- Record: found
- Abstract: found
- Article: found
Dengue and Other Common Causes of Acute Febrile Illness in Asia: An Active Surveillance Study in Children
Read this article at
Abstract
Background
Common causes of acute febrile illness in tropical countries have similar symptoms, which often mimic those of dengue. Accurate clinical diagnosis can be difficult without laboratory confirmation and disease burden is generally under-reported. Accurate, population-based, laboratory-confirmed incidence data on dengue and other causes of acute fever in dengue-endemic Asian countries are needed.
Methods and principal findings
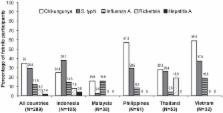
This prospective, multicenter, active fever surveillance, cohort study was conducted in selected centers in Indonesia, Malaysia, Philippines, Thailand and Vietnam to determine the incidence density of acute febrile episodes (≥38°C for ≥2 days) in 1,500 healthy children aged 2–14 years, followed for a mean 237 days. Causes of fever were assessed by testing acute and convalescent sera from febrile participants for dengue, chikungunya, hepatitis A, influenza A, leptospirosis, rickettsia, and Salmonella Typhi. Overall, 289 participants had acute fever, an incidence density of 33.6 per 100 person-years (95% CI: 30.0; 37.8); 57% were IgM-positive for at least one of these diseases. The most common causes of fever by IgM ELISA were chikungunya (in 35.0% of in febrile participants) and S. Typhi (in 29.4%). The overall incidence density of dengue per 100 person-years was 3.4 by nonstructural protein 1 (NS1) antigen positivity (95% CI: 2.4; 4.8) and 7.3 (95% CI: 5.7; 9.2) by serology. Dengue was diagnosed in 11.4% (95% CI: 8.0; 15.7) and 23.9% (95% CI: 19.1; 29.2) of febrile participants by NS1 positivity and serology, respectively. Of the febrile episodes not clinically diagnosed as dengue, 5.3% were dengue-positive by NS1 antigen testing and 16.0% were dengue-positive by serology.
Author Summary
Acute febrile episodes are common in children living in tropical countries. Diagnosis can be challenging because symptoms of the more common infectious causes are similar and often mimic those of dengue. Asia Pacific has over 70% of the worldwide dengue disease burden, although dengue incidence is generally underestimated because most surveillance systems are passive or based on clinical diagnosis without laboratory confirmation. Understanding the local etiology of febrile illness and the incidence of dengue is important when planning large-scale vaccine trials. This prospective, active fever surveillance, cohort study was carried out in children in five dengue-endemic Asian countries – Indonesia, Malaysia, Philippines, Thailand and Vietnam – during 2010–2011. Acute febrile episodes occurred in 289 (19.3%) of the cohort of 1,500 children. Among the diseases for which antibodies were tested using commercial kits, the top three causes of acute fever were chikungunya, Salmonella Typhi and dengue, followed by influenza A, rickettsia and hepatitis A. Dengue was confirmed in 11.4% of the febrile children by viral protein detection and in 23.9% by serology. Clinical diagnosis was not sufficient to detect all dengue cases. These findings are of relevance to those planning clinical studies of vaccines against these infectious agents in Southeast Asia.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
A study of typhoid fever in five Asian countries: disease burden and implications for controls.
- Record: found
- Abstract: found
- Article: not found
From research to phase III: preclinical, industrial and clinical development of the Sanofi Pasteur tetravalent dengue vaccine.
- Record: found
- Abstract: found
- Article: not found