and with cultures in 39 samples. In one case, the result of Diafactory
tinea unguium® was negative and the culture was positive (T. rubrum). In three cases,
the result of Diafactory tinea unguium® was negative and the direct examination was
positive. In the 13 cases of moulds or yeasts isolation, the immunochromatographic
test was negative. The positive and negative predictive value of Diafactory tinea
unguium® were respectively 75% and 86%.
Conclusion: In order to reinforce the importance of confirmation in the diagnosis
of mycological onychomycosis, Diafactory tinea unguium® provides a reliable, convenient
and quick method (5 to 30 minutes). Because of its good detection capacity, this device
improved the accuracy of diagnosis of tinea inguium in complement of conventional
fungus testing methods.
Gazit
Z.
1
Gefen-Halevi
S.
2
Belausov
N.
1
Keller
N.
1
Smollan
G.
1
1Clinical Microbiology, Sheba medical center, Ramat Gan, Israel
2Clinical Microbiology, Sheba medical center, ramat gan, Israel
P481. Molecular identification of Dermatophytes from clinical samples
Objectives: Dermatophytes are fungi that can invade and infect the keratinized layers
of skin, hair, and nails. Three genera of fungi, Trichophyton (T), Microsporum (M),
and Epidermophyton (E), account for most Dermatophytic infections and cause a pathology
called tinea (ringworm fungi). The gold-standard method for dermatophyte identification
is microscopy and culture. Microscopy has low specificity and culture has low
Methods: (1) The gold-standard methods routinely used in the laboratory were microscopy
using 30% KOH and culture using DTM (dermatophyte test medium) agar incubated at 28
°C for up to 4 weeks. For 12 months, from 1.1.2016 until 31.12.2016 samples from skin,
nail and hair from patients with suspected tinea were analyzed using the gold-standard
methods. (2) A new Israeli commercial molecular kit called DERMADYN Dermatophyes detection
test V.01 (Dyn R&D) kit that identifies 7 dermatophytes; T. rubrum complex, T. tonsurans,
T. violaceum, M. gypseum, E. floccosum, M. canis and T. mentagrophytes complex was
evaluated compared to the gold-standard on 103 samples. (3) From 1.8.18 until 31.12.18,
1179 samples were analyzed for the presence of Dermatophytes using the molecular kit
only.
Results: Using the gold-standard only 480 (23.6%) samples were positive for dermatophytes
with a distribution of 462 (23%), 12 (0.6%), 2 (0.1%), 2 (0.1%) 1 (0.05%), 1 (0.05%)
positive for T. rubrum complex, T. mentagrophytes complex, M. canis, Scopulariopsis
brevicaulis, T. tonsurans, and M. gypseum, respectively. Discrepancies between microscopy
and culture were found in 27% of the samples. (2) There was an agreement between the
gold-standard and the molecular kit in 60 out of the 103 samples tested (58%), a complete
disagreement in 9 (9%) and a partial disagreement in 34 (33%) of them. (3) Using the
molecular approach 578 (49%) samples were positive for dermatophytes with the distribution
of 551 (46.7%), 2 (0.2%), 8 (0.7%), 6 (0.5%), 1 (0.08%) and 10 (0.85%) positive for
T. rubrum complex, T. mentagrophytes complex, M. canis, T. tonsurans, M. gypseum and
E. floccosum, respectively.
Conclusion: Twice the number of dermatophytes were identified using the molecular
approach compared to the gold-standard with a similar distribution of species having
T. rubrum complex the main pathogen. The molecular assay has a much shorter training
period, a high capacity of performance and high sensitivity. Although the kit contains
only 7 dermatophytes, these are the most prevalent species in Israel. As discrepancies
between microscopy and culture is a common problem and as culture has low sensitivity
Labs should consider evaluating a molecular based diagnostic method for dermatophyte
identification to increase detection rates.
Gits-Muselli
M.
1
2
Hamane
S.
2
Verillaud
B.
3
Cherpin
E.
2
Guigue
N.
2
Garcia-Hermoso
D.
1
Alanio
A.
4
Bretagne
S.
4
1Molecular Mycology, Institut Pasteur, PARIS, France
2Parasitology-mycology Laboratory, Lariboisière Saint-Louis Fernand Widal Hospital,
Assistance Publique-Hôpitaux de Paris (AP-HP), PARIS, France
3Ent Department, Lariboisière Saint-Louis Fernand Widal Hospital, Assistance Publique-Hôpitaux
de Paris (AP-HP), PARIS, France
4Paris-diderot, Sorbonne Paris Cité University, Institut Pasteur, Molecular Mycology
Unit, Cnrs Cmr2000, Parasitology-Mycology Laboratory, Lariboisière Saint-Louis Fernand
Widal Hospitals, Assistance Publique-Hôpitaux de Paris, Paris, France
P483. In the Aspergillus section Nigri, Aspergillus welwitschiae is more often responsible
for otomycosis than Aspergillus tubingensis and Aspergillus niger
Objectives: Fifteen percent of external otitis are otomycosis (Ninkovic et al. 2008).
Aspergillus section Nigri are the most frequent molds isolated from external ear conduct
(EEC) samples followed by Aspergillus section Flavi (R. Sarvan et al. 2012). Aspergillus
section Nigri include several cryptic species difficult to differentiate using phenotypic
features. We wondered whether some species of the Nigri section could be more frequently
observed in some specific anatomical sites.
Methods: We identified at the species level all the isolates of Aspergillus section
Nigri consecutively collected between 2010 and 2017 in our laboratory and stored frozen
at -20 C°. After thawing and subculture on Sabouraud medium, DNA was extracted using
a MagnaPure DNA extractor and species identification was performed upon the partial
sequence of the calmodulin gene. The two primers used for amplification of the targeted
gene were CMD5 (CCGAGTACAAGGARGCCTTC) and CMD6 (CCGATRGAGGTCATRACGTGG) (Hong et al.
2005). The sequences were compared to the Mycobank database. The sequences obtained
were aligned to build a phylogenic tree using Geneious software.
Results: A total of 186 strains were sequenced: 75 EEC (64 patients), 78 respiratory
samples (78 patients), and 33 air environmental isolates. When considering the first
strain per patient, we identified by decreasing frequency: Aspergillus tubingensis
(78/175; 45 %), Aspergillus welwitschiae (59/175; 34 %), Aspergillus niger (34/175;
19 %), Aspergillus luchuensis (2/175; 1%), and Aspergillus japonicus (2/175; 1%).
When several isolates were available for a given patients, the following isolates
belonged to the same species. The species repartition was statistically different
when comparing EEC samples to respiratory and air samples with A. welwitschiae overrepresented
in otomycosis (53% 34/64) (p<0.001). In contrast, the distribution of the species
was not different between respiratory and air samples, with 46% and 61% A. tubingensis
respectively (Figure 1).
