- Record: found
- Abstract: found
- Article: found
Human Immunodeficiency Virus Type 1 Nef protein modulates the lipid composition of virions and host cell membrane microdomains
Read this article at
Abstract
Background
The Nef protein of Human Immunodeficiency Viruses optimizes viral spread in the infected host by manipulating cellular transport and signal transduction machineries. Nef also boosts the infectivity of HIV particles by an unknown mechanism. Recent studies suggested a correlation between the association of Nef with lipid raft microdomains and its positive effects on virion infectivity. Furthermore, the lipidome analysis of HIV-1 particles revealed a marked enrichment of classical raft lipids and thus identified HIV-1 virions as an example for naturally occurring membrane microdomains. Since Nef modulates the protein composition and function of membrane microdomains we tested here if Nef also has the propensity to alter microdomain lipid composition.
Results
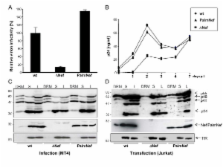
Quantitative mass spectrometric lipidome analysis of highly purified HIV-1 particles revealed that the presence of Nef during virus production from T lymphocytes enforced their raft character via a significant reduction of polyunsaturated phosphatidylcholine species and a specific enrichment of sphingomyelin. In contrast, Nef did not significantly affect virion levels of phosphoglycerolipids or cholesterol. The observed alterations in virion lipid composition were insufficient to mediate Nef's effect on particle infectivity and Nef augmented virion infectivity independently of whether virus entry was targeted to or excluded from membrane microdomains. However, altered lipid compositions similar to those observed in virions were also detected in detergent-resistant membrane preparations of virus producing cells.
Related collections
Most cited references70
- Record: found
- Abstract: found
- Article: not found
1H, 13C and 15N chemical shift referencing in biomolecular NMR.
- Record: found
- Abstract: found
- Article: not found
Importance of the nef gene for maintenance of high virus loads and for development of AIDS.
- Record: found
- Abstract: found
- Article: not found