- Record: found
- Abstract: found
- Article: not found
Mesenchymal Stem Cell-Based Tissue Regeneration is Governed by Recipient T Lymphocyte via IFN-γ and TNF-α
Abstract
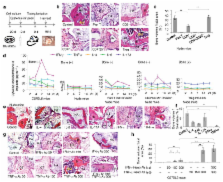
Stem cell-based regenerative medicine is a promising approach for tissue reconstruction. Here, we showed that pro-inflammatory T cells in the recipients inhibited bone marrow mesenchymal stem cell (BMMSC)-mediated bone formation via T helper 1 (Th1) cytokine interferon (IFN)-γ induced down-regulation of runt-related transcription factor 2 (Runx-2) pathway and tumor necrosis factor (TNF)-α-regulated BMMSC apoptosis. TNF-α converted IFN-γ-activated non-apoptotic Fas to a caspase 3/8-associated apoptotic signaling in BMMSCs through inhibition of nuclear factor kappa B (NFκB), resulting in BMMSC apoptosis. Conversely, reduction of IFN-γ and TNF-α levels by systemic infusion of Foxp3 + regulatory T cells (Tregs) markedly improved BMMSC-based bone regeneration and calvarial defect repair in C57BL6 mice. Furthermore, we showed that local administration of aspirin reduced levels of IFN-γ and TNF-α at the implantation site and significantly improved BMMSC-based calvarial defect repair. These data collectively uncover a previously unrecognized role of recipient T cells in BMMSC-based tissue engineering.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Adult mesenchymal stem cells for tissue engineering versus regenerative medicine.
- Record: found
- Abstract: not found
- Article: not found
Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo.
- Record: found
- Abstract: found
- Article: not found
