- Record: found
- Abstract: found
- Article: found
Serum under- O-glycosylated IgA1 level is not correlated with glomerular IgA deposition based upon heterogeneity in the composition of immune complexes in IgA nephropathy
Read this article at
Abstract
Background
Although serum under- O-glycosylated IgA1 in IgA nephropathy (IgAN) patients may deposit more preferentially in glomeruli than heavily- O-glycosylated IgA1, the relationship between the glomerular IgA deposition level and the O-glycan profiles of serum IgA1 remains obscure.
Methods
Serum total under- O-glycosylated IgA1 levels were quantified in 32 IgAN patients by an enzyme-linked immunosorbent assay (ELISA) with Helix aspersa (HAA) lectin. Serum under- O-glycosylated polymeric IgA1 (pIgA1) was selectively measured by an original method using mouse Fcα/μ receptor (mFcα/μR) transfectant and flow cytometry (pIgA1 trap). The percentage area of IgA deposition in the whole glomeruli (Area-IgA) was quantified by image analysis on the immunofluorescence of biopsy specimens. Correlations were assessed between the Area-IgA and data from HAA-ELISA or pIgA1 trap. The relationships between clinical parameters and data from HAA-ELISA or pIgA1 trap were analyzed by data mining approach.
Results
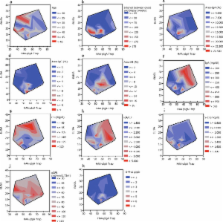
While the under- O-glycosylated IgA1 levels in IgAN patients were significantly higher than those in healthy controls when measured (p < 0.05), there was no significant difference in under- O-glycosylated pIgA1. There was neither a correlation observed between the data from HAA-ELISA and pIgA1 trap (r 2 = 0.09) in the IgAN patients (r 2 = 0.005) nor was there a linear correlation between Area-IgA and data from HAA-ELISA or the pIgA1 trap (r 2 = 0.005, 0.03, respectively). Contour plots of clinical parameters versus data from HAA-ELISA and the pIgA1 trap revealed that patients with a high score in each clinical parameter concentrated in specific areas, showing that patients with specific O-glycan profiles of IgA1 have similar clinical parameters. A decision tree analysis suggested that dominant immune complexes in glomeruli were consisted of: 1) IgA1-IgG and complements, 2) pIgA1 and complements, and 3) monomeric IgA1-IgA or aggregated monomeric IgA1.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies.
- Record: found
- Abstract: found
- Article: not found
Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity.
- Record: found
- Abstract: not found
- Article: not found