- Record: found
- Abstract: found
- Article: found
False friends: Phagocytes as Trojan horses in microbial brain infections
review-article
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Introduction
Humans are constantly exposed to pathogenic microbes. The first line of cellular host
defense is composed of “professional” phagocytes, cells that efficiently recognize
pathogens, internalize them, and then marshal an array of antimicrobial mechanisms
to destroy them. Nevertheless, successful pathogens evade or survive such attack.
A particularly subversive strategy is to manipulate normal phagocyte behaviors to
benefit the microbe, sometimes even turning the phagocyte from a threat to a safe
haven. In this environment, the microbes can multiply while protected from immune
surveillance, and in some cases, even travel to the most protected host site, the
brain. This gives rise to the Trojan horse analogy: like the wooden horse that carried
hidden enemies through the gates into the walled city of Troy, phagocytes carry intracellular
microbes through the blood–brain barrier (BBB) into the central nervous system (CNS).
Immune cells in the brain
Traditionally, the brain has been considered an immune-privileged site because it
lacks the normal robust inflammatory responses to antigenic challenges. However, it
does have an active immune surveillance system [1] that involves the extravasation
of leukocytes, mostly monocytes and lymphocytes, into the meninges and cerebrospinal
fluid (CSF). This process follows the same general events that occur in other tissues:
rolling of the leukocyte, arrest, crawling, and then transendothelial migration [2].
Because the brain microvascular endothelial cells (BMECs) of the BBB are joined by
tight junctions and embedded in a proteinaceous matrix [3], transmigrating leukocytes
rarely cross the BBB directly. Instead, they cross into the outer meningeal spaces,
where the vasculature is devoid of tight junctions, and from this site they monitor
the CSF for the presence of immune signals. Additionally, a recently discovered brain
lymphatic system samples the perivascular spaces, bypassing the physical cellular
barrier composed of BMECs [4, 5]. Even in healthy individuals, therefore, phagocytes
are in close proximity to brain tissue, poised to act upon immune signals.
CNS phagocytes actively respond to signals generated by developmental changes, injury,
disease, or infection. Such signals include interferons produced by endothelial cells
in response to viral pathogens, chemotactic peptides like N-formyl-methionyl-leucyl-phenylalanine
(fMLP) generated by bacterial pathogens, and inflammatory cytokines released by epithelial
cells in response to fungal pathogens [6]. Microglia, phagocytes that are the only
resident immune cells in the brain, also produce cytokines and chemokines to recruit
other effector cells to that site. Rapid response to these signals is enabled by the
normal presence of phagocytes and lymphocytes in the meninges. However, tight regulation
of this response is crucial because adult neurons in the CNS generally do not regenerate;
if these cells are damaged by any activities of infiltrating phagocytes, they cannot
be replaced, potentially resulting in permanent damage. The BBB helps to limit immune
infiltration from the blood, aiding the host to mount an immune response that is robust
enough to contain infection yet limited to prevent tissue damage. For the most part,
this balance is maintained, and brain infection is prevented or controlled.
Phagocytes as Trojan horses
A model for Trojan horse transit into the brain
In the absence of trauma, pathogens that cause lethal brain infections (e.g., those
in Table 1) reach it from remote sites, generally traveling in the bloodstream. For
microbes that use Trojan horse transit, the first step is infection of a phagocyte
in the periphery (Fig 1A). Once internalized, the pathogen may actively manipulate
the phagocyte to promote migration towards the brain [7]. Alternatively, it may suppress
phagocyte activation (and consequent sequestration in the tissue of origin), allowing
the infected cell to circulate normally throughout the body (Fig 1B). Once an infected
phagocyte reaches the brain, it adheres to the luminal side of brain capillaries (with
or without activation of BMECs) and crosses the BBB, either paracellularly (between
BMECs) or transcellularly (through BMECs) (Fig 1C). After brain entry, the pathogen
may exit its Trojan horse to infect other neural structures (Fig 1D). This model has
been elucidated in the most detail for HIV and other viruses [8, 9], but studies reviewed
below suggest that similar strategies are used by other microbes that are the focus
of this review: bacteria, fungi, and parasites. Aspects of this model may also apply
to non-CNS pathogens, such as mucosal pathogens that use phagocytes to disseminate
(see “Perspectives and conclusions”).
