- Record: found
- Abstract: found
- Article: found
Interim Results of a Multicenter Trial with the New Electronic Subretinal Implant Alpha AMS in 15 Patients Blind from Inherited Retinal Degenerations
Read this article at
Abstract
Purpose: We assessed the safety and efficacy of a technically advanced subretinal electronic implant, RETINA IMPLANT Alpha AMS, in end stage retinal degeneration in an interim analysis of two ongoing prospective clinical trials. The purpose of this article is to describe the interim functional results (efficacy).
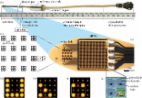
Methods: The subretinal visual prosthesis RETINA IMPLANT Alpha AMS (Retina Implant AG, Reutlingen, Germany) was implanted in 15 blind patients with hereditary retinal degenerations at four study sites with a follow-up period of 12 months ( www.clinicaltrials.gov NCT01024803 and NCT02720640). Functional outcome measures included (1) screen-based standardized 2- or 4-alternative forced-choice (AFC) tests of light perception, light localization, grating detection (basic grating acuity (BaGA) test), and Landolt C-rings; (2) gray level discrimination; (3) performance during activities of daily living (ADL-table tasks).
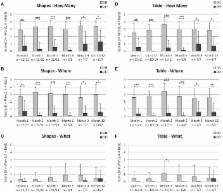
Results: Implant-mediated light perception was observed in 13/15 patients. During the observation period implant mediated localization of visual targets was possible in 13/15 patients. Correct grating detection was achieved for spatial frequencies of 0.1 cpd (cycles per degree) in 4/15; 0.33 cpd in 3/15; 0.66 cpd in 2/15; 1.0 cpd in 2/15 and 3.3 cpd in 1/15 patients. In two patients visual acuity (VA) assessed with Landolt C- rings was 20/546 and 20/1111. Of 6 possible gray levels on average 4.6 ± 0.8 (mean ± SD, n = 10) were discerned. Improvements (power ON vs. OFF) of ADL table tasks were measured in 13/15 patients. Overall, results were stable during the observation period. Serious adverse events (SAEs) were reported in 4 patients: 2 movements of the implant, readjusted in a second surgery; 4 conjunctival erosion/dehiscence, successfully treated; 1 pain event around the coil, successfully treated; 1 partial reduction of silicone oil tamponade leading to distorted vision (silicon oil successfully refilled). The majority of adverse events (AEs) were transient and mostly of mild to moderate intensity.
Conclusions: Psychophysical and subjective data show that RETINA IMPLANT Alpha AMS is reliable, well tolerated and can restore limited visual functions in blind patients with degenerations of the outer retina. Compared with the previous implant Alpha IMS, longevity of the new implant Alpha AMS has been considerably improved. Alpha AMS has meanwhile been certified as a commercially available medical device, reimbursed in Germany by the public health system.
Related collections
Most cited references21
- Record: found
- Abstract: found
- Article: found
Subretinal electronic chips allow blind patients to read letters and combine them to words
- Record: found
- Abstract: found
- Article: not found
Interim results from the international trial of Second Sight's visual prosthesis.
- Record: found
- Abstract: found
- Article: not found