Inside each species, the sequence identity was high (96.5% for A. tubingensis, 99.3%
A. welwitschiae, and 99.4% A. niger isolates) with no difference according to the
isolation site. The phylogenetic tree based on the calmodulin gene partial sequences
showed a stackable organization to the tree generated using whole genome sequencing
(Vesth et al. (2018)), with Aspergillus luchuensis included within the A. tubingensis
clade, and A. welwitschiae closed to the A. niger clade (Figure 2).
Conclusion: Among the 175 Aspergillus species belonging to the Nigri section, A. welwitschiae
was found significantly associated with otomycosis whereas the species distribution
was similar between respiratory and environmental isolates. This finding suggests
specific physical properties of A. welwitschiae to thrive in the external ear. One
hypothesis could be a better fitness to infect EEC containing different lipids linked
to the ear-wax.
Aloui
D.
1
Tbini
M.
2
Jaafoura
H.
2
Cheikhrouhou
S.
1
Bouchekoua
M.
1
Trabelsi
S.
1
Salah
M. Ben
2
Khaled
S.
1
1Laboratory of Parasitology and Mycology, Charles Nicolle Hospital, tunis, Tunisia
2Oto-rhino-laryngology, Charles Nicolle Hospital, tunis, Tunisia
P484. Fungal necrotizing external otitis
Objectives: Malignant otitis externa is an uncommon, but potentially fatal, complication
of otitis externa affecting immunocompromised and elderly patients, particularly those
with diabetes. Fungal infection is rare, severe and the clinical presentations pose
significant problems in diagnosis and treatment. The purpose of our study is to report
the diagnostic and therapeutic difficulties of fungal necrotizing external otitis.
Methods: It is a retrospective study spread over 5 years conducted between January
2012 and December 2017. It included patients with documented diagnosis of fungal necrotizing
external otitis.
Results: There were eight men and three women, all diabetic with a mean age of 64
years. The reason for the consultation was treatment-resistant otalgia and otorrhea.
The clinical examination and the TDM (tomodensitometry) were in favor of a necrotizing
external ear infection. All patients were treated with antipyocyanic therapy antibiotic
(ceftazidime and ciprofloxacin) for an average duration of one month. A swab of the
infected ear was taken for mycological examination and a mycosic agent was revealed
within an average of 45 days. Candida albicans was isolated in four cases and Candida
parapsilosis in one case. Concerning molds, Aspergillus flavus was isolated in four
cases, Aspergillus fumigatus and Rhizopus spp in one case respectively. Treatment
was based on Amphotericin B in two cases and on Amphotericin B followed by Voriconazole
in the other cases. The average duration of treatment was four months. Seven patients
evolved well, two were lost to follow-up and two died. The average follow-up period
was 11 months.
Conclusion: When a necrotizing external ear infection, occurs in an immunocompromised
patient, with no response to antibiotic therapy, a fungal infection should be suspected
and treated early and effectively to ensure a better prognosis.
Ramirez
E. Alvarado
Vargas
L.
Stenström
C.
Forsblom
S.
Girestam
B.
Loko
G.
Chryssanthou
E.
Clinical Microbiology, Karolinska University Hospital, Stockholm, Sweden
P485. Chromagars for differentiation between Trichophyton benhamiae, Microsporum canis
and M. audouinii
Objectives: Objective: Trichophyton benhamiae has increasingly been isolated in Europe
during the last years. In culture, this dermatophyte is difficult to differentiate
macroscopically from immature colonies of Microsporum canis and sometimes from non-sporulating
form of M. audouinii. Here we have used different culture conditions aiming to establish
the macroscopic differences between these species, which could be useful for identification.
Methods: Method: T benhamiae (6 isolates), M. canis (8 isolates), and M. audouinii
(5 isolates) were cultured at 28 °C on Trichophyton agars for nutritional requirements
(5 d), urease (5 d), Chromagar CandiSelect™ (BioRad) (7-10 d) and CHROMAgar™Candida
(CHROMAgar) (7-10 d). Identification of T. benhamiae, M. canis and non-sporulating
form of M. audouinii were confirmed by ITS sequencing.
Results: Results: Five of 6 T. benhamiae isolates had requirement for vitamins (inositol
and thiamine) and had positive urease test after 5 days incubation at 28 °C. M. canis
and M. audouinii isolates showed no vitamin requirements. M. audouinii showed weak
or negative urease test. The tested dermatophyte species could be differentiated by
colony colour on Chromagar CandiSelect™: T. benhamiae presented turquoise diffuse
pigment, meanwhile M. canis had light yellow and M. audouinii purple pigment around
the colony. On CHROMAgar™Candida the colonies showed macroscopical differences: T.
benhamiae had colonies with white centre and yellow margin, and the other two dermatophytes
had brownish colonies.
Conclusion: Conclusion: Identification and differentiation between T. benhamiae, M.canis
(immature colonies) and M. audouinii (non-sporulating form) by conventional methods
is difficult. Using biochemical tests like vitamin requirements, urease and Chromagars,
may be helpful for identification.
Colom
M.F.
1
Ferrer
C.
1
Leting
S.
2
Ramírez
L.
1
Ekai
J.
2
Hernández
C.
3
1Medical Mycology Lab, University Miguel Hernandez, Sant Joan d’Alacant, Spain
2Medical Diagnosis Laboratory, Lodwar County Referral Hospital, Lodwar, Kenya
3General Surgery, Hospital Clínico San Carlos, Madrid, Spain
P487. First results in the study of mycetoma in Turkana (Kenya)
Objectives: The aim of the study was to assess the prevalence of mycetoma in the county
of Turkana (Northwest Kenya), as well as to describe the main causative agents involved
in the disease and their possible environmental origin, using simple methods that
can be applied locally.
Methods: Based on the data collected by the team of cooperative medicine Cirugia en
Turkana, a specific study for mycetoma was started during the 16th campaign of that
team in February 2019. Patients with suspected mycetoma were studied at the Lodwar
County Referral Hospital (LCRH). After informing the patient and getting their consent,
the lesions were examined and sampled (mainly by biopsy) and clinical data were recorded.
Samples were washed in sterile saline solution and cut in fragments. Some of these
were inoculated in Sabouraud Dextrose Agar and Malt Extract Agar plates. One fragment
of each sample was used for DNA extraction. The DNA and the rest of the fragments
of samples were kept at -20ºC. Environmental samples were also taken from soils, trees
and shrubs in different locations of the county. These samples were also fragmented
and inoculated in the same culture media. All cultures were incubated at room temperature
at the LCRH laboratory. The DNA obtained from clinical samples was submitted to PCR
amplification of the ITS-5.8S and the V4-V5 16S rRNA gene region, for the detection
and identification of fungi and bacteria respectively.