10.1371/journal.ppat.1006680.g001
Fig 1
The role of phagocytes as Trojan horses for CNS pathogens.
Most neuroinvasive pathogens first infect organs outside the CNS, such as the lungs
or the intestines. Cryptococcus neoformans infection of the lungs is shown here as
an example. Once infection is established, phagocytes (pink cells) are recruited to
these sites (A), where they engulf the pathogen. Some infected phagocytes leave the
site of infection and enter the bloodstream, facilitated by the highly permeable vasculature
(green) of peripheral organs (B). Through a process that is poorly understood, many
of these home to the CNS. Once there, infected phagocytes may act as Trojan horses,
traversing the BBB (blue cells) with the pathogen as a passenger (C). Although both
paracellular (top) and transcellular (bottom) transmigration can occur, the latter
is most likely due to the presence of tight junctions in the BBB (C). Once inside
the brain, pathogens can potentially exit their Trojan horses and infect other neural
structures (D). Parts of this model (A and B) also apply to phagocyte-assisted dissemination
and infection outside of the CNS (see text). BBB, blood–brain barrier; CNS, central
nervous system; ECs, endothelial cells. Arrows indicate movement; broken arrow indicates
fungal egress.
10.1371/journal.ppat.1006680.t001
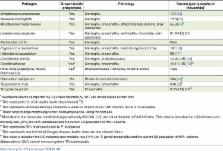
Table 1
Deaths due to CNS infection by select obligate and facultative intracellular microbes.
Pathogen
Growth insidephagocytes
Pathology
Burden (yearly deaths, in thousands)
1
Streptococcus pneumoniae
Yes
Meningitis
113 [35]
Neisseria meningitidis
Yes
Meningitis
73.3 [35]
Mycobacterium tuberculosis
Yes
Meningitis, encephalitis, intracranial tuberculoma, brain abscesses
55.6 [35]
2
Listeria monocytogenes
Yes
Meningitis, encephalitis, ventriculitis, choroiditis, brain abscesses
0.19 (US) [36]
Escherichia coli K1
Yes
Meningitis
Rare
Cryptococcus neoformans
Yes
Meningitis, encephalitis, cerebral cryptococcomas
181 [18]
Histoplasma capsulatum
Yes
Meningitis, encephalitis
80 [37]
3
Coccidioides immitis
Yes
Meningitis, brain abscesses
<0.20 (US) [38]
Candida albicans
Yes
4
Meningitis, encephalitis
<0.10 (US) [39]
5
Other fungi (Aspergillus, Mucor, Blastomyces)
Yes
4
Brain abscesses, meningitis, cerebral stroke
Rare
Plasmodium falciparum
No
Brain microvessel obstruction
584 [35]
6
Trypanosoma cruzi
Yes
Meningitis, encephalitis
3.0 [35]
7
Toxoplasma gondii
Yes
Encephalitis
0.20 (US) [39]
8
1 Worldwide deaths as reported in [35] unless denoted (by “US”) as United States burden
only.
2 This represents 5% of all deaths due to disseminated TB.
3 This represents all extrapulmonary infections; a value for deaths due to CNS infection
alone is not available.
4 These fungi change morphology when inside phagocytes, killing the host cell.
5 Most deaths due to invasive candidiasis (approximately 350,000; [37]) are not attributable
to CNS infection. This value is based on the US incidence and mortality rate (24%)
for CNS candidiasis and the current US population of HIV+ patients.
6 This represents 80% of all deaths due to P. falciparum.
7 This represents one-third of all Chagas disease deaths; most are due to heart failure.
8 This value is based on the US incidence and mortality rate (14%) for T. gondii encephalitis
and the current US population of HIV+ patients.
Abbreviations: CNS, central nervous system; TB, tuberculosis.
Bacterial infections
The most common causes of bacterial meningitis are the facultative intracellular pathogens
Streptococcus pneumoniae and Neisseria meningitidis. Because they survive in the blood
and can independently interact with BMECs to enter the brain, these microbes do not
require Trojan horse transit, although this mechanism may contribute to S. pneumoniae
infection [10]. In contrast, Trojan horses play a central role in infections by another
leading cause of bacterial meningitis, Listeria monocytogenes.
L. monocytogenes is a pathogen of humans and domesticated animals that invades the
brain parenchyma, unlike most neuroinvasive bacteria, which are limited to the meninges
(Table 1). This distinct pathology may relate to its use of Trojan horse invasion,
which was first suggested by histological studies showing parasitized phagocytes in
the brain tissue of infected mice [11]. Further suggestive of a Trojan horse mechanism
were reports that phagocytosis of L. monocytogenes causes the release of immune signals
and activation of BMECs [12], both of which would promote the recruitment of additional
leukocytes to the site of infection. More direct support for this mechanism came from
the observation that infected mice treated with gentamicin to kill extracellular bacteria
still developed CNS infection [13]. This occurred regardless of the initial route
of infection, consistent with a general model whereby phagocytes are recruited to
the site of infection, engulf the pathogen, and then disseminate (Fig 1). Bolstering
this observation, injection of L. monocytogenes-infected bone marrow myeloid cells
caused faster and greater brain colonization than injection of free bacteria [14].
In this study, which used chimeric mice that expressed a fluorescent protein in their
bone marrow cells, an increase in fluorescent signal was observed in the brain as
the infection progressed, also consistent with a Trojan horse model. The bone marrow
and spleen are among the first organs infected by L. monocytogenes. Interestingly,
phagocytes infected in these tissues cannot kill the bacteria but do up-regulate chemokine
receptors, making them ideal Trojan horses [15].
Fungal infections
Fungal infections are responsible for up to 1.6 million deaths every year [3], and
the ones affecting the CNS have the highest morbidity and mortality [16]. Although
several fungal pathogens cause meningitis (Table 1), the only one to frequently do
so is Cryptococcus neoformans. Most people have been exposed to this environmental
yeast [17]. While healthy individuals are generally asymptomatic, in immunocompromised
individuals, the initial pulmonary infection can subsequently disseminate to the CNS.
As a result, C. neoformans is the most common causative agent of meningitis in sub-Saharan
Africa and a leading cause of death in HIV+ individuals, killing close to 200,000
people each year [18].
As with L. monocytogenes, early evidence for Trojan horse transit of C. neoformans
came from histological examination of brains from infected mice [19]. This work was
complemented by studies supporting the role of phagocytes in cryptococcal dissemination
from the lungs to the brain. For example, depletion of alveolar macrophages reduced
dissemination from the lungs [20], systemic monocyte depletion after lung infection
reduced fungal burden in other organs [21], and intravenous administration of C. neoformans-associated
macrophages caused higher brain burden than infection with free cryptococci. More
recently, direct evidence for Trojan horse transit has come from two studies using
in vitro models of brain endothelia. Both groups cultured human cerebral microvascular
endothelial cell (hCMEC) monolayers on permeable membranes separating the upper (“blood”)
and lower (“brain") compartments of tissue culture wells. In one study, a monocytic
cell line was first incubated with C. neoformans, which was engulfed by or adhered
to the phagocytes, and the samples were then stained to mark any externally adherent
fungi. This mixture was added to the upper chamber, and one day later, monocytes containing
unstained fungi were found in the lower chamber, suggesting that Trojan horse crossing
had occurred [22]. (Interestingly, the same experiments performed with Cryptococcus
gattii, a species that primarily causes lung infections, showed less barrier crossing.)
In the other study, our group used a flow cytometry strategy to isolate primary human
monocytes or macrophages that contained only a single internalized cryptococcal cell.
We used this population to directly compare Trojan horse and free fungal transit across
a similar BBB model and found that both mechanisms contribute to overall transmigration
[23]. We further showed that immune signals that are normally generated during cryptococcal
infection preferentially stimulate Trojan horse transit and that this mode of entry
provides an alternative for fungal mutants that cannot otherwise traverse the BBB.