Results: In this 2019 campaign 11 patients were studied. All were men between 13 and
70 Yo (mean age 34). Ten of them were shepherds and one worked as a builder. Lesions
were mainly located at the feet (91%). Three of them referred black grains in the
exudate and 6 yellow or cream coloured. Culture of clinical and environmental samples
yield 5 and 24 fungal colonies respectively. For clinical samples, most of cultures
were negative. From environmental samples, fungi morphologically compatible with Madurella
mycetomatis and Trematosphaeria grisea were obtained as well as other mycetoma-related
genera (Alternaria and Aspergillus). Molecular identification of showed Aspergillus
terreus, Aspergillus flavus, and Paecilomyces formosus in the environment. Cellulosimicrobium
cellulans, an actinobacteria that has not been previously reported as causative agent
of mycetoma, was detected in two of the clinical samples, Alternaria sp. in one and
Penicillium spp in 5 (P. glabrum, P. spinulosum, P. corylophilus). After DNA amplification,
three samples remained negative.
Conclusion: The detection of Cellulosimicrobium cellulans could be a significative
result of this study. Nevertheless, it should be confirmed in more samples and patients.
For Alternaria sp, and Aspergillus sp, they had been reported as mycetoma agents previously.
The lack of results from some of the yellow grain mycetomas could be since some patients
were under antibiotic treatment and, for the black grain ones, this is probably due
to the low sensitivity of the method in the detection of these fungi. The isolation
of mycetoma agents from the environmental samples confirms that not only Acacia thorns,
but also other vegetal products, can be the origin and vehicle of inoculation of the
pathogens
Samaras
K.
Markantonatou
A.-M.
Vyzantiadis
T.-A.
First Department of Microbiology, Medical School, Aristotle University of Thessaloniki,
Thessaloniki, Greece
P488. Examining the efficacy of Origanum heracleoticum essential oil against fungal
pathogens of the nails: preliminary results.
Objectives: Fungal infections of the nails (onychomycoses) are very common and often
need long term treatments, not always effective. In addition to the available chemical
antifungal agents, natural products have been used as well. Scope of this study was
to test the possible efficacy of commercial antifungal regimens based on Origanum
heracleoticum essential oil as well as the efficacy of their main antifungal substance,
carvacrol, against common fungal pathogens of the nails.
Methods: Three different regimens were tested. Regimen (A), contains Origanum heracleoticum
essential oil (purity>99%, carvacrol >86%), whereas regimen (B) contains the essential
oil (28% v/v) in combination with other natural essential oils. Both formulations
are suitable for external use. The antifungal activity of pure carvacrol was also
tested. Possible indication of the aforementioned regimens (A and B) is the treatment
of microbial pathogens of the nails. The proposed dosage for regimen (A) is 4-6 drops
(20 μL per drop) oregano essential oil diluted in a teaspoon (5 mL) of another oil
(for example olive oil), which is equivalent to carvacrol concentration of approximately
13.76-20.64 mg/ml. Regimen (B) is proposed to be used undiluted (carvacrol concentration
approximately 240 mg/ml). In order to demonstrate their possible antifungal activity,
serial dilutions of regimens (A), (B) and pure carvacrol from 1,024 mg/L to 0.031
mg/L were tested against dermatophytic (Trichophyton rubrum and Trichophyton interdigitale)
and non dermatophytic isolates (Candida albicans, Candida parapsilosis, Candida glabrata
and Scopulariopsis brevicaulis) which are all common causes of onychomycoses. The
fungal strains were examined also for their sensitivity in fluconazole and itraconazole.
The susceptibility testing was performed according to the proposed EUCAST methodology
(microdilution method).
Results: All tested regimens exhibited antifungal activity in high concentrations.
Depending on the fungal strain the range of MICs was 64-256 mg/L for regimen (A),
64-512 mg/L for regimen (B) and 64-512 mg/L for pure carvacrol. The ranges of MICs
were 0.063-4 mg/L for itraconazole and 0.5-64 mg/L for fluconazole.
Conclusion: The results of the study demonstrate that the regimens tested may have
antifungal activity against both dermatophytic and non dermatophytic isolates, although
in much higher concentrations than the commonly used antifungal drugs. However, being
substances of only local use on the fungal lesions, there is the probability that
the above mentioned concentrations could be achieved on the nail application. Further
experimental data and studies of clinical use are required in order to specify potential
benefit, duration of treatment and possible toxicity.
Ge
G.
1
Shi
D.
2
1Jining Medical University, Jining, China
2Jining No. 1 People’s Hospital, Jining, China
P489. Cutaneous ulcer due to Candida parasilosis associated with skin trauma in an
adult patient with psoriasis vulgaris
Case Report: The Candida parapsilosis (C. parapsilosis) family has emerged as a major
opportunistic and nosocomial pathogen. It causes multifaceted pathology in immuno-compromised
and normal hosts. Common symptoms of Candida skin infections include thickening of
the skin, hyperkeratosis, and erythema. C. parapsilosis rarely induced the skin ulcer
without assistance from other pathogens. Here, we present 27-year-old female with
cutaneous ulcer on her left foot due to C. parapsilosis after trauma. She has a 6-month
history of psoriasis vulgaris without treatment. C. parapsilosis was isolated from
skin lesion for several times and was confirmed by morphological and cultural characteristics
as well as by DNA molecular analysis. Patient was successfully treated with oral itraconazole
in 200 mg daily for seven weeks. To our knowledge, it was the first report that C.
parapsilosis induced cutaneous ulcer in a patient with psoriasis; Cutaneous candidiasis
maybe associated with the skin barrier damage (the trauma) or psoriasis.
Pchelin
I.
Mochalov
Y.
Chilina
G.
Vasilyeva
N.
Taraskina
A.
Kashkin Research Institute of Medical Mycology, North-Western State Medical University
named after I.I. Mechnikov, Saint Petersburg, Russian Federation
P490. rTwo genetic lines of Trichophyton rubrum population in Russia
Objectives:
Trichophyton rubrum is the most prevalent agent of onychomycosis and tinea pedis in
most countries, including Russia. However, data on its population structure remain
controversial, with some studies reporting the absence of the structure and others
reporting bipartite structure. We aimed at studying T. rubrum population by microsatellite
analysis and typing of TERG_02941 locus.
Methods: The study included 59 clinical isolates of T. rubrum from Saint Petersburg
and Yekaterinburg, Russia. Species identification was done by ITS rDNA region sequencing.
Typing was performed by real time PCR of the locus TERG_02941 and microsatellite analysis
of 12 loci, including 4 original ones. The data were processed in the R software using
the polysat and poppr packages. Genetic distances were calculated by Bruvo method.
Results: Ribosomal ITS region sequencing revealed nucleotide sequence, identical to
that one, deposited under KT285224 accession in all but two isolates. Real-time PCR
of the TERG_02941 locus revealed one SNP at position 793. At this position, adenine
was found in 59% of isolates and guanine was found in 34% of isolates. The scatterplot,
based on the results of the analysis of the lengths of microsatellite loci by the
method of principal components, contained two separate clouds of points. One of the
clouds was formed by isolates with TERG_02941 793A genotype, the other — by 793G isolates
(Fig. 1). The value of the Simpson index, calculated for equal samples, was not significantly
different for TERG_02941 genotypes. The association index, rˉd
= 0.02483, was calculated using the Multilocus Style Permutation method after deleting
one non-informative locus and performing clonal correction. The value laid beyond
the right boundary of the distribution of expected frequencies. The probability rˉd
was estimated as 0.002, which made it possible to reject the null hypothesis of the
absence of connections between the markers. The χ
2 test showed no connection between the TERG_02941 isolate genotype and the localization
of the lesion on the patient’s body.
Conclusion: Microsatellite analysis with an extended set of loci confirms the presence
of two genetic lines of the fungus T. rubrum in the territory of the Russian Federation,
with the same diversity. In the population of the fungus there is a significant deviation
from the Hardy-Weinberg equilibrium.
Abdillah
A.
1
Khelaifia
S.
2
Raoult
D.
2
Bittar
F.
3
Ranque
S.
1
1Vitrome, Aix Marseille Univ, IRD, AP-HM, SSA, Marseille, France
2Culturomics, IHU Méditerranée Infection, Marseille, France
3Mephi, Aix Marseille Univ, IRD, AP-HM, SSA, Marseille, France
P491. Comparison of three skin sampling methods and two media for culturing Malassezia
yeast
Objectives:
Malassezia species are lipid-dependent basidiomycetous yeast. They are resident commensals
of the human skin but also cause common skin diseases, such as pityriasis versicolor,
seborrheic dermatitis, etc. Whether they are associated with other human diseases
remains an open question, partly because of the lack of efficient medium to culture
these fastidious yeast. Besides, the absence of a standardized skin sampling technique
introduces a major bias in culture-based descriptive studies. The objectives of this
study were: 1) to compare three methods of skin sampling for the detection of Malassezia
yeast and 2) to evaluate the performances of « FastFung » a new culture medium based
on the Schædler agar, for the growth of Malassezia spp. by culture compared to the
reference Dixon medium.
Methods: A total of 10 healthy volunteers were included in this study. Fifteen skin
samples were collected at 5 different body sites for each participant using three
sampling methods: a) dry cotton swabs, b) swabs in a liquid transport medium « Transwab®»,
and c) a sterile gauze. The three methods were applied successively in a random order,
which was noted, at each sampling site. Each skin sample was then plated in parallel,
in a random order on both Dixon and FastFung media. All plates were incubated aerobically
at 30°C and cultures were examined each day for one week for Malassezia spp. growth.
Results:
Malassezia yeast grew in 93/300 (31%) culture plates, corresponding to 150 samples.
A total of 1 082 Malassezia spp. colonies were isolated and identified by MALDI-TOF
Mass Spectrometry, of which 455 (42%) were M. globosa, 424 (40%) M. sympodialis, and
203 (18%) M. restricta. Multivariate statistical analysis found a significant independent
effect (P<10-3) of the sampling methods. of the 93 positive culture, 62 (67%) were
sampled using a sterile gauze, 17 (18%) with Transwab®, and 14 (15%) with the dry
cotton swab. We also found a statistically significant independent effect (P = 0.003)
of the culture medium, with the FastFung and Dixon media yielding 58 (62%) and 35
(38%) positive culture, respectively. Interestingly, the majority of the fastidious
M. globosa and M. restricta species were isolated on the FastFung medium.
Conclusion: Our findings demonstrate that sterile gauze is the most efficient and
reliable skin sampling technique to culture Malassezia yeast; and that the novel FastFung
culture medium is suitable, and more efficient than the Dixon medium for Malassezia
spp. culture. We propose implementing sterile gauze skin sampling and the FastFung
culture medium in the routine Malassezia spp. cultivation procedures.
Packeu
A.
1
Baert
F.
1
Goens
K.
1
Roesems
S.
1
Stubbe
D.
2
Hendrickx
M.
3
1Mycology & Aerobiology, Sciensano, Brussels, Belgium
2Mycology & Aerobiology, Bccm/ihem Collection, Sciensano, Brussels, Belgium
3Mycology, Sciensano, BCCM/IHEM, Brussels, Belgium
P495. Morphological and molecular characterization of the T. benhamiae isolates.
Objectives: Dermatophytes are keratinophilic fungi responsible for causing dermatophytosis
in both man and animals. Recently, several genera were (re)introduced in addition
to Epidermophyton, Microsporum, and Trichophyton and depending on their primary habitat,
divided into geophilic, zoophilic and anthropophilic species. Due to the fact that
they are affecting more than 20-25% of the world’s population, able to affect healthy
individuals and in some cases are communicable makes this specific group of fungi
of great importance for public health. Recent taxonomical changes (de Hoog et al.,
2017) proposed an increase in the number of genera, simplifying identification of
dermatophytes in routine diagnostics. The implementation of these changes necessitated
a taxonomic re-evaluation of the dermatophytes within the Belgian BCCM/IHEM culture
collection. and furthermore, an evaluation of such a large dataset leads to further
insights in the taxonomy of this specific group of dermatophytes.
Methods: In the present study, the members of the T. benhamiae and T. bullosum clade
were evaluated, using different molecular methods (sequencing and MALDI-TOF MS). The
obtained results were coupled with conventional physiological, morphological, clinical,
and geographical data of the strains in order to obtain a correct nomenclature for
each analysed isolate. As proposed by de Hoog et al., T. benhamiae strains can be
categorized into two subclades: the T. bullosum clade and the T. benhamiae clade.
The T. bullosum clade comprises the type strain of this zoophilic species isolated
from cutaneous lesion of a horse described for the first time in 1933 by Lebasque.
The T. benhamiae clade comprises isolates from the zoophilic species T. benhamiae,
T. erinacei, T. verrucosum and T. eriotrephon and the anthropohilic T. concentricum
species. However, in both clades delineation of the different species still remains
unclear. Especially for T. benhamiae a lot of confusion still exists, where 2 different
phenotypes (white and yellow) have been described.
Results: Preliminary molecular results (ITS and BT), obtained with the BCCM-IHEM isolates,
point to the existence of only one T. benhamiae clade with well supported clades for
the species T. erinacei, T. verrucosum and T. eriotrephon. The remaining species are,
however, not well resolved. While most T. benhamiae strains clustered together with
T. concentricum as a subclade in the ITS+BT phylogeny, some strains clustered with
T. bullosum forming a highly supported clade.
Conclusion: In order to better delineate the different taxonomic entities and to define
criteria for their identification, a combining of all results obtained via the proposed
polyphasic approach will be primordial.
Cano-Lira
J.F.
1
Rodriguez-Andrade
E.
1
Wiederhold
N.
2
Stchigel
A.
1
1Mycology, Universitat Rovira i Virgili, Reus, Spain
2Pathology, The University of Texas Health Science Center at San Antonio, San Antonio,
United States of America
P496. Phenotypic and phylogenetic characterization of malbranchea-like isolates from
clinical specimens in United States
Objectives: The main objective of this work was to identify phenotypically and molecularly
twenty-two strains from clinical specimens in USA, preliminary classified as Malbranchea
spp.
Methods: For cultural characterization, the strains were grown onto potatoes extract
agar (PDA), phytone yeast extract agar and oatmeal agar, and the colonies measured
and described after two weeks at 25°C. Vegetative and reproductive structures were
observed and documented from slide cultures mounted on water and 60% lactic acid by
a BH1 Olympus microscope. Photo micrographs were taken using a Zeiss Axio-Imager M1
light microscope with a DeltaPix Infinity X digital camera using Nomarski differential
interference contrast. For phylogenetic analysis, total DNA was extracted from colonies
after 7-10 days of growth on PDA at 25 °C. DNA was used to amplify and to sequence
a fragment of the 28S nrRNA gene (LSU) using the primer pair LR0R and LR5, and the
ribosomal internal transcribed spacers (ITS) by using the primer pair ITS5 and ITS4.
A preliminary molecular identification was performed with ITS and LSU sequences using
the Basic Local Alignment Search Tool (BLAST; https://blast.ncbi.nlm.nih.gov/Blast.cgi).
A maximum level of identity of ≥ 98% respect to the sequences of type or reliable
reference strains deposited in the GenBank/EMBL database, and the phenotypic characteristics,
were used for identification at species-level. LSU sequences generated by us were
aligned with other from known taxa belonging to the order Onygenales by ClustalW application
of MEGA v. 6.06. The phylogenetic tree was built using maximum-likelihood (ML) and
Bayesian-inference (BI) methods with RAxML v. 8.2.10 and MrBayes v3.2.6 computer programs,
respectively. Oidiodendron truncatum and Myxotrichum deflexum (Myxotrichaceae family)
were used as outgroup.
Results: Hitherto, taking into account the phylogenetic data and the phenotypic features,
eleven strains were identified at species level. We report the finding of Auxarthron
alboluteum (two strains), A. conjugatum (two strains), A. umbrinum (three strains)
and A. zuffianum (one strain); and of Malbranchea aurantiaca (two strains) and M.
flocciformis (one strain). One strain of Arachnomyces, two of Auxarthron, one of Malbranchea
and two of Spiromastigoides could not be identified at species-level. In addition,
two strains did not matched at genus-level.
Conclusion: The phenotypic characterization and the phylogenetic analysis carried
out by us only allowed us to identify at species-level 11/19 strains previously identified
as Malbranchea spp. Most of these strains finally resulted known species of the genus
Auxarthron, and only three of them could be identified as Malbranchea species. This
indicates that the presumptive morphological identification is unreliable, and molecular
data are needed for the correct identification of this sort of fungi. Surprisingly,
eight of nineteen strains were identified at genus-level or not identified, which
suggests that these malbranchea-like fungi could be putatively new taxa
Mucek
K.
Maier
T.
Timke
M.
Kostrzewa
M.
Research and Development, Microbiology and Diagnostics, Bruker Daltonik GmbH, Bremen,
Germany
P497. Fast mold identification with MALDI-TOF MS: The importance of the spectra acquisition
method
Objectives: Invasive fungal infections are a major cause for morbidity and mortality
in ill, immunocompromised and paediatric patients [1, 2]. Clinical diagnosis of mold
infections and identification of fungal contamination is often based on morphological
criteria which encounter issues like robustness of phenotypic procedures, often sporulation
as a prerequisite for identification and very few skilled mycologists that are capable
of differentiating molds in the medical environment. In order to solve this situation
one of the most promising alternative to identify clinically and environmental relevant
molds is MALDI-TOF MS. Through the generation of protein spectra and the comparison
with a reference library, molds can be identified fast and reliable. However, in some
cases the acquisition of mass spectra particular from filamentous fungi is challenging
since the frond mycelium often results in reduced spectra quality.
Methods: A minimum about 56 filamentous fungi was measured with MALDI-TOF in duplicates
with at least two different operators. Each strain was cultivated on Sabouraud agar
for 24 – 48 h, front mycel was smeared on a MALDI target plate. One of two spots was
pre-treated with formic acid before all spots were overlaid with HCCA matrix and the
measurement started. Two different acquisition methods were applied. The Bruker ‘standard’
method and the ‘filamentous fungi’ method was compared. Subsequently all spectra were
matched against the Filamentous Fungi Library 3.0.
Results: After cultivation of filamentous fungi biological material could be transferred
successfully to a MALDI-TOF target plate in duplicates for each strain. The results
showed that the rate of spectra acquisition by using the filamentous fungi method
was significant higher (about 25%) compared to the standard acquisition method. It
could be shown additionally that the acquired spectra resulted in an increased identification
rate.
Conclusion: The MALDI Biotyper Filamentous Fungi Module with adapted acquisition settings
for filamentous fungi improves the identification rate for molds. MALDI-TOF based
differentiation is less error prone and faster compared to classical identification.
Results can be easily obtained in one hour from cultivation plate. Moreover, the adapted
filamentous fungi method compared to the standard spectra acquisition method is a
vast advantage. Time consuming repetition of target preparation and re-measurement
is reduced as well as previous protein extraction for MALDI-TOF measurement. Thus,
mold infections like aspergillosis or mucormycosis or fungal contaminations are identified
more reliable and faster. [1] Prattes, J., Heldt, S., Eigl, S. et al. Curr Fungal
Infect Rep (2016) 10: 43. https://doi.org/10.1007/s12281-016-0254-5 [2] Gullo, A.
Drugs (2009) 69(Suppl 1): 65. https://doi.org/10.2165/11315530-000000000-00000
Uhrlass
S.
1
Sithach
M.
2
Koch
D.
1
Wittig
F.
1
Muetze
H.
1
Krueger
C.
1
Nenoff
P.
1
1Partnership Dr. C. Krueger & Prof. P. Nenoff, Laboratory of medical microbiology,
Roetha OT Moelbis, Germany
2Department of Dermatology, Preah Kossamak Hospital, Phnom Penh, Cambodia
P498. Trichophyton mentagrophytes – a new genotype in Cambodia
Objectives: According to the currently suggested classification and new taxonomy of
the dermatophytes, the former Trichophyton mentagrophytes (TM)-complex is now differentiated
in TM (zoophilic strains) and Trichophyton interdigitale (TI, anthropophilic strains).
Methods: Mycological diagnostics was done during a survey of superficial dermatophytoses
in Cambodia.
Results: 1) The new genotype “Cambodia” of TM (strain 201341/18) was found in a 8
years old male child with skin lesions starting already 3 months ago, prior the dermatology
consultation. Inflammatory papules on the right part of the scalp were to be seen.
2) A 31 years old male suffered from a tinea cruris. The dermatophyte isolate was
found to be the new TM genotype Cambodia (strain 218292/17). 3) A 26 years old male
farmer showed lesions appearing since one year, started with scaly pruriginous plaques
on both hips. Clinical diagnosis was tinea corporis. By mycological diagnostics, from
skin scrapings, a dermatophyte was isolated. By sequencing of the DNA it was identified
as TM genotype VIII India (strain 218294/17). 4) A one-year-old male was diagnosed
as tinea corporis. The dermatophyte which was growing from skin scrapings of the baby´s
cutaneous lesions was identified as T. interdigitale (TI strain 250037/18 genotype
II* - mixed type between TI and TM).
Conclusion: Today, due to sequencing of the ITS region of the rDNA, at least 10 genotypes
of TM and TI are known. These genotypes are mostly associated to the geographic origin
or to the source of infection. Currently, two genotypes of TI (ITS Type I [Europe]
and ITS Type II [Cosmopolite]), were described. TM, however, comprises the common
zoophilic “German” ITS Type III (Europe) and III* (Cosmopolite), genotype IV (UK,
USA, South Africa and Feancde), V (Asia, Egypt, Iran, Iraq and Japan), VI (Europe,
Russia and Finland), VII (from Thailand originating isolates in Switzerland and Germany,
USA, Australia, Georgia, Russia, and Vietnam), VIII (Asia, India, Iran, Oman, Australia),
and last but not least the Australian genotype (ITS Type IX). Based on results of
sequencing preferably of the ITS region of the rDNA, we are able to demonstrate that
2 out of 3 TM strains in Cambodia represent a new, until now not described, ITS genotype
which is called now the “Cambodian” genotype of TM. Surprisingly, we found one additional
TM strain of the Indian genotype VIII. An association between this dermatophytosis
in Cambodia and the current Indian outbreak of superficial dermatophytosis can only
be suspected. The fourth here isolated dermatophyte belongs to the until now very
rarely described genotype TI II*. Phylogenetic, this genotype is the so called mixed
type between anthropophilic TI and zoophilic TM. Each genotype of the TM/TI complex
seems to be specific for e.g. a geographic area, for a country, or for a distinct
animal which represents the source of infection, speaking for a special way of transmission
of the infection.
Hallur
V.
1
Sable
M.
2
C
P.
3
Rudramurthy
S. M
4
Sharma
P.
5
Prakash
H.
4
Chakrabarti
A.
4
1Microbiology, All India Institute of Medical Sciences, Bhubaneswar, Bhubaneswar,
India
2Pathology, All India Institute of Medical Sciences, Bhubaneswar, Bhubaneswar, India
3Ent, All India Institute of Medical Sciences, Bhubaneswar, Bhubaneswar, India
4Medical Microbiology, Postgraduate Institute of Medical Education and Research, Chandigarh,
India
5Ent, All India Institute of Medical Sciences Bhubaneswar, Bhubaneswar, India
P499. Novel Cunninghamella species causing mucormycosis in an apparently immunocompetent
individual
Case Report: Title: Novel Cunninghamella species causing mucormycosis in an apparently
immunocompetent individual Mucormycosis due to Cunninghamella spp. is extremely rare
clinical entity. We report here a case of mucormycosis due to Cunninghamella sp. in
an apparently immunocompetent individual. Material & Methods: Patient & microbiological
methods: Tissue biopsy from the lesion was subjected to histopathology, and culture.
Formalin fixed paraffin embedded tissue (FFPE) and culture were sent to Mycology Reference
Laboratory at PGIMER Chandigarh for species identification & susceptibility testing.
Molecular identification: DNA was extracted from FFPE tissue and cultures as described
previously by Rickerts et. al.(1) & Prakash et.al. (2) DNA from FFPE tissue was PCR
amplified using mucoromycetes specific primers. The ITS and 26s genes of ribosomal
DNA of the isolate were amplified using pan fungal primers. Consensus sequences obtained
from amplified products were analyzed using the National Center for Biotechnology
Information (NCBI) Basic Local Alignment Search Tool (BLAST). Phylogenetic characterization:
The 26S rDNA and ITS sequences of different Cunninghamella were retrieved from NCBI
nucleotide database. Phylogenetic analysis was performed as per Prakash et.al.(2)
Results: Patient & microbiological methods: A 26-year-old healthy man presented with
slowly progressive swelling on the left cheek for 2 years and ulceration over the
swelling for two months. Laboratory investigations revealed a normal blood sugar,
hemogram, CD4 counts, liver and renal function tests. He was non-reactive for HIV.
Contrast enhanced computed tomography scan of the head and neck showed mild enhancing
lesions on left side of the face along with thickening of floor of maxilla. Biopsy
from the lesion showed focal areas of chronic inflammation with multinucleated giant
cells containing broad aseptate fungal hyphae. A rapidly growing white mould with
aseptate hyphae & erect sporangiophores ending in a swollen vesicle, with 1-spored
sporangiola suggestive of Cunninghamella was isolated. The isolate had minimum inhibitory
concentrations (MICs) of 4µg/ml for amphotericin B, itraconazole, and posaconazole;
and 3µg/ml for Terbinafine as per CLSI M38A2. He was managed with repeated debridement
over 7.5 months, amphotericin B and posaconazole for 1.5 months & 6 months respectively.
As there was no improvement, he was finally treated with oral Itraconazole. At present
the ulcer has healed, but the swelling is still persisting. Molecular identification:
The fungus was identified as Cunnighamella sp. by analysis of DNA sequence of the
FFPE tissue. ITS sequences of the isolate showed 97.54% base identity with uncultured
Cunninghamella spp. (MG571234.1) isolated from Thailand. Phylogenetic analysis: Phylogenetic
analysis of ITS and 26s regions of all known Cunninghamella species with the present
isolate suggested that the isolate may be a new or undefined species under genus Cunninghamella.
Conclusion: We report recalcitrant mucormycosis due to a Cunninghamella sp., which
is phylogenetically different from other described species in this genus. Further
molecular characterization is essential to identify the species. References: Eur J
Clin Microbiol Infect Dis. 2006 Jan;25(1):8-13. Med Mycol. 2016 Aug 1;54(6):567-75.
doi: 10.1093
Cmokova
A.
1
2
Kolarik
M.
1
Stubbe
D.
3
Dobias
R.
4
Lyskova
P.
5
Hoyer
L.
6
Janouskovcova
H.
7
Wiegand
C.
8
Malatova
N.
9
Kano
R.
10
Nenoff
P.
11
Uhrlass
S.
12
Peano
A.
13
Mencl
K.
14
Maier
T.
15
Kuklova
I.
16
Hubka
V.
1
17
1Institute of Microbiology, Czech Academy of Science, Prague, Czech Republic
2Department of Botany, Charles University, Prague, Czech Republic
3Mycology & Aerobiology, Sciensano, Brussels, Belgium
4Bacteriology and Mycology, Public Health Institute in Ostrava, Ostrava, Czech Republic
5Institute of Health in Usti nad Labem, Usti nad Labem, Czech Republic
6College of Veterinary Medicine, University of Illinois at Urbana-Champaign, Urbana,
United States of America
7University Hospital in Pilsen, Pilsen, Czech Republic
8Department of Parasitology, Humboldt University, Berlin, Germany
9Hospital Ceske Budejovice, Ceske Budejovice, Czech Republic
10Nihon University School of Veterinary Medicine, Tokyo, Japan
11Laboratory For Medical Microbiology, Mölbis, Mölbis, Germany
12Laboratory For Medical Microbiology, Mölbis, Tallinn, Germany
13Universita degli Studi di Torino, Turin, Italy
14Pardubice Regional Hospital, Pardubice, Czech Republic
15Research and Development, Microbiology and Diagnostics, Bruker Daltonik GmbH, Bremen,
Germany
16First Faculty of Medicine, Charles University in Prague, Prague, Czech Republic
17Department of Botany, Charles University, Prague, Czech Republic
P500. rResolving Trichophyton benhamiae complex: zoonotic pathogens on the rise
Objectives: Species of Trichophyton benhamiae complex have a worldwide distribution
and their host range includes many pet and livestock animals. Nowadays, this complex
has become very important due to epidemic spread of T. benhamiae complex species in
children and their pet animals in Europe. No causal mechanism has been found that
would explain this increase. Moreover, the incidence of several other species from
this complex is also increasing in Europe due to higher interest of people in pet
hedgehogs (host of T. erinacei), insufficient prophylactic vaccination (T. verrucosum)
or increasing use of molecular methods in the dermatological diagnostics leading to
more accurate identification (T. bullosum, T. eriotrephon). A considerable genetic
and phenotypic variability has been revealed in these emerging pathogens, but the
species limits are not always clearly defined. Therefore, the aim of this study was
to elucidate species boundaries and phylogenetic relationships between species belonging
to the T. benhamiae complex.
Methods: A total of 352 clinical isolates from T. benhamiae complex associated with
human and animal dermatophytoses were analysed using molecular markers (DNA sequence
data and microsatellites), and morphological and physiological methods.
Results: Members of the T. benhamiae complex were resolved into three major clades
(designated T. benhamiae, T. erinacei and T. africanum clades). All analyses supported
delimitation of four species in the T. benhamiae clade, i.e., T. benhamiae, T. concentricum,
and two novel species, T. europaeum and T. japonicum. In contrast to DNA sequence
and based phylogeny, the population genetic and phenotypic data distinguished North
American strains of T. benhamiae (mostly isolated from dogs) and emerging European
strains associated with quinea pigs. The status of new variety, T. benhamiae var.
luteum, is proposed for these strains. The T. erinacei clade consisted of three species,
namely T. erinacei, T. verrucosum and T. eriotrephon. Trichophyton bullosum and one
new species, T. africanum (previously African race of Arthroderma benhamiae) together
with T. bullosum formed the T. bullosum clade
Conclusion: Species boundaries in the T. benhamiae clade were resolved by using polyphasic
approach. This approach supported recognition of nine species, including three new
zoophilic species. Trichophyton benhamiae was split into two varieties; T. benhamiae
var. luteum is currently responsible for the European outbreak of zoonotic infections.
Video abstracts
Colman
S.
Cattoir
L.
Boel
A.
Microbiology, OLV Hospital, Aalst, Belgium
V01. Subcutaneous nodule caused by Phaeoacremonium fuscum in a non-immunocompromised
patient
Objectives:
Phaeohyphomycosis (PHM) is a chronic infection caused by dematiaceous fungi which
usually involves the skin and subcutaneous tissue. As a result of the use of molecular
diagnostic techniques, previously unrecognized causative agents of phaeohyphomycosis
have been discovered. Here we report the first case of phaeohyphomycosis caused by
Phaeoacremonium fuscum in an immunocompetent patient.
Methods: Case presentation A 60 year old Filipino male presented with rotator cuff
pathology to the orthopedics consultation. On physical examination a painless, soft,
subcutaneous mass above the right patella was noted, which didn’t cause the patient
any complaints. The suprapatellar nodule had been growing slowly for 3 years. The
patient denied any apparent antecedent trauma, arthropod bite, family history or contact
with a person with similar lesions. The man still visits the Philippines on an infrequent
basis. He had no fever, weight loss or other symptoms. Detailed physical examination
further revealed a 4x3 cm, immobile, non-pulsatile light red mass. There were no other
signs of inflammation of the skin. A serous effusion was aspirated from the mass.
Results: Fungal culture of the aspirated fluid showed growth of a filamentous fungus
on chocolate blood agar and Sabouraud’s dextrose agar. Molecular identification was
performed by sequence analysis of the internal transcribed spacer region 2 (ITS2).
BLAST search revealed a similarity of 100% with Phaeoacremonium fuscum. The antifungal
susceptibility profile was established by EUCAST broth microdilution: itraconazole
>16 mg/L, posaconazole 0.25 mg/L, amphotericin B 0.50 mg/L, voriconazole 0.50 mg/L.
This susceptibility profile is similar to those previously published. A wide local
excision of the lesion was performed a couple of weeks later. The patient had an uneventful
postoperative recovery. No antifungal therapy was started postoperatively since the
patient was not immunocompromised and there was lack of reimbursement. Histopathological
examination of the specimen showed fibrous tissue with a mixed cellular infiltrate
including lymphocytes, plasma cells, macrophages and neutrophils. Periodic acid-schiff
stain and Grocott stain showed evidence of branching septate hyphae.
Conclusion: PHM is caused by brown pigmented fungi containing melanin in their cell
wall. Phaeoacremonium species are known as vascular plant pathogens, responsible for
wilting and dieback of woody plants. They are also opportunistic pathogens in humans,
mainly causing cutaneous or subcutaneous PHM. However, sporadic cases of respiratory
tract infection, septic arthritis and disseminated infection are described. The true
prevalence of Phaeoacremonium species is probably underestimated due to the asymptomatic
nature of most subcutaneous lesions and the difficult identification. There is no
standard antifungal regimen or evidence regarding the necessity for antifungal treatment
as concomitant with surgery for localized infections. Long courses of antifungals,
such as voriconazole treatment, are often employed to reduce the risk of recurrence.
Case reports suggest that surgery can be curative if the infection is localized and
adequately removed, as was the case in our patient. Regardless of the immune status
of the patient, clinicians should consider the possibility of a fungal infection when
they encounter chronic subcutaneous nodules without major symptoms.
Premamalini
T.
1
Kumar
P. Kennedy
1
Kumaran
N. Anish
1
Kindo
A.J.
2
1Microbiology, Sri Ramachandra Medical College and Research Institute, SRIHER, Chennai,
India
2Microbiology, Sri Ramachandra Medical college and Research Institute, chennai, India
V02. Trichosporon diagnosis- The Right path
Objectives: To discuss about the correct path in identifying Trichosporon sp. To emphasize
the importance of performing conventional laboratory diagnostic methods like microscopy,
along with molecular methods in the diagnosis of Trichosporon sp.
Methods: Yeast-like colonies were observed for microscopic characteristics by Gram
staining. The isolates which showed the presence of budding yeast cells, pseudohyphae
and arthroconidia were tested for production of the enzyme urease. Urease positive
test isolates were provisionally identified as belonging to the genus Trichosporon.
To confirm the identity of all phenotypically identified Trichosporon isolates, Trichosporon
genus specific colony PCR was performed. This pair of primer is Trichosporon specific
and it amplified part of the nucleotide sequences of the rDNA small subunit (18S).
Results: The study of colony morphology of the Trichosporon isolates on SDA at 25
°C after 10 days of incubation revealed varied colony morphology. Most commonly, they
may appear as white to cream, farinose or cerebriform colonies, with furrows. The
important characteristic feature of this yeast is the production of arthroconidia
and enzyme urease. Performing Gram stain or Dalmau technique to search for arthroconidia
is a very useful tool for screening of Trichosporon spp. Finally, the accurate identification
of this yeast was done using DNA based methods like Trichosporon genus specific colony
PCR. The members of the genus Trichosporon produced DNA bands of approximately 170
bp.
Conclusion:
Trichosporon sp. has been so far reported as the second most common cause of disseminated
yeast infections in humans, next to the genus Candida. They are emerging opportunistic
pathogens causing disseminated infections in immunocompromised patients. Such infections
are usually difficult to diagnose, do not respond to treatment with routinely used
antifungal agents, and are associated with high mortality rates. Hence, correct identification
of Trichosporon sp. helps the clinician in providing appropriate treatment. This also
applies for other emerging yeast infections, where combination of conventional and
molecular methods may help in timely and accurate diagnosis.
Chander
J.
1
Punia
R.P.S.
2
Dass
A.
3
Attri
A.K.
4
1Microbiology, Government Medical College Hospital, Chandigarh, India
2Pathology, Government Medical College Hospital, Chandigarh, India
3Ent, Government Medical College Hospital, Chandigarh, India
4General Surgery, Government Medical College Hospital, Chandigarh, India
V03. Disseminated rhinosporidiosis with different morphological lesions involving
various anatomical sites.
Objectives: Rhinosporidiosis is a chronic granulomatous disease caused by Rhinosporidium
seeberi, which is a taxonomically debated endosporulating aquatic eukaryotic organism
since long. Rhinosporidiosis predominantly involves the mucous membranes of the nose
and eyes mainly conjunctiva. In recent past, giant lesions and disseminated type of
disease, has also been reported in some of the clinical settings. This disease is
hyperendemic in the Indian subcontinent, particularly in Sri Lanka and southern India.
However, sporadic cases have occasionally been reported from other continents.
Methods: A case report of rare disseminated rhinosporidiosis with varied morphological
lesions involving simultaneously three anatomical sites i.e. nasal, cutaneous and
bones in a hepatitis B virus surface antigen (HBsAg) positive patient. A 35-years
old male, resident of district Madhubani, Bihar, fisherman by occupation presented
with complaints of left sided nasal obstruction, insidious in onset, gradually progressive
and associated with change in voice since the last 6 years. Also, there was slowly
progressive swelling over medial aspect of left ankle for the last 1 year and a similar
looking swelling, smaller in size also appeared in the left post-auricular region
four months back and has gradually increased in size. On examination, a polypoidal
fungating mass was present over left medial aspect of foot which was non-tender, highly
vascular, large, hemi-spherical in shape and bled on touch. On anterior rhinoscopy,
a warty, irregular, non-tender mass, not bleeding on touch, occupying whole of left
nasal cavity was seen. Oral cavity showed bulging soft palate and a bilobed pale polypoidal
mass with smooth surface hanging from nasopharynx to oropharynx. There was a solitary
ovoid growth measuring 2 x 2 cm with normal appearing overlying skin in the left upper
post-auricular region. Serum of the patient on testing for HBsAg was positive. The
patient was known chronic alcoholic and smoker.
Results: A wedge biopsy of the lesion when examined in normal saline showed sporangia
of R.seeberi in different stages of development. Routine haematoxylin and eosin (H
& E) staining, periodic acid-Schiff (PAS) staining as well as mucicarmine staining
of the tissue also revealed findings of rhinosporidiosis with many sporangia and scattered
endospores seen in a background of inflammatory infiltrate composed of polymorphs,
lymphocytes and plasma cells including the bony involvement. A final diagnosis of
disseminated rhinosporidiosis presenting simultaneously with three different kinds
of lesions; nasal, cutaneous and osteomyelitis of medial malleolus and distal end
of tibia was made. The patient was treated surgically by performing below knee amputation;
transnasal and transoral excision with electrocoagulation as well as excision of postauricular
mass was also performed. Histopathology of the excised bone and other growths was
consistent with the preoperative diagnosis. The patient was discharged and advised
dapsone therapy 100 mg bd to prevent recurrence. Presently, the patient is on follow
up and there is no recurrence of the disease.
Conclusion: This is prudent for clinicians, microbiologists and pathologists to keep
rhinosporidiosis as a differential diagnosis when managing patients with polypoidal
growths even in non-endemic areas. In such a background high index of suspecion is
the key to reach the final diagnosis.