Finally, we used live microscopy to directly visualize C. neoformans-infected phagocytes
as they crossed model BBB by forming transendothelial pores in the hCMEC. Our microscopic
observations also suggested that phagocytes may serve as “taxis” in addition to Trojan
horses, contributing to brain infection by picking up the cryptococci (which survive
poorly in blood) at distal sites and delivering them to the BBB, where the free fungi
can cross independently.
Parasitic infections
Parasitic infections cause high burdens of disease in low- and middle-income countries,
with almost 800,000 deaths in 2015 (Table 1). Several parasites cause devastating
CNS pathology, either while remaining in the vasculature—like the parasite that causes
malaria—or by crossing the BBB. Here we focus our discussion on a parasite that is
estimated to infect one-third of the world, Toxoplasma gondii [24].
T. gondii is acquired orally and colonizes the gastrointestinal tract. In healthy
humans, a robust immune response halts the rapid parasite proliferation that would
cause severe acute disease in an immunocompromised host. However, even immunocompetent
individuals do not completely clear the infection and remain chronically infected
with quiescent parasite cysts, mainly in tissues of the CNS and skeletal muscle. Support
for Trojan horse transport of T. gondii derives from studies similar to those mentioned
above for other pathogens, mainly adoptive transfer studies showing that parasitized
monocytes or dendritic cells cause brain infection faster than free parasites [25].
Consistent with these observations, the injection of intracellular parasites together
with antibodies against CD11b, which blocks phagocyte migration, reduced brain burden
2-fold. Furthermore, enhanced transendothelial migration of infected leukocytes has
been observed in some, although not all, in vitro studies [26, 27]; another study
using a robust BBB model consisting of brain endothelia and astrocytes reported the
preferential transmigration of infected monocytes [28]. Lastly, T. gondii Trojan horse
transit has been visualized in vitro, although these studies used an activated non-brain
endothelial cell line (human umbilical vein endothelial cells [HUVECs])[29].
As with the other pathogens discussed here, free T. gondii likely also cross the BBB,
although the relative frequency of the two processes is not known. Notably, infection
causes endothelial cells to become activated, with up-regulation of adhesion molecules
and down-regulation of junctional complexes [28]; both of these processes could stimulate
phagocyte transmigration and thus promote Trojan horse transit. Intravital microscopy
has also shown that BMECs serve as a replicative niche for T. gondii and that intracellular
replication is required for egress (through host cell lysis) into the CNS [30]. Interestingly,
the same experiments did not show Trojan horse movement, although they did reveal
infected phagocytes trapped on the vascular side of brain vessels; these may act as
taxis (as with C. neoformans), serving as a source of free parasites to infect BMECs
or cross the BBB. This idea has recently been supported by the observation that adhesion
of infected leukocytes to endothelial cells in vivo triggers parasite egress [31].
Perspectives and conclusions
Only a few pathogens cause significant pathology in the brain, yet they collectively
lead to over 1 million deaths every year (Table 1). Understanding how these microbes
cross the BBB has implications not only for the development of new treatments for
these diseases but also for our understanding of the basic immunobiology of the CNS.
Here we have presented a general model for Trojan horse infection of the brain and
discussed three pathogens that exploit this mechanism. While most of the experimental
support for this process comes from in vitro studies, new technologies like real-time
in vivo imaging are beginning to offer exciting insights into this and related processes.
Beyond the CNS, bloodstream phagocytes play other critical roles in infection, such
as assisting in the dissemination of Salmonella from the gut [32], providing a protected
replicative niche for Leishmania parasites [33], and harboring latent Mycobacterium
reservoirs [34]. Clearly, understanding the complex interactions between phagocytes
and pathogens is of the utmost importance if we wish to elucidate important steps
in pathogenesis that can be targeted for efficient control of these deadly infections.
Related collections
Most cited references24
- Record: found
- Abstract: not found
- Article: not found
How leukocytes cross the vascular endothelium.
Dietmar Vestweber (2015)
- Record: found
- Abstract: found
- Article: not found
Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans.
Caroline Charlier, Kirsten Nielsen, Samira Daou … (2009)
- Record: found
- Abstract: found
- Article: not found
Innate cell communication kick-starts pathogen-specific immunity.
Amariliz Rivera, Mark Siracusa, George Yap … (2